
Comprehensive survival nomograms for locally advanced gastric cancer: a large population-based real-world study
Highlight box
Key findings
• The nomograms for overall survival and cancer-specific survival in locally advanced gastric cancer (LAGC) were built and validated based on the therapeutic selection and pathological and demographic variables using a national database.
What is known and what is new?
• Gastric cancer ranks as the fourth most common cancer and the third leading cause of global mortality.
• In this study, nomograms were constructed and verified regarding the prediction of overall survival and cancer-specific survival for LAGC based on therapeutic selection, demographic factors, and pathological features.
What is the implication, and what should change now?
• This study can help clinicians make better clinical decisions and encourage LAGC patients to actively receive treatment.
Introduction
Currently, gastric cancer (GC) ranks as the fourth most common cancer and the third leading cause of global mortality (1). Patients with GC are usually diagnosed as locally advanced stage (2) and even advanced stage (3,4). There is an urgent need to reduce the recurrence rate and improve the prognosis of GC patients. The adjuvant radiotherapy with fluorouracil/leucovorin, which was demonstrated by the landmark Intergroup 0116 trial, provides survival benefits for locally advanced gastric cancer (LAGC; T3–4, and/or N+) (5). Accordingly, chemoradiotherapy is recommended as a normative treatment for LAGC patients receiving radical resection by the National Comprehensive Cancer Network (NCCN) guidelines (6). Considering the consistency of treatment and poor prognosis, this study focused on LAGC.
As a general evaluation method for gastric cancer, the American Joint Committee on Cancer (AJCC) staging system plays a critical role in clinical practices but hardly makes accurate individualized predictions for patients with GC since certain prognostic factors are missing, such as therapeutic methods, primary tumor size, age at diagnosis, and so on (7-12). Therefore, comprehensive risk-stratified tools involving treatment selection and demographic factors is recommended to be created for LAGC, which is conducive to making individual decisions in clinical practices and further improving survival rates. A nomogram is a two-dimensional diagram from a computation of mathematical functions which allow the estimation of specific endpoints to be made for the estimation of specific endpoints. In addition, nomograms offer convenient and prompt predictions for clinical practice.
In this study, nomograms were constructed and verified regarding the prediction of overall survival (OS) and cancer-specific survival (CSS) for LAGC based on therapeutic selection, demographic factors, and pathological features. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1255/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Surveillance, Epidemiology, and End Results (SEER) database, which is an open, free, and authoritative source library that includes annually updated information on the clinical features, cancer incidence and survival rates in the United States (U.S.), was applied to generate data for the retrospective analysis. The research was limited to the patients with non-metastatic LAGC (ICD-O-3: 8140, 8142, 8143, 8144, 8145, 8201, 8210, 8211, 8230, 8255, 8260, 8261, 8262, 8263, 8290, 8310, 8323, 8480, 8481, 8490) diagnosed from 2004 to 2016, accounting for 35,316 patients in total. Based on CS extension (http://web2.facs.org/cstage0205/stomach/Stomachschema.html and http://web2.facs.org/cstage0205/esophagusgejunction/EsophagusGEJunctionschema.html), T stage was re-classified to align with the 8th AJCC staging system. Therefore, the current study defined LAGC as non-metastatic patients with clinical T-stage T3–4 or N-stage N1–3, corresponding to AJCC clinical stage IB: T1N1M0, all stages IIA, IIB, IIIA, IIIB, and IIIC. The exclusion criteria were as follows: T1–2N0 (n=12,512); survival months is 0 (n=844); autopsy/death certificate only cases (n=14); without positive histology (n=75); missing detailed information for transforming to the 8th AJCC staging (n=114). The final study sample involved 21,757 patients with LAGC (T3–4 and/or N+) (Figure 1).

For each patient, the following demographic, clinical, pathological, and therapeutic variables were acquired: gender, age, ethnicity, marital status, primary tumor size, tumor location, pathological grade, T and N stage, surgery for the primary tumor, chemotherapy, radiotherapy, regional nodes examined (RNE), and follow-up information. All qualified patients were randomly separated into training (n=14,505) and validation (n=7,252) groups at a ratio of 2:1.
Statistical analysis
The 95% confidence interval (CI) and hazard ratio (HR) were calculated by Cox regression models. The licensed prognostic factors in the univariate Cox regression model were incorporated into the multivariate analysis. Then, nomograms were constructed and assessed to predict 2-, 3-, and 5-year OS and CSS in the LAGC patients using R software following the results of the multivariate Cox regression analysis. The distinguishing ability of the novel nomograms was verified by various methods, involving the concordance index (C-index), time-dependent receiver operating characteristic (ROC) curve, and the value of the area under the ROC curve (AUC). The calibration curves were plotted to compare the nomogram-predicted survival with the actual survival. The decision curve analysis (DCA) was performed to determine the clinical usefulness by quantifying the net benefits at different threshold probabilities.
X-tile software was used to determine the optimal cut-off values. Statistical analyses were performed with R software (version 3.6.1) and IBM SPSS software (version 25.0) (IBM, Armonk, NY, USA). The related R packages ‘rms’, ‘survival’, ‘magick’, ‘timeROC’, ‘ggplotify’, and ‘cowplot’ were introduced for the creation and evaluation of the nomograms. P<0.05 was considered significant in all statistical analyses.
