
Oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma: a literature review
Introduction
Radical nephroureterectomy (RNU) still represents the gold standard treatment for non-metastatic upper tract urothelial cancer (UTUC) (1), despite an increasing role of kidney-sparing procedures (2). While the RNU procedure was traditionally performed with open surgery, there has been a major shift towards minimally invasive techniques over the past two decades, first with laparoscopy and more recently with robotic assisted surgery (3). This shift has translated into lower surgical morbidity and faster postoperative recovery (4). In general, the assessment and treatment of UTUC patients has evolved over the years, and multi-disciplinary management is required to optimize the oncologic outcomes for what remains a potentially deadly disease (5).
The aim of the present non-systematic review is to provide a comprehensive analysis of reported oncologic outcomes of the RNU procedure, as well as an overview of factors that might impact these oncologic outcomes. We present this article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-882/rc).
Methods
This is a non-systematic review of the literature focusing on the studies reporting on oncologic outcomes of RNU. An electronic literature search was performed using PubMed using “radical nephroureterectomy” and “oncologic outcomes” as free text search terms (Table 1). Both original articles and systematic reviews were considered. Search was limited to articles published in the last 20 years, and only articles in English were considered. Articles cited in review articles from the original literature search were also considered.
Table 1
| Items | Specification |
|---|---|
| Date of search | 11/14/22–4/2/23 |
| Databases and other sources searched | PubMed |
| Search terms used | Free text search terms: radical nephroureterectomy, upper tract urothelial carcinoma, oncologic outcomes |
| Timeframe | 2000–2023 |
| Inclusion criteria | Inclusion criteria: Original articles and systematic reviews, written in English |
| Selection process | The selection process was performed by two of the authors (GG, DR) and consensus was obtained when needed with the assistance of the senior author (RA) |
The impact of surgical technique
From open to laparoscopic RNU and the role of bladder cuff excision
Since the early 2000s, there has been a significant amount of literature dedicated to comparing outcomes for open and laparoscopic RNU. The overwhelming majority of retrospective studies (6-29), as well as the only reported randomized prospective trial (30), concluded that these two techniques have comparable oncologic outcomes for organ-confined disease (Table 2).
Table 2
| Author | Study period | No of cases (technique) | Median FU, mo | 5-yr RFS, % | 5-yr CSS, % | Comment |
|---|---|---|---|---|---|---|
| Bariol (9) | 1992–1999 | 42 (open) | 96 | 82.1* | nr | – |
| 26 (lap) | 101 | 72* | nr | |||
| Muntener (11) | 1993–2001 | 39 (lap) | 74 | 59 | 68 | – |
| Rouprêt (14) | 1994–2004 | 26 (open) | 78 | 51.2 | 61.5 | – |
| 20 (lap) | 68.5 | 71.6 | 90 | |||
| Capitanio (15) | 1987–2007 | 979 (open) | 73 | 76.2 | 73.1 | Lap group: more favorable path stage and lower rate of LVI |
| 270 (lap) | 31 | 86.8 | 85.8 | |||
| Greco (16) | 1999–2003 | 70 (open) | 60 | 73 | – | RFS decreased as path stage increased |
| 70 (open) | 75 | |||||
| Waldert (17) | 1999–2006 | 59 (open) | 41 | 76 | 80 | T stage and grade independent factors for progression and cancer specific mortality |
| 43 (lap) | 79 | 85 | ||||
| Simone (18) | 2003–06 | 40 (open) | 44 | – | 89.9 | Prospective randomized trial—for pT3 and high-grade, CSS and MFS in favor of open |
| 40 (lap) | 79.8 | |||||
| Walton (20) | 1987–2008 | 703 (open) | 36 | 73.7 | 75.4 | – |
| 70 (lap) | 17 | 63.4 | 75.2 | |||
| Stewart (21) | 1992–2000 | 39 (open) | 163 | 79^ | – | – |
| 23 (lap) | 76^ | |||||
| Ariane (22) | 1995–2010 | 459 (open) | 27 | 50.7 | – | Oncologic outcomes for locally advanced disease similar between open and lap |
| 150 (lap) | 52.2 | |||||
| Fairey (23) | 1994–2009 | 403 (open) | 26.4 | 43 | 73 | – |
| 446 (lap) | 33 | 76 | ||||
| Zou (25) | 1999–2013 | 101 (open) | 53 | – | 87.1 | pT stage, tumor grade and LVI independent predictors of cancer specific mortality |
| 21 (lap) | 85.7 | |||||
| Liu (26) | 2000–2013 | 213 (open) | 60 | 47 | 63 | – |
| 52 (lap) | 59 | 70 | ||||
| Moschini (28) | 2006–2018 | 3,227 (open) | 62 | – | – | Lap not inferior to open |
| 757 (lap) | ||||||
| Veeratterapillay (31) | 2004–2018 | 422 (any) | 110.4 | – | 70.5 | – |
*, Metastasis free survival; ^, 10-yr progression free survival. FU, follow-up; RFS, recurrence-free survival; CSS, progression free survival; LVI, lymphovascular invasion; MFS, metastasis free survival.
