
Osteosarcoma at the site of a previously treated simple bone cyst
Introduction
Simple bone cyst (SBC), also known as solitary or unicameral bone cyst, is a mildly expansile, benign lytic bone lesion. It mostly affects children and adolescents with a 2:1 male predominance. Any bone of the extremities could be involved, but the most common locations are the proximal humerus and femur (1-3).
Malignant tumors developed at a previously treated bone cyst without a history of radiation is a rare clinic event that hardly been reported in the literature. In total, only five cases were reported (4-7), and four of them had the initial lesion of aneurysmal bone cyst (ABC), three patients developed osteosarcoma and one patient developed undifferentiated high-grade pleomorphic sarcoma of bone (UHGPS). Only one patient with the initial lesion of SBC was reported, and an UHGPS developed at the same site afterwards (6). The pathogenesis for the malignancy formation is still controversial. In some view, the malignant tumors arose due to malignant transformation of the previous bone cysts. However, as fluorescence in situ hybridization (FISH) was never done on these cases, the possibility that the bone cyst was a secondary lesion from the beginning still cannot be ruled out.
In the case report, we present a 23-year-old patient who developed an osteosarcoma at the site of a previous SBC that was treated with extensive curettage and bone-grafting without a history of radiotherapy. The patient was informed that information regarding his case would be presented for publication, and he provided consent.
Case presentation
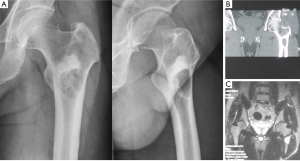
A 23-year-old man was admitted to our hospital in September 2011 because of a mild pain of the left coxofemoral region. Anteroposterior and lateral radiographs (Figure 1A) revealed a well-defined lytic lesion in the intertrochanteric area of the left femur surrounded by a shell of sclerotic bone. Proximal to the lucency, a high-density shadow was presented, which suggested an enhanced sclerotic process of bone in the area. Computed tomography (CT) (Figure 1B) of the left proximal femur revealed a radiolucent lesion in the intertrochanteric area with a rim of bone surrounding the cyst. No periosteal reaction was observed and no soft tissue mass was presented. The high-density shadow area showed homogeneous osteosclerosis performance under CT imaging. Magnetic resonance imaging (MRI) (Figure 1C) showed homogeneous low to intermediate T1 signal and high T2 signal of the lesion, with continuous low signal of bone shell surrounding it. The sclerotic area showed low T1 and T2 signal on MRI imaging. Routine laboratory tests were within normal range.
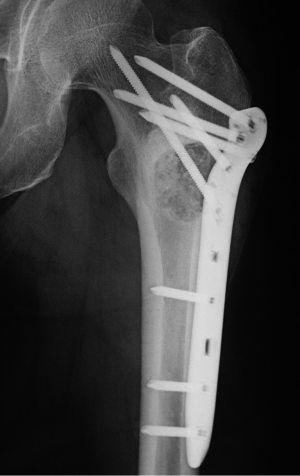
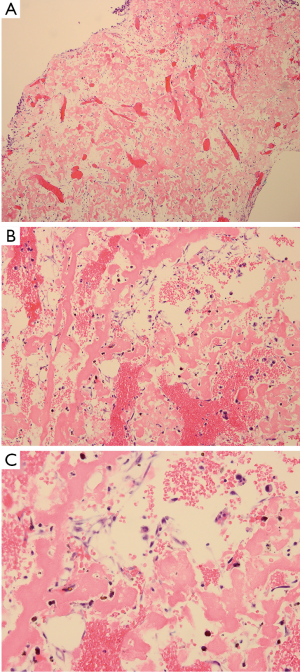
The patient underwent surgery in October 2011. A lateral window was made to expose the lesion, and a clear yellow cystic fluid was observed in the cyst cavity. Cyst wall with thin membranous lining was seen after suction of the cystic fluid. Then, extensive, meticulous curettage was performed, followed by burring of the margins. Anhydrous alcohol was used to soak the cyst cavity. After that, autogenous iliac bone graft implantation and internal fixation was performed to reconstruct the defect (Figure 2). The histological examination revealed fibrovascular tissue with fragments of immature bone, mesenchymal cells, and occasional lymphocytes and osteoclast-like giant cells (Figure 3). The immunohistochemical (IHC) staining results showed: S-100 (−), CD1a (−), PCK (−), CK7 (−), AMACR (−), CD68 (PG-M1) foam cells (+). Pathological diagnosis of SBC was made.