Results
Patient characteristics
The entire cohort comprised 21,757 patients with histologically confirmed LAGC, who were randomly distributed into training and verification groups at a ratio of 2:1. Table 1 summarizes the demographic, clinical, and pathological characteristics of the study cohort. The cohort was predominantly male (65.14%) and white (70.27%). LAGC patients with a married status accounted for 60.35% of the patients, and 23.26% were diagnosed as mucinous cell carcinoma (MCC) or signet ring cell carcinoma (SRCC). Patients with LAGC who received chemotherapy accounted for 64.88% of the patients, and 78.08% underwent gastrectomy. Overall, the median OS was 17 months (8–39 months) and the median CSS was 18 months (8–41 months).
Table 1
| Characteristics | Total (n=21,757) | Training group (n=14,505) | Validation group (n=7,252) |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 7,585 (34.86) | 5,041 (34.75) | 2,544 (35.08) |
| Male | 14,172 (65.14) | 9,464 (65.25) | 4,708 (64.92) |
| Age (years), n (%) | |||
| ≤50 | 2,556 (11.75) | 1,694 (11.68) | 862 (11.89) |
| 51–60 | 3,977 (18.28) | 2,639 (18.19) | 1,338 (18.45) |
| 61–70 | 5,840 (26.84) | 3,926 (27.07) | 1,914 (26.39) |
| 71–80 | 5,923 (27.22) | 3,956 (27.27) | 1,967 (27.12) |
| >80 | 3,461 (15.91) | 2,290 (15.79) | 1,171 (16.15) |
| Marital status, n (%) | |||
| Married | 13,131 (60.35) | 8,699 (59.97) | 4,432 (61.11) |
| Unmarried/NOS | 8,626 (39.65) | 5,806 (40.03) | 2,820 (38.89) |
| Race, n (%) | |||
| White | 15,288 (70.27) | 10,181 (70.19) | 5,107 (70.42) |
| Black | 2,763 (12.70) | 1,854 (12.78) | 909 (12.53) |
| Other/NOS | 3,706 (17.03) | 2,470 (17.03) | 1,236 (17.04) |
| Tumor location, n (%) | |||
| Cardia | 7,440 (34.20) | 4,932 (34.00) | 2,508 (34.58) |
| Body and fundus | 5,379 (24.72) | 3,632 (25.04) | 1,747 (24.09) |
| Antrum and pylorus | 5,540 (25.46) | 3,654 (25.19) | 1,886 (26.01) |
| Overlapping lesion | 1,663 (7.64) | 1,129 (7.78) | 534 (7.36) |
| NOS | 1,735 (7.97) | 1,158 (7.98) | 577 (7.96) |
| Pathological grade, n (%) | |||
| I–II | 5,751 (26.43) | 3,847 (26.52) | 1,904 (26.25) |
| III/IV | 14,432 (66.33) | 9,629 (66.38) | 4,803 (66.23) |
| Unknown | 1,574 (7.23) | 1,029 (7.09) | 545 (7.52) |
| Histological type, n (%) | |||
| Adenocarcinomas | 16,696 (76.74) | 11,085 (76.42) | 5,611 (77.37) |
| MCC/SRCC | 5,061 (23.26) | 3,420 (23.58) | 1,641 (22.63) |
| T stage, n (%) | |||
| T1 | 1,562 (7.18) | 1,029 (7.09) | 533 (7.35) |
| T2 | 1,789 (8.22) | 1,201 (8.28) | 588 (8.11) |
| T3 | 10,887 (50.04) | 7,284 (50.22) | 3,603 (49.68) |
| T4a | 5,362 (24.64) | 3,570 (24.61) | 1,792 (24.71) |
| T4b | 2,157 (9.91) | 1,421 (9.80) | 736 (10.15) |
| N stage, n (%) | |||
| N0 | 5,326 (24.48) | 3,509 (24.19) | 1,817 (25.06) |
| N1 | 11,200 (51.48) | 7,516 (51.82) | 3,684 (50.80) |
| N2 | 3,739 (17.19) | 2,505 (17.27) | 1,234 (17.02) |
| N3 | 1,492 (6.86) | 975 (6.72) | 517 (7.13) |
| Surgery, n (%) | |||
| Gastrectomy | 16,988 (78.08) | 11,340 (78.18) | 5,648 (77.88) |
| Non-gastrectomy/NOS | 4,769 (21.92) | 3,165 (21.82) | 1,604 (22.12) |
| Radiotherapy, n (%) | |||
| Neoradiotherapy | 2,282 (10.49) | 1,537 (10.60) | 745 (10.27) |
| Radiotherapy† | 7,642 (35.12) | 5,121 (35.31) | 2,521 (34.76) |
| No/unknown | 11,833 (54.39) | 7,847 (54.10) | 3,986 (54.96) |
| Chemotherapy, n (%) | |||
| Yes | 14,117 (64.88) | 9,385 (64.70) | 4,732 (65.25) |
| No/unknown | 7,640 (35.12) | 5,120 (35.30) | 2,520 (34.75) |
| Tumor size, n (%) | |||
| ≤2 cm | 1,926 (8.85) | 1,276 (8.80) | 650 (8.96) |
| 2–5 cm | 8,223 (37.79) | 5,553 (38.28) | 2,670 (36.82) |
| 5–10 cm | 6,131 (28.18) | 4,071 (28.07) | 2,060 (28.41) |
| >10 cm | 1,027 (4.72) | 677 (4.67) | 350 (4.83) |
| NOS | 4,450 (20.45) | 2,928 (20.19) | 1,522 (20.99) |
| RNE, n (%) | |||
| <5 | 6,331 (29.10) | 4,201 (28.96) | 2,130 (29.37) |
| 5–10 | 2,697 (12.40) | 1,815 (12.51) | 882 (12.16) |
| 11–15 | 3,295 (15.14) | 2,182 (15.04) | 1,113 (15.35) |
| ≥15 | 9,169 (42.14) | 6,138 (42.32) | 3,031 (41.80) |
| NOS | 265 (1.22) | 169 (1.17) | 96 (1.32) |
| OS (months), 95% CI | 17 [8–39] | 17 [8–40] | 17 [8–39] |
| CSS (months), 95% CI | 18 [8–41] | 18 [8–41] | 18 [8–40] |
†, not neoadjuvant. LAGC, locally advanced gastric cancer; NOS, not otherwise specified; MCC, mucinous cell carcinoma; SRCC, signet ring cell carcinoma; RNE, regional nodes examined; OS, overall survival; CSS, cancer-specific survival.