This is also confirmed in a recent large meta-analysis involving over 20,000 patients by Liu et al., who found no difference in 2-5-year recurrence-free survival, cancer-specific survival, or overall survival between patients undergoing open or laparoscopic RNU (32).
However, when looking at patients with advanced stage disease, a recent systematic review by the European Association of Urology Guidelines panel suggested that laparoscopic bladder cuff excision might translate into worse oncologic outcomes, including increased rate of intravesical recurrence (33). In two recent multicenter propensity score matched analyses from Japan, Shigeta et al. found more atypical recurrence sites and an increased risk of subsequent intravesical recurrence in the “pure” laparoscopic group (34), as well worse oncologic outcomes when only considering T3N0M0 UTUC populations (35). On the other hand, in a smaller study by Lee et al., the oncologic outcomes of laparoscopic RNU in patients with high stage disease were comparable to those of open surgery (24). In one of the largest studies with the longest follow-up to date, Veeratterapillay et al. performed a retrospective analysis of RNU at a UK tertiary referral center. Median follow-up was 9.2 years. The 5- and 10-year CSS rates were 70.5% and 67.1%, respectively (31).
The advent of robotic RNU
In more recent years, the discussion has de facto shifted towards the “oncological safety” of robotic RNU, which seems to offer less surgical morbidity when compared to open RNU (4). A small number of retrospective studies have shown robotic RNUs to produce comparable oncologic outcomes to laparoscopic and open RNUs (36-40) (Table 3). In a large meta-analysis involving over 80,000 patients, no correlation was found between surgical technique—including open, laparoscopic, and robotic—and recurrence-free and cancer-specific survival outcomes (4).
Table 3
| Author | Study period | No of cases | Median FU, mo | 5-yr RFS, % | 5-yr CSS, % | Comment |
|---|---|---|---|---|---|---|
| Lim (36) | 2007–10 | 32 | 45.5 | 68.1 | 75.8 | Female gender and stage ≥ pT2 associated with shorter RFS |
| Aboumohamed (37) | 2008–14 | 65 | 25 | 57.1 | 69.5 | LVI associated with worse CSS |
| Lee (38) | 2004–17 | 161 (open) | 41.71 | – | – | Surgery type not significantly associated with survival outcomes |
| 137 (lap) | 38.1 | |||||
| 124 (robotic) | 23.7 | |||||
| De Groote (39) | 2008–17 | 78 | 15 | 53* | – | – |
| Zeuschner (40) | 2009–19 | 65 (open) | 30.9 | 55.3^ | 68.4# | Lymph node metastases and patient age with strongest impact on PFS |
| 66 (robotic) | 66.7^ | 76.2# |
*, 4-yr; ^, 2-yr progression free survival; #, 2-yr overall survival. FU, follow-up; RFS, recurrence-free survival; CSS, progression free survival; LVI, lymphovascular invasion.