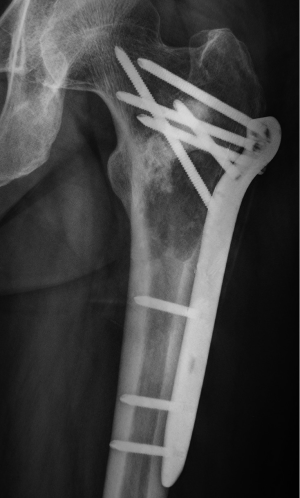
After the curettage surgery, no other adjuvant treatment was given and the wound healed well. Regular follow-up until 22 months post-surgery did not found any clinical or radiographic evidence of recurrence. In August 2013, the patient visited doctors because of persistent pain at the previous surgical site. Radiographs revealed a recurrent osteolytic lesion at almost the same site of the femur (Figure 4). However, the osteolytic area was significantly larger than the previous lesion and the margins were ambiguous. The patient was hospitalized in September 2013, and an open biopsy was performed. Histological examination showed spindle cell component producing osteoid with high grade hypercellularity, abundant mitotic figures, and marked nuclear pleomorphism (Figure 5). A pathological diagnosis of osteosarcoma was made. To ensure the validity of the initial diagnosis of SBC at the previous surgery, the pathology department re-examined the pathological tissues under all-staff consultation. The final pathological diagnosis was still SBC.
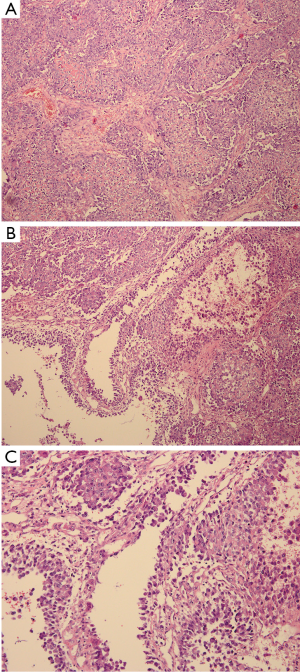
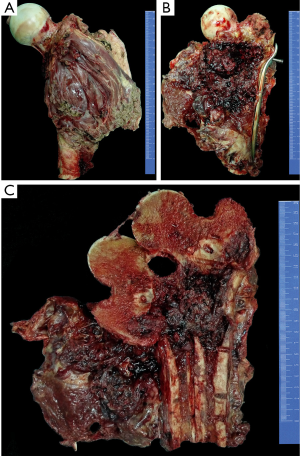
The chemotherapy protocol of this patient was an intensified chemotherapy protocol according to a Randomized Phase III Trial of the European Osteosarcoma Intergroup (8), with a total of 600 mg/m2 of cisplatin and 450 mg/m2 of Adriamycin plus granulocyte colony stimulating factor (G-CSF) separated into six 2-week administration cycles (three pre-surgical cycles and three post-surgical cycles). Three cycles of pre-operative chemotherapy were given to the patient, which continued for nearly two months. In November 2013, a wide resection of the tumor was performed. The defect was reconstructed by a custom-made prosthesis. The gross appearance was presented in Figure 6. After surgery, the patient received three additional courses of chemotherapy. After completion of the post-surgical chemotherapy, the patient had satisfactory function ability. At the latest follow-up in May 2016, the patient was alive with no evidence of disease.
Discussion
Malignant bone lesion development at the site of a previously treated bone cyst without a history of radiotherapy is a rare clinic event with only five patients reported in the literature (4-7). Among them, ABC was the initial lesion in four patients, and only one patient’s previous lesion was SBC with a UHGPS developed at the same site afterwards (6). To our knowledge, it is the first case report that an osteosarcoma developed at the site of a previously treated SBC.
For the patient described in this paper, several possible mechanisms existed to explain the development of the subsequent osteosarcoma. Firstly, the osteosarcoma may be a second primary tumor that arise de novo at the same site of the previous SBC. As the osteosarcoma occurred at the previous surgical site that had been treated with bone-grafting, it may be possible that the grafted bone promoted the development of the osteosarcoma. Although the role has not been firmly clarified, it may be comparable with that a malignant lesion occurring within an area of bone infarction (9-11). In both situations, the reparative and proliferative changes in the border of a dead bone area could form the basis of malignant tumor formation (9). It is suspected that the unbalance in the remodelling process between progenitor cells and the scaffold which provides three-dimensional support was correlated with spontaneous malignant transformation of the progenitor cells (12). As suggested by laboratory studies, a three-dimensional scaffold, including bone graft and engineered scaffold, could form the niche for mesenchymal stem cells (MSC) to develop tumors (12-14).