Screening independent prognostic factors
The weight of each variable affecting OS and CSS was calculated by the univariable and multivariable Cox regression models. The qualified factors in the univariate analysis were brought into the Cox regression model for the multivariate analysis. According to the results of the multivariate Cox regression models, 13 variables (i.e., age, marital status, race, tumor location, pathological grade, histological type, T and N stage, surgery for the primary tumor, radiotherapy, chemotherapy, tumor size, and RNE) were confirmed as independent predictors for both OS (Table 2) and CSS (Table 3).
Table 2
| Characteristics | Univariable analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI lower | 95% CI upper | P value | HR | 95% CI lower | 95% CI upper | P value | ||
| Gender | 0.798 | ||||||||
| Female | Reference | NA | |||||||
| Male | 0.995 | 0.954 | 1.037 | 0.798 | |||||
| Age (years) | <0.001 | <0.001 | |||||||
| ≤50 | Reference | Reference | |||||||
| 51–60 | 1.074 | 0.993 | 1.162 | 0.076 | 1.053 | 0.973 | 1.140 | 0.202 | |
| 61–70 | 1.161 | 1.079 | 1.249 | <0.001 | 1.181 | 1.097 | 1.272 | <0.001 | |
| 71–80 | 1.477 | 1.374 | 1.587 | <0.001 | 1.437 | 1.334 | 1.547 | <0.001 | |
| >80 | 2.107 | 1.951 | 2.275 | <0.001 | 1.667 | 1.536 | 1.809 | <0.001 | |
| Marital status | <0.001 | <0.001 | |||||||
| Married | Reference | Reference | |||||||
| Unmarried/NOS | 1.274 | 1.224 | 1.326 | <0.001 | 1.128 | 1.083 | 1.176 | <0.001 | |
| Race | <0.001 | <0.001 | |||||||
| White | Reference | Reference | |||||||
| Black | 0.985 | 0.929 | 1.045 | 0.626 | 1.024 | 0.963 | 1.088 | 0.455 | |
| Other/NOS | 0.741 | 0.701 | 0.784 | <0.001 | 0.789 | 0.744 | 0.836 | <0.001 | |
| Tumor location | <0.001 | <0.001 | |||||||
| Cardia | Reference | Reference | |||||||
| Body and fundus | 0.838 | 0.795 | 0.883 | <0.001 | 0.827 | 0.779 | 0.878 | <0.001 | |
| Antrum and pylorus | 0.876 | 0.832 | 0.923 | <0.001 | 0.832 | 0.782 | 0.884 | <0.001 | |
| Overlapping lesion | 1.160 | 1.075 | 1.252 | <0.001 | 0.949 | 0.874 | 1.031 | 0.218 | |
| NOS | 1.186 | 1.101 | 1.278 | <0.001 | 0.955 | 0.880 | 1.035 | 0.263 | |
| Pathological grade | <0.001 | <0.001 | |||||||
| I–II | Reference | Reference | |||||||
| III/IV | 1.260 | 1.203 | 1.320 | <0.001 | 1.237 | 1.178 | 1.299 | <0.001 | |
| Unknown | 1.575 | 1.450 | 1.711 | <0.001 | 1.080 | 0.991 | 1.177 | 0.078 | |
| Histological type | <0.001 | <0.001 | |||||||
| Adenocarcinomas | Reference | Reference | |||||||
| MCC/SRCC | 1.124 | 1.074 | 1.177 | <0.001 | 1.118 | 1.066 | 1.173 | <0.001 | |
| T stage | <0.001 | <0.001 | |||||||
| T1 | Reference | Reference | |||||||
| T2 | 0.952 | 0.855 | 1.061 | 0.375 | 1.014 | 0.909 | 1.130 | 0.804 | |
| T3 | 1.173 | 1.078 | 1.276 | <0.001 | 1.352 | 1.238 | 1.476 | <0.001 | |
| T4a | 1.604 | 1.469 | 1.751 | <0.001 | 1.685 | 1.538 | 1.847 | <0.001 | |
| T4b | 2.397 | 2.173 | 2.643 | <0.001 | 2.228 | 2.010 | 2.469 | <0.001 | |
| N stage | <0.001 | <0.001 | |||||||
| N0 | Reference | Reference | |||||||
| N1 | 1.074 | 1.022 | 1.129 | 0.005 | 1.446 | 1.372 | 1.525 | <0.001 | |
| N2 | 1.398 | 1.314 | 1.487 | <0.001 | 2.252 | 2.105 | 2.408 | <0.001 | |