In a recent NCDB (2010-16) analysis including 2,631 patients, the robotic RNU group showed increased rates of lymph node dissection (41). Soria et al. recently proposed a “tetrafecta” composite outcome—including lymph node dissection, bladder cuff excision, lack of complications, negative surgical margins—which they found to be associated with higher 5-year overall survival rates (42). In another recent propensity score matched analysis, Veccia et al. found robotic RNU to be associated with higher rates of “tetrafecta” achievement when compared to laparoscopic RNU (43).
Factors associated with oncologic outcomes
Several studies have looked at the potential impact of different treatment-, patient- and tumor-related factors on the oncologic outcomes of RNU (Figure 1).
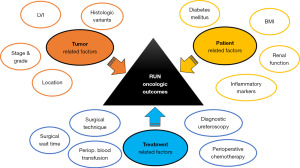
Treatment related factors
Diagnostic ureteroscopy
Multiple meta-analyses have found diagnostic ureteroscopy prior to RNU to be associated with increased risk of intravesical recurrence (IVR) (44,45). Marchioni et al. analyzed 6 studies including 2,382 patients, 765 of which underwent diagnostic URS prior to RNU. All examined studies were retrospective, and the majority examined Asian populations. The IVR rate ranged from 39.2% to 60.7% and from 16.7% to 46% in patients with and without prior URS, respectively. In the pooled analysis, a significant association was found between performance of URS prior to RNU and IVR (HR 1.56; P<0.001) (44). Despite higher risk of IVR, no association between ureteroscopy and long-term survival outcomes seems to be proven in the metanalysis by Guo et al., which included 8 eligible studies containing 3,975 patients (45).
In a more recent UK study (not included in the above meta-analyses), prior URS, T2 stage, proximal ureter tumor and bladder cancer history were predictors of metachronous intravesical recurrence (31). Also, in a recent analysis of 485 cases of minimally invasive RNU from the ROBUUST collaborative group, a diagnostic ureteroscopic biopsy was found to be associated to a 50% higher chance of developing IVR (46).
It has been suggested that future studies examine if immediate administration of intravesical chemotherapy following ureteroscopy might reduce the rates of intravesical recurrence (45).
Surgical wait time
A systematic review by Nowak et al. investigated surgical wait time following UTUC diagnosis and found inconsistent results, suggesting that a safe delay in RNU may be different for different subsets of UTUC patients (47). A review of 138 patients by Lee et al. also found no significant impact of surgical wait time after UTUC diagnosis on cancer-specific survival and recurrence-free survival (48). Interestingly, in a subgroup of 80 of these patients who had urothelial carcinoma of the ureter, cancer-specific survival and recurrence-free survival were significantly higher for those who underwent RNU within a month of their diagnosis than those that waited longer than one month for surgery (48). This suggests that surgical wait time should be minimized for those with ureteral tumors.
Perioperative blood transfusion (PBT)
A few studies have reported an association between PBTs during or after RNU and oncologic outcomes. A study by Rink et al. involving 285 UTUC patients found PBT associated with advanced tumor stage and higher tumor grade, and worse overall survival on multivariate analysis (49). Rieken et al. found PBT to be associated with disease recurrence, cancer-specific survival, and overall survival in univariate analysis, but no association was found in a multivariate Cox regression (50). A study by Bagrodia et al. retrospectively separated patients into groups based on if they received an intraoperative blood transfusion, postoperative blood transfusion, or no blood transfusion, and found no association with survival outcomes on multivariate analysis (51). The varied results suggest that the relationship between PBT and survival in UTUC patients requires further investigation.