Secondly, the osteosarcoma may develop from malignant transformation of the SBC. Kyriakos et al. hypothesized that, for ABCs, the varied cell population including proliferating fibroblasts, osteoblasts and osteoclast-type cells support the possibility for the malignant transformation (7). Similarly, varied cells in the membranous lining and cyst wall of SBCs, such as fibroblasts, immature osteoclast-like giant cells and other mesenchymal cells, may also serve as the nidus for malignant tumor formation. It may be comparable with that secondary sarcomas like secondary chondrosarcoma arose due to malignant transformation of osteochondroma (15). The reparative microenvironments and changing growth factors after the initial curettage and bone-grafting may also influence the metabolism of the residual SBC cell components and contribute to the malignant tumor occurrence (12).
For the patient, after extensive curettage of the initial lesion, all the tissue that was removed was under histological examinations. The tissue was found to be typical of SBC, either at the initial examination or at the re-examination under all-staff consultation of pathological department, and no evidence of malignancy was found. It needs to be mentioned that, compared with ABC, the distinguishing between SBC and osteosarcoma is easier for pathologists. A rare form of osteosarcoma named low-grade aneurysmal bone cyst-like osteosarcoma has a totally mimic histological features of ABC (4,16,17), which could make the diagnosis difficult in some cases. Nevertheless, the pathological difference between SBC and osteosarcoma is more distinct, which may be able to confirm that the initial lesion, at least partly, was SBC.
However, we still cannot totally rule out the possibility that the initial lesion contained osteosarcoma from the beginning, and the SBC was secondary to the malignant lesion. The radiograph appearance of the initial lesion was not very typical of SBC, because of a sclerotic appearance just proximal to the lucency area. Besides, the sclerotic area was present after curettage and bone grafting, which suggests that the curettage of the proximal part was possibly incomplete and that the sampling for biopsy of this area was insufficient.
But on the other hand, SBC is hardly reported to be secondary to a malignant lesion, while ABC is sometimes found to be the secondary lesion of sarcomas (18-21) due to their different pathogenesis mechanisms. It is widely accepted that SBC was caused by a focal defect in metaphyseal remodeling which blocks interstitial fluid drainage, thus leading to increased pressure, focal bone necrosis and fluid accumulation (22-24); while ABC was caused by a local circulatory disturbance which leads to increased venous pressure, local hemorrhage and production of blood-filled cavities (25,26). It would very unusual that a SBC occurred as a secondary lesion of an osteosarcoma, and it is hard to explain that why a SBC not an ABC was secondary to the osteosarcoma if the bone cyst was a secondary lesion. We are unable to verify whether an osteosarcoma existed in the initial lesion as the sampling for biopsy of the sclerotic area was insufficient in this patient, and we expected it to be further clarified in future studies when the similar lesion is encountered and reported in the future.
In conclusion, we reported the first case that an osteosarcoma developed at the site of a previously treated SBC. The underlying mechanisms are still nuclear and needed to be further clarified with more cases to be reported in the future.