| N3 | 1.928 | 1.772 | 2.098 | <0.001 | 3.276 | 2.987 | 3.594 | <0.001 | |
| Surgery | <0.001 | <0.001 | |||||||
| Gastrectomy | Reference | Reference | |||||||
| Non-gastrectomy/NOS | 2.514 | 2.401 | 2.632 | <0.001 | 1.921 | 1.774 | 2.079 | <0.001 | |
| Radiotherapy | <0.001 | <0.001 | |||||||
| Neoradiotherapy | Reference | Reference | |||||||
| Radiotherapy† | 1.202 | 1.114 | 1.297 | <0.001 | 0.870 | 0.799 | 0.947 | 0.001 | |
| No/unknown | 1.680 | 1.562 | 1.807 | <0.001 | 1.065 | 0.975 | 1.163 | 0.163 | |
| Chemotherapy | <0.001 | <0.001 | |||||||
| Yes | Reference | Reference | |||||||
| No/unknown | 1.623 | 1.559 | 1.690 | <0.001 | 1.561 | 1.483 | 1.643 | <0.001 | |
| Tumor size | <0.001 | <0.001 | |||||||
| ≤2 cm | Reference | Reference | |||||||
| 2–5 cm | 1.210 | 1.117 | 1.312 | <0.001 | 1.142 | 1.053 | 1.239 | 0.001 | |
| 5–10 cm | 1.362 | 1.254 | 1.479 | <0.001 | 1.197 | 1.100 | 1.303 | <0.001 | |
| >10 cm | 1.798 | 1.605 | 2.014 | <0.001 | 1.368 | 1.216 | 1.539 | <0.001 | |
| NOS | 2.123 | 1.952 | 2.310 | <0.001 | 1.377 | 1.261 | 1.503 | <0.001 | |
| RNE | <0.001 | <0.001 | |||||||
| <5 | Reference | Reference | |||||||
| 5–10 | 0.577 | 0.541 | 0.615 | <0.001 | 0.914 | 0.842 | 0.993 | 0.033 | |
| 11–15 | 0.502 | 0.471 | 0.534 | <0.001 | 0.744 | 0.685 | 0.807 | <0.001 | |
| ≥15 | 0.458 | 0.437 | 0.480 | <0.001 | 0.605 | 0.562 | 0.652 | <0.001 | |
| NOS | 0.882 | 0.744 | 1.046 | 0.150 | 1.073 | 0.902 | 1.278 | 0.426 | |
†, not neoadjuvant. OS, overall survival; HR, hazard ratio; CI, confidence interval; NA, unavailable; NOS, not otherwise specified; MCC, mucinous cell carcinoma; SRCC, signet ring cell carcinoma; RNE, regional nodes examined.
Table 3
| Characteristics | Univariable analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI lower | 95% CI upper | P value | HR | 95% CI lower | 95% CI upper | P value | ||
| Gender | 0.396 | ||||||||
| Female | Reference | NA | |||||||
| Male | 0.979 | 0.931 | 1.029 | 0.396 | |||||
| Age (years) | <0.001 | <0.001 | |||||||
| ≤50 | Reference | Reference | |||||||
| 51–60 | 1.036 | 0.951 | 1.128 | 0.416 | 1.012 | 0.929 | 1.103 | 0.787 | |
| 61–70 | 1.042 | 0.961 | 1.130 | 0.314 | 1.088 | 1.002 | 1.181 | 0.044 | |
| 71–80 | 1.257 | 1.159 | 1.363 | <0.001 | 1.265 | 1.164 | 1.375 | <0.001 | |
| >80 | 1.728 | 1.581 | 1.890 | <0.001 | 1.435 | 1.304 | 1.578 | <0.001 | |
| Marital status | <0.001 | <0.001 | |||||||
| Married | Reference | Reference | |||||||
| Unmarried/NOS | 1.241 | 1.183 | 1.303 | <0.001 | 1.097 | 1.043 | 1.153 | <0.001 | |
| Race | <0.001 | <0.001 | |||||||
| White | Reference | Reference | |||||||
| Black | 0.952 | 0.886 | 1.024 | 0.189 | 0.977 | 0.906 | 1.054 | 0.553 | |
| Other/NOS | 0.751 | 0.702 | 0.802 | <0.001 | 0.813 | 0.759 | 0.871 | <0.001 | |
| Tumor location | <0.001 | <0.001 | |||||||
| Cardia | Reference | Reference | |||||||
| Body and fundus | 0.791 | 0.742 | 0.844 | <0.001 | 0.771 | 0.717 | 0.829 | <0.001 | |
| Antrum and pylorus | 0.843 | 0.791 | 0.897 | <0.001 | 0.800 | 0.743 | 0.862 | <0.001 | |
| Overlapping lesion | 1.144 | 1.044 | 1.253 | 0.004 | 0.877 | 0.794 | 0.968 | 0.009 | |