Perioperative chemotherapy
There is growing evidence that platinum-based perioperative chemotherapy improves survival for UTUC patients. The only randomized controlled trial to date that has investigated this is the POUT trial (Peri-Operative chemotherapy versus sUrveillance in upper Tract urothelial cancer), which found in a study of 261 participants that adjuvant chemotherapy significantly improved disease-free survival (52). Birtle et al. argued that adjuvant chemotherapy should be the standard of care for patients with pT2-T4 pN0-N3 M0 or pTany N1–3 M0 disease (52). Multiple retrospective studies found that perioperative chemotherapy offers a survival benefit specifically for UTUC patients with pathologic vascular invasion (53) and positive lymph nodes (54,55), but not for patients without these disease features.
Compared to adjuvant chemotherapy, there is less evidence to support neoadjuvant chemotherapy for UTUC patients, especially considering the challenge of accurately staging UTUC prior to RNU (56,57). Pinar et al. argued that there is a strong rationale for neoadjuvant chemotherapy because of the impact of RNU on renal function, however there is insufficient evidence to fully recommend it over adjuvant chemotherapy at this time (58).
Patient-related factors
Baseline renal function
In a meta-analysis of over 4,000 patients by Kim et al., there was a significant negative association between preoperative renal function and survival (59). Another large meta-analysis noted similar findings after dividing patients into two groups: those with chronic kidney disease (CKD; eGFR <60 mL/min/1.73 m2) and those with normal kidney function. The CKD group had worse five-year overall (67.5% vs. 79.4%), cancer-specific (73.6% vs. 83.5%), and progression-free survival (61.5% vs. 74.6%) than the normal kidney function group (60). A linear relationship between eGFR and prognostic survival was suggested also in the study by Li et al. (61). These findings stress the importance of identifying UTUC patients with renal insufficiency prior to RNU.
Diabetes mellitus (DM)
A retrospective multi-institutional study by Rieken et al. evaluated the impact of DM and metformin use among UTUC patients. Their analysis found that diabetic patients who did not use metformin had a significantly higher risk of disease recurrence and worse cancer-specific mortality than diabetic patients who used metformin as well as non-diabetic patients (62). Another study by Gao et al. evaluated DM among UTUC patients, and while no correlation with survival outcomes was identified, they did find higher rates of IVR among patients with DM (63).
Body mass index (BMI)
A single-institutional study of 237 patients found that obesity (measured as BMI ≥30 kg/m2) was independently associated with higher risk of disease recurrence and cancer-specific mortality in patients treated with RNU for UTUC (64). In a study by Murakami et al., obesity was also associated with worse cancer-specific survival, although it was not found to be a prognostic factor on multivariate analysis (65). Interestingly, a study by Inamoto et al. in a Japanese population actually found obesity to be associated with improved cancer specific survival (66), suggesting that the impact of BMI may be controversial. On the other end of the spectrum, multiple studies have found being underweighted (67,68) or having a smaller BMI (69) to be associated with worse cancer-specific survival among UTUC patients.
Markers of systemic inflammation
The neutrophil/lymphocyte ratio (NLR), which is a marker of stress and systemic inflammation, has gained attention in recent years due to its prognostic value for various cancers, including urothelial cancer (70). In UTUC patients, a “high” or “altered” preoperative NLR has been associated with poorer oncologic outcomes as well as worse pathologic features including more advanced tumor stage, lymph node metastasis and lymphovascular invasion (71-75). The NLR may even be able to subclassify patients within a clinical stage and identify a “poor prognostic group”, which may help guide future clinical decision-making (76).
Another marker of systemic inflammation, C-reactive protein (CRP), has been evaluated among UTUC patients. Obata et al. found that patients with an elevated preoperative CRP, >0.5 mg/dL, had significantly lower 5-year recurrence-free and cancer-specific survival rates than those without elevated CRP (77). Similarly, Aziz et al. found that serum CRP above 0.9 mg/dL was associated with more aggressive tumor biology and poorer survival (78). Interestingly, postoperative normalization of CRP level has been associated with improved survival (79).
The Glasgow Prognostic Score (GPS) and modified GPS, which are based on CRP and albumin levels, have also been associated with clinicopathologic features and survival outcomes in UTUC patients (80,81).