Acknowledgments
The authors gratefully acknowledge the staff in the Department of Orthopedics, Department of Oncology, and Department of Pathology, West China Hospital, Sichuan University.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neer CS 2nd, Francis KC, Marcove RC, et al. Treatment of unicameral bone cyst. A follow-up study of one hundred seventy-five cases. J Bone Joint Surg Am 1966;48:731-45. [Crossref] [PubMed]
- Sung AD, Anderson ME, Zurakowski D, et al. Unicameral bone cyst: a retrospective study of three surgical treatments. Clin Orthop Relat Res 2008;466:2519-26. [Crossref] [PubMed]
- Cha SM, Shin HD, Kim KC, et al. Does fracture affect the healing time or frequency of recurrence in a simple bone cyst of the proximal femur? Clin Orthop Relat Res 2014;472:3166-76. [Crossref] [PubMed]
- Hsu CC, Wang JW, Huang CH, et al. Osteosarcoma at the site of a previously treated aneurysmal bone cyst. A case report. J Bone Joint Surg Am 2005;87:395-8. [Crossref] [PubMed]
- Anract P, de Pinieux G, Jeanrot C, et al. Malignant fibrous histiocytoma at the site of a previously treated aneurysmal bone cyst: a case report. J Bone Joint Surg Am 2002;84-A:106-11. [Crossref] [PubMed]
- Picci P, Sieberova G, Alberghini M, et al. Late sarcoma development after curettage and bone grafting of benign bone tumors. Eur J Radiol 2011;77:19-25. [Crossref] [PubMed]
- Kyriakos M, Hardy D. Malignant transformation of aneurysmal bone cyst, with an analysis of the literature. Cancer 1991;68:1770-80. [Crossref] [PubMed]
- Lewis IJ, Nooij MA, Whelan J, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 2007;99:112-28. [Crossref] [PubMed]
- Sakkers RJ, van der Heul RO, Kroon HM, et al. Late malignant transformation of a benign giant-cell tumor of bone. A case report. J Bone Joint Surg Am 1997;79:259-62. [Crossref] [PubMed]
- Endo M, Yoshida T, Yamamoto H, et al. Low-grade central osteosarcoma arising from bone infarct. Hum Pathol 2013;44:1184-9. [Crossref] [PubMed]
- Duong S, Sallis JG, Zee SY. Malignant fibrous histiocytoma arising within a bone infarct in a patient with sickle cell trait. Int J Surg Pathol 2004;12:67-73. [Crossref] [PubMed]
- Tasso R, Augello A, Carida' M, et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis 2009;30:150-7. [Crossref] [PubMed]
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007;130:601-10. [Crossref] [PubMed]
- Ramasamy R, Lam EW, Soeiro I, et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia 2007;21:304-10. [Crossref] [PubMed]
- Bovée JV, Sakkers RJ, Geirnaerdt MJ, et al. Intermediate grade osteosarcoma and chondrosarcoma arising in an osteochondroma. A case report of a patient with hereditary multiple exostoses. J Clin Pathol 2002;55:226-9. [Crossref] [PubMed]
- Adler CP. Case report 111. Skeletal Radiol 1980;5:56-60. [Crossref] [PubMed]
- Reed RJ, Rothenberg M. Lesions of bone that may be confused with aneurysmal bone cyst. Clin Orthop Relat Res 1964;150-62. [PubMed]
- Anoop TM, Geetha N, Babanrao SA, et al. Primary osteosarcoma of rib mimicking lung mass with secondary aneurysmal bone cyst formation. J Thorac Oncol 2014;9:738-9. [Crossref] [PubMed]
- Amukotuwa SA, Choong PF, Smith PJ, et al. Femoral mesenchymal chondrosarcoma with secondary aneurysmal bone cysts mimicking a small-cell osteosarcoma. Skeletal Radiol 2006;35:311-8. [Crossref] [PubMed]
- Tay T, Wong SB. Clear cell chondrosarcoma with secondary aneurysmal bone cyst changes. Singapore Med J 2014;55:e49-51. [Crossref] [PubMed]
- Tomoyuki K, Susa M, Nakayama R, et al. Secondary aneurysmal bone cyst following chondroblastoma of the patella. Rare Tumors 2013;5:e43 [Crossref] [PubMed]
- Kadhim M, Thacker M, Kadhim A, et al. Treatment of unicameral bone cyst: systematic review and meta analysis. J Child Orthop 2014;8:171-91. [Crossref] [PubMed]
- Kaelin AJ, MacEwen GD. Unicameral bone cysts. Natural history and the risk of fracture. Int Orthop 1989;13:275-82. [Crossref] [PubMed]
- Lee JH, Reinus WR, Wilson AJ. Quantitative analysis of the plain radiographic appearance of unicameral bone cysts. Invest Radiol 1999;34:28-37. [Crossref] [PubMed]
- Tsagozis P, Brosjö O. Current Strategies for the Treatment of Aneurysmal Bone Cysts. Orthop Rev (Pavia) 2015;7:6182. [Crossref] [PubMed]
- Cottalorda J, Bourelle S. Current treatments of primary aneurysmal bone cysts. J Pediatr Orthop B 2006;15:155-67. [Crossref] [PubMed]