| NOS | 1.154 | 1.054 | 1.264 | 0.002 | 0.894 | 0.810 | 0.986 | 0.025 | |
| Pathological grade | <0.001 | <0.001 | |||||||
| I–II | Reference | Reference | |||||||
| III/IV | 1.414 | 1.334 | 1.498 | <0.001 | 1.351 | 1.271 | 1.436 | <0.001 | |
| Unknown | 1.817 | 1.645 | 2.008 | <0.001 | 1.160 | 1.047 | 1.287 | 0.005 | |
| Histological type | <0.001 | <0.001 | |||||||
| Adenocarcinomas | Reference | Reference | |||||||
| MCC/SRCC | 1.200 | 1.136 | 1.267 | <0.001 | 1.134 | 1.071 | 1.201 | <0.001 | |
| T stage | <0.001 | <0.001 | |||||||
| T1 | Reference | Reference | |||||||
| T2 | 1.004 | 0.871 | 1.158 | 0.955 | 1.071 | 0.928 | 1.235 | 0.350 | |
| T3 | 1.374 | 1.228 | 1.536 | <0.001 | 1.555 | 1.385 | 1.745 | <0.001 | |
| T4a | 1.975 | 1.760 | 2.215 | <0.001 | 2.037 | 1.809 | 2.294 | <0.001 | |
| T4b | 3.073 | 2.712 | 3.483 | <0.001 | 2.788 | 2.447 | 3.178 | <0.001 | |
| N stage | <0.001 | <0.001 | |||||||
| N0 | Reference | Reference | |||||||
| N1 | 1.123 | 1.055 | 1.195 | <0.001 | 1.519 | 1.422 | 1.622 | <0.001 | |
| N2 | 1.552 | 1.440 | 1.673 | <0.001 | 2.531 | 2.334 | 2.746 | <0.001 | |
| N3 | 2.198 | 1.992 | 2.426 | <0.001 | 3.629 | 3.257 | 4.044 | <0.001 | |
| Surgery | <0.001 | <0.001 | |||||||
| Gastrectomy | Reference | Reference | |||||||
| Non-gastrectomy/NOS | 2.735 | 2.590 | 2.888 | <0.001 | 1.961 | 1.780 | 2.161 | <0.001 | |
| Radiotherapy | <0.001 | <0.001 | |||||||
| Neoradiotherapy | Reference | Reference | |||||||
| Radiotherapy† | 1.198 | 1.097 | 1.309 | <0.001 | 0.880 | 0.797 | 0.973 | 0.012 | |
| No/unknown | 1.602 | 1.471 | 1.745 | <0.001 | 1.091 | 0.984 | 1.210 | 0.098 | |
| Chemotherapy | <0.001 | <0.001 | |||||||
| Yes | Reference | Reference | |||||||
| No/unknown | 1.465 | 1.394 | 1.539 | <0.001 | 1.476 | 1.387 | 1.571 | <0.001 | |
| Tumor size | <0.001 | <0.001 | |||||||
| ≤2 cm | Reference | Reference | |||||||
| 2–5 cm | 1.246 | 1.125 | 1.380 | <0.001 | 1.175 | 1.060 | 1.303 | 0.002 | |
| 5–10 cm | 1.461 | 1.317 | 1.621 | <0.001 | 1.256 | 1.129 | 1.398 | <0.001 | |
| >10 cm | 2.068 | 1.802 | 2.373 | <0.001 | 1.500 | 1.301 | 1.731 | <0.001 | |
| NOS | 2.387 | 2.148 | 2.652 | <0.001 | 1.443 | 1.294 | 1.610 | <0.001 | |
| RNE | <0.001 | <0.001 | |||||||
| <5 | Reference | Reference | |||||||
| 5–10 | 0.523 | 0.483 | 0.566 | <0.001 | 0.861 | 0.777 | 0.954 | 0.004 | |
| 11–15 | 0.460 | 0.427 | 0.497 | <0.001 | 0.696 | 0.628 | 0.771 | <0.001 | |
| ≥15 | 0.433 | 0.409 | 0.458 | <0.001 | 0.560 | 0.510 | 0.614 | <0.001 | |
| NOS | 0.908 | 0.744 | 1.108 | 0.343 | 1.129 | 0.921 | 1.384 | 0.244 | |
†, not neoadjuvant. CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval; NA, unavailable; NOS, not otherwise specified; MCC, mucinous cell carcinoma; SRCC, signet ring cell carcinoma; RNE, regional nodes examined.
The nomograms predicting 2-, 3- and 5-year OS and CSS were created using the 13 qualified variables (Figure 2A,2B). Based on the nomograms, the N stage and T stage had the largest contribution to prognosis, followed by surgery and RNE. By adding up the points related to each variable and projecting the total points to the bottom scales, it was easy to calculate the estimated 2-, 3-, and 5-year OS and CSS probabilities.