Tumor related factors
Tumor stage and grade
A meta-analysis by Cha et al. across 23 institutions found T classification independently associated with disease recurrence and cancer-specific mortality on univariate and multivariate analysis (82). They found high tumor grade associated with these outcomes only on univariate analysis. An analysis across 12 institutions by Margulis et al. found high tumor grade and advancing pathologic T stage associated with disease recurrence and cancer-specific survival (83). Multiple other studies have also reported the significance of tumor stage and grade (84).
One thing to keep in mind is that UTUC might be often underestimated on initial biopsy. A study by Koll et al. found that 61% of patients with < pT1 disease on endoscopic biopsy were upstaged to ≥ pT1 in final pathology, and 30% of patients with low-grade disease were upgraded to high-grade (56). A larger analysis of over 1,200 patients found clinical under-staging to occur in 59.5% of patients and under-grading to occur in 42.4% of patients (57). These findings suggest that one should not solely rely on ureteroscopic biopsy results when stratifying patients based on risk, especially if considering more conservative treatment options.
Tumor location
A difference in risk based on primary tumor location was found in a meta-analysis by Krajewski et al. of over 16,000 patients (85). Patients with ureteral tumors were found to have significantly worse cancer-specific survival, overall survival, and disease-free survival than patients with renal pelvic tumors (85). Another meta-analysis by Wu et al. reported similar findings, except for patients with pT3/4 and pN+ tumors (86). Conversely, a study by Tai et al. found ureteral tumor location to negatively impact survival outcomes only for patients with pT3 disease (87). A related finding by Inamoto et al. is that patients with lower ureteral tumors have a higher rate of urothelial recurrence than those with upper ureteral and pelvic tumors, after adjusting for many prognostic factors (88). This may play a role in the association between ureteral tumor location and poorer survival outcomes.
Tumor multifocality has also been shown to negatively impact survival outcomes. A study by Chromecki et al. involving almost 2,500 patients identified 23.7% to have tumor multifocality at the time of RNU and found this to be an independent predictor of disease progression and cancer specific mortality in patients with organ-confined disease (89). The large meta-analysis by Wu et al. (86) and a single institution study by Milojevic et al. (90) also found multifocal tumors to be negatively associated with survival outcomes.
Lymphovascular invasion (LVI)
After patients undergo RNU, one of the most significant pathological findings is LVI. In a large meta-analysis of almost 30,000 patients by Stangl-Kremser et al., patients with LVI were found to be 43% more likely to have IVR and have 53% lower cancer-specific survival (91). Other smaller retrospective studies also found that LVI predicted or was associated with adverse progression free survival and decreased cancer-specific survival (92,93). This suggests that adjuvant therapies should be strongly considered for patients with a diagnosis of LVI following RNU.
Histologic variants
Another noteworthy pathologic finding following RNU is the presence of a histologic variant, such as a micropapillary, squamous, or sarcomatoid tumor. Rates of histologic variants have been reported between 7.9% and 11.8% (94,95). In a propensity score matched analysis of 1,173 patients by Chung et al., variant histology was independently associated with worse recurrence-free survival, cancer-specific survival, and overall survival (94). In this same study, when they only analyzed patients with variant histology who underwent adjuvant chemotherapy, no significant differences in survival were found, which suggests adjuvant therapy may mitigate the dangers of variant histology.