Calibration and verification of the prognostic nomograms
To identify the discriminating superiority of the nomograms, various methods were used in this study, including C-index values, time-dependent ROC curves, DCA curves, and calibration curves. The C-indexes of the nomogram for the prediction of OS were 0.711 (95% CI: 0.706–0.716) and 0.709 (95% CI: 0.702–0.717) in the training and verification groups, respectively, which were higher than those of the AJCC stage for OS [0.588 (95% CI: 0.581–0.595) in the training cohort and 0.589 (95% CI: 0.579–0.599) in the verification cohort]. The differences between the nomogram and AJCC stage for the prediction of CSS were similar. The C-indexes of the nomogram for predicting CSS were 0.722 (95% CI: 0.715–0.728) in the training cohort and 0.719 (95% CI: 0.710–0.728) in the verification group. Additionally, the AJCC stage illustrated an inferior value for the C-index [0.608 (95% CI: 0.600–0.616) in the training cohort and 0.606 (95% CI: 0.595–0.618) verification cohort (Table 4)].
Table 4
| Groups | OS | CSS | |||
|---|---|---|---|---|---|
| C-index | 95% CI | C-index | 95% CI | ||
| Nomogram-training group | 0.711 | 0.706–0.716 | 0.722 | 0.715–0.728 | |
| AJCC stage-training group | 0.588 | 0.581–0.595 | 0.608 | 0.600–0.616 | |
| Nomogram-validation group | 0.709 | 0.702–0.717 | 0.719 | 0.710–0.728 | |
| AJCC stage-validation group | 0.589 | 0.579–0.599 | 0.606 | 0.595–0.618 | |
OS, overall survival; CSS, cancer-specific survival; C-index, index of concordance; CI, confidence interval; AJCC, American Joint Committee on Cancer.
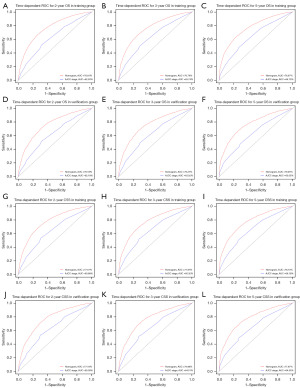
Time-dependent ROC at 2-, 3-, and 5-year were conducted to confirm that the nomograms had higher sensitivities and specificities when predicting the prognosis of OS and CSS compared to the AJCC staging system. The 2-, 3-, and 5-year AUC values of the nomogram for OS were 76.81%, 76.74%, and 76.97%, respectively, compared with 62.50%, 63.74%, and 64.13%, respectively, for that of AJCC stage in the training group (Figure 3A-3C). The AUC values of the nomogram were also superior to the AJCC stage (2-year OS: 76.18% vs. 62.18%; 3-year OS: 76.27% vs. 63.02%; 5-year OS: 76.95% vs. 63.05%) for the validation group (Figure 3D-3F). In addition, the predictive performance of the nomogram for CSS was superior to the AJCC stage in both the training cohort (2-year CSS: 77.57% vs. 63.88%; 3-year CSS: 77.87% vs. 65.32%; 5-year CSS: 78.13% vs. 66.16%) and validation cohort (2-year CSS: 77.16% vs. 63.38%; 3-year CSS: 76.98% vs. 64.51%; 5-year CSS: 77.67% vs. 64.50%) (Figure 3G-3L).
The calibration curves showed no obvious deviations from the reference line, which described an optimal agreement between the actual observations and model prediction for 2-, 3-, and 5-year OS (Figure 4A-4F) and CSS (Figure 4G-4L) in the training and validation cohorts. Moreover, DCA demonstrated the excellent clinical utility of the nomogram, showing superior net benefits and a net reduction in interventions per 100 patients compared to the current AJCC staging system across a wider range of reasonable threshold probabilities for OS and CSS (Figure 5).


Performance of the nomograms in stratifying based on risk points
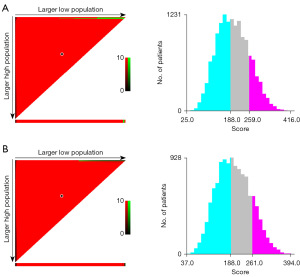
The prognostic points of all the independent variables were assigned based on the established nomogram, and the optimal cut-off values were calculated using X-tile based on the total points (13). According to the cut-off values of the nomogram for OS, the LAGC were divided into low-risk (points <188), moderate-risk (188≤ points <259), and high-risk (points ≥259) (Figure 6A). Similarly, patients were classified as the three subgroups based on the total points (<188, 188 to 261, and ≥261) for CSS (Figure 6B).
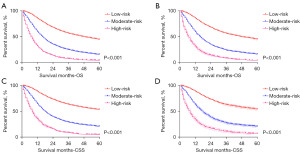
Additionally, the Kaplan-Meier survival curves were subsequently delineated and are shown in Figure 7. In the training cohort, the low-risk group had the longest median survival (OS: 48 months; CSS: 77 months), followed by the moderate-risk group (OS: 16 months; CSS: 18 months), and the high-risk group (both of OS and CSS: 8 months) (Figure 7A,7B). A significant statistical distinction in survival outcomes was observed between the three groups. Similar results were also observed in the verification group (median OS: 48 months for the low-risk group; 16 months for the moderate-risk group; 7 months for the high-risk group; median CSS: 77 months for the low-risk group; 18 months for the moderate-risk group; 8 months for the high-risk group) (Figure 7C,7D).
Discussion
This study, which was based on a national group of LAGC patients receiving treatment between 2004 and 2016, made a remarkable effort to screen independent prognostic factors of OS and CSS and then develop nomograms providing individualized survival assessments and improving personalized management decisions.
The prognosis of gastric cancer is associated with the AJCC staging system. The nomograms revealed that the T stage and N stage contributed the most to discriminating for OS and CSS prediction regarding LAGC. Nonetheless, patients with LAGC usually have an obviously divergent prognosis because of discrepant therapeutic, demographic, and epigenetic backgrounds, even though some LAGC patients are in the same AJCC stage. Moreover, the AJCC staging system cannot accurately reflect the survival benefits from radiotherapy, chemotherapy, and surgical resection for LAGC. The nomograms successfully made up for the shortcoming and intuitively displayed the effects of surgery, chemotherapy, and radiotherapy on prolonging survival. In addition, RNE, as an indicator reflecting the quality of surgery (14), was found to be positively correlated with survival benefits. Schwarz et al. reported that LAGC patients with RNE ≥15 had the best long-term survival (15), which was consistent with the nomograms. Furthermore, the removal of an adequate number of lymph nodes (≥15) is generally considered to be beneficial for staging purposes (6). Therefore, RNE, as a powerful supplement to the N stage, improved the predictive effect of the nomograms in the study.
Several clinical studies confirmed that tumor size influences tumor response to radiochemotherapy and the prognosis of patients with different tumor types, such as rectal cancer (16), neck and head cancer (17,18), and non-small-cell lung cancer (19,20). In addition, an increasing number of studies have focused on discriminating tumor response to radiochemotherapy regarding various types of cancer. Several studies reported that magnetic resonance imaging (MRI)-based radiomics predict pathological response to radiochemotherapy (21-23). However, it is impossible to assess whether the tumor size is related to survival by affecting radiochemosensitivity in the study since this is a limitation of the SEER database.
The prognosis of gastric cancer usually depends on the infiltration depth of the primary tumor and the metastatic status of regional lymph nodes, while it remains unclear about the effect of tumor location on survival outcomes. This study demonstrated that cardiac tumors had a relatively poor prognosis. Another study also revealed that tumor location can be used as a prognostic factor by analyzing the SEER database (24). In addition, the nomograms, with more relevant factors, including grade, histological type, age at diagnosis, marital status, and race, showed higher sensitivities and specificities than AJCC. Furthermore, DCA identifies predictive models that help clinicians make better decisions (25). The superior net benefits and a net reduction in interventions per 100 patients revealed that the nomograms in this study had an excellent value for clinical application compared to the AJCC stage.
To better incorporate these findings into clinical practice, this study classified LAGC into low-, moderate-, and high-risk based on the nomograms. Although the demographic and pathological factors cannot be changed, aggressive treatment can reduce the risk points and downgrade stratification. For example, a patient with 262 total points (75-year-old: 30 points; unmarried: 10 points; white: 20 points; cardia: 16 points; III grade: 18 points; adenocarcinomas: 0 points; T4a: 25 points; N1: 31 points; gastrectomy: 0 points; without radiotherapy and chemotherapy: 17 and 38 points; 8 cm: 15 points; RNE =3: 42 points; using the OS nomogram), who belonged to the high-risk group, can be downgraded to the moderate-risk group (207 total points) after chemotherapy and radiotherapy. Meanwhile, the nomograms can also help avoid overtreatment. Radiotherapy and/or chemotherapy, especially combined chemotherapy, are questionable for LAGC with low total points based on the nomograms. In addition, visual survival differences in the nomograms can encourage patients in need of radiochemotherapy to receive treatment actively, which may increase the radiochemotherapeutic ratio and then prolong the survival of LAGC patients.
Previously, several studies constructed nomograms regarding gastric cancer (24,26,27). However, the nomograms in the current study have clear advantages. First, the multiple validation methods better determined the effectiveness of clinical practice. Second, the values of C-indexes regarding the nomograms in this study were superior to those in previous studies (0.680–0.707) (24,26,27). Moreover, the nomograms possessed better sensitivities and specificities compared with the research of Wang et al. (AUC value of predicting 5-year CSS: 78.13% vs. 74.60% in the training cohort; 77.67% vs. 74.70% in the verification) (26).
The limitations of this study include: (I) the use of retrospective data and (II) some important information is missing. First, there is a lack of specific radiotherapy and chemotherapy regimens. In particular, it is not possible to determine whether a patient had undergone neoadjuvant radiotherapy, and the different treatment regimens would seriously affect the patient’s prognosis. In addition, some important tumor markers, such as carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), and carbohydrate associated antigen 19–9 (CA19–9), as well as MSI and HER-2 gene tests are also important for the treatment and prognosis of gastric cancer. The SEER database lacks a description of those important information. Similarly, the overall health status influences the treatment choices and prognosis, but the SEER database does not record information on this aspect (e.g., the Charlson-Deyo score). Despite all those aforementioned limitations, the superior specificity, sensitivity, and excellent clinical value of the nomograms constructed in this study cannot be masked.
Conclusions
Nomograms regarding OS and CSS for LAGC were built and validated based on therapeutic selection and pathological and demographic variables using a national database. Moreover, this study can help clinicians make better clinical decisions and encourage LAGC patients to actively receive treatment.
Acknowledgments
The authors would like to express their gratitude towards the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries for their valuable contributions towards the development of this database.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1255/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1255/coif). Z.Z. reports funding from the Natural Scientific Foundation of China (No. 81702956), the Natural Science Foundation of Hunan Province (Nos. 2020JJ4903 and 2020JJ5920), the Construction of Innovative Ability of National Clinical Research Center for Geriatric Disorders (No. 2019SK2335), the Strategy-Oriented Special Project of Central South University of China (No. ZLXD2017003), and the Colorectal Cancer Medical Seed Research Fund of Beijing Bethune Public Welfare Foundation Named “Effect and Mechanism of YAP1 on EGFR Resistance in K-ras Wild-type Metastatic Colorectal Cancer”. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Sert F, Yalman D, Özkök S. Lymphopaenia and accidental splenic doses: Do they have any prognostic value for locally advanced gastric cancer patients treated with radiochemotherapy? Contemp Oncol (Pozn) 2019;23:226-33. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Hunt RH, Camilleri M, Crowe SE, et al. The stomach in health and disease. Gut 2015;64:1650-68. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Son T, Hyung WJ, Lee JH, et al. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer 2012;118:4687-93. [Crossref] [PubMed]
- Zu H, Wang F, Ma Y, et al. Stage-stratified analysis of prognostic significance of tumor size in patients with gastric cancer. PLoS One 2013;8:e54502. [Crossref] [PubMed]
- Kunisaki C, Makino H, Kimura J, et al. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery 2010;147:204-11. [Crossref] [PubMed]
- Li C, Oh SJ, Kim S, et al. Macroscopic Borrmann type as a simple prognostic indicator in patients with advanced gastric cancer. Oncology 2009;77:197-204. [Crossref] [PubMed]
- Kunisaki C, Akiyama H, Nomura M, et al. Clinicopathologic characteristics and surgical outcomes of mucinous gastric carcinoma. Ann Surg Oncol 2006;13:836-42. [Crossref] [PubMed]
- Talamonti MS, Kim SP, Yao KA, et al. Surgical outcomes of patients with gastric carcinoma: the importance of primary tumor location and microvessel invasion. Surgery 2003;134:720-7; discussion 727-9. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Li Y, Zhao L, Güngör C, et al. The main contributor to the upswing of survival in locally advanced colorectal cancer: an analysis of the SEER database. Therap Adv Gastroenterol 2019;12:1756284819862154. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317-28. [Crossref] [PubMed]
- Liu W, Li Y, Zhu H, et al. The Relationship between Primary Gross Tumor Volume and Tumor Response of Locally Advanced Rectal Cancer: pGTV as a More Accurate Tumor Size Indicator. J Invest Surg 2021;34:181-90. [Crossref] [PubMed]
- Studer G, Rordorf T, Glanzmann C. Impact of tumor volume and systemic therapy on outcome in patients undergoing IMRT for large volume head neck cancer. Radiat Oncol 2011;6:120. [Crossref] [PubMed]
- Strongin A, Yovino S, Taylor R, et al. Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012;82:1823-30. [Crossref] [PubMed]
- Basaki K, Abe Y, Aoki M, et al. Prognostic factors for survival in stage III non-small-cell lung cancer treated with definitive radiation therapy: impact of tumor volume. Int J Radiat Oncol Biol Phys 2006;64:449-54. [Crossref] [PubMed]
- Bradley JD, Ieumwananonthachai N, Purdy JA, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2002;52:49-57. [Crossref] [PubMed]
- Li Y, Liu W, Pei Q, et al. Predicting pathological complete response by comparing MRI-based radiomics pre- and postneoadjuvant radiotherapy for locally advanced rectal cancer. Cancer Med 2019;8:7244-52. [Crossref] [PubMed]
- Cui Y, Yang X, Shi Z, et al. Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 2019;29:1211-20. [Crossref] [PubMed]
- Sun C, Tian X, Liu Z, et al. Radiomic analysis for pretreatment prediction of response to neoadjuvant chemotherapy in locally advanced cervical cancer: A multicentre study. EBioMedicine 2019;46:160-9. [Crossref] [PubMed]
- Yuan SQ, Wu WJ, Qiu MZ, et al. Development and Validation of a Nomogram to Predict the Benefit of Adjuvant Radiotherapy for Patients with Resected Gastric Cancer. J Cancer 2017;8:3498-505. [Crossref] [PubMed]
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565-74. [Crossref] [PubMed]
- Wang CY, Yang J, Zi H, et al. Nomogram for predicting the survival of gastric adenocarcinoma patients who receive surgery and chemotherapy. BMC Cancer 2020;20:10. [Crossref] [PubMed]
- Hirabayashi S, Kosugi S, Isobe Y, et al. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol 2014;25:1179-84. [Crossref] [PubMed]