Conclusions
RNU is a procedure with measured long-term oncologic outcomes. Minimally invasive techniques have gained an established role as they seem to offer comparable “oncologic safety”, although special attention is needed in relation to the method of bladder cuff excision. Robotic RNU is gaining popularity, and while evidence remains limited, the current literature suggests this to be a valid surgical option. Several factors might impact the oncologic outcomes of UTUC patients undergoing RNU. Because of the non-systematic nature of the present review, not all of them are discussed herein. These can be broadly categorized in treatment-related, patient-related, and tumor-related factors. These factors can provide crucial information to help stratify patients based on their relative risk of disease recurrence and overall mortality, which may guide clinical decision-making.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ram A. Pathak and Ashok K. Hemal) for the series “Upper Tract Urothelial Cancer” published in Translational Andrology and Urology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-882/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-882/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-882/coif). The series “Upper Tract Urothelial Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rouprêt M, Babjuk M, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol 2021;79:62-79. [Crossref] [PubMed]
- Saini S, Lukas V, Pathak RA, et al. Robot-Assisted Laparoscopic Ureteral Reconstruction for Malignant Pathology: Single-Center Experience with Analysis of Perioperative, Functional, and Oncologic Outcomes. J Endourol 2023;37:42-9. [Crossref] [PubMed]
- Saini S, Pathak RA, Hemal AK. Robotic nephroureterectomy in the management of upper tract urothelial cancer: inching toward standard of care? Int Urol Nephrol 2022;54:1777-85. [Crossref] [PubMed]
- Veccia A, Antonelli A, Francavilla S, et al. Robotic versus other nephroureterectomy techniques: a systematic review and meta-analysis of over 87,000 cases. World J Urol 2020;38:845-52. [Crossref] [PubMed]
- Kenigsberg AP, Meng X, Ghandour R, et al. Oncologic outcomes of radical nephroureterectomy (RNU). Transl Androl Urol 2020;9:1841-52. [Crossref] [PubMed]
- Gill IS, Sung GT, Hobart MG, et al. Laparoscopic radical nephroureterectomy for upper tract transitional cell carcinoma: the Cleveland Clinic experience. J Urol 2000;164:1513-22. [Crossref] [PubMed]
- Jarrett TW, Chan DY, Cadeddu JA, et al. Laparoscopic nephroureterectomy for the treatment of transitional cell carcinoma of the upper urinary tract. Urology 2001;57:448-53. [Crossref] [PubMed]
- Klingler HC, Lodde M, Pycha A, et al. Modified laparoscopic nephroureterectomy for treatment of upper urinary tract transitional cell cancer is not associated with an increased risk of tumour recurrence. Eur Urol 2003;44:442-7. [Crossref] [PubMed]
- Bariol SV, Stewart GD, McNeill SA, et al. Oncological control following laparoscopic nephroureterectomy: 7-year outcome. J Urol 2004;172:1805-8. [Crossref] [PubMed]
- Rassweiler JJ, Schulze M, Marrero R, et al. Laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma: is it better than open surgery? Eur Urol 2004;46:690-7. [Crossref] [PubMed]
- Muntener M, Nielsen ME, Romero FR, et al. Long-term oncologic outcome after laparoscopic radical nephroureterectomy for upper tract transitional cell carcinoma. Eur Urol 2007;51:1639-44. [Crossref] [PubMed]
- Schatteman P, Chatzopoulos C, Assenmacher C, et al. Laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma: results of a Belgian retrospective multicentre survey. Eur Urol 2007;51:1633-8; discussion 1638. [Crossref] [PubMed]
- Manabe D, Saika T, Ebara S, et al. Comparative study of oncologic outcome of laparoscopic nephroureterectomy and standard nephroureterectomy for upper urinary tract transitional cell carcinoma. Urology 2007;69:457-61. [Crossref] [PubMed]
- Rouprêt M, Hupertan V, Sanderson KM, et al. Oncologic control after open or laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma: a single center experience. Urology 2007;69:656-61. [Crossref] [PubMed]
- Capitanio U, Shariat SF, Isbarn H, et al. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur Urol 2009;56:1-9. [Crossref] [PubMed]
- Greco F, Wagner S, Hoda RM, et al. Laparoscopic vs open radical nephroureterectomy for upper urinary tract urothelial cancer: oncological outcomes and 5-year follow-up. BJU Int 2009;104:1274-8. [Crossref] [PubMed]
- Waldert M, Remzi M, Klingler HC, et al. The oncological results of laparoscopic nephroureterectomy for upper urinary tract transitional cell cancer are equal to those of open nephroureterectomy. BJU Int 2009;103:66-70. [Crossref] [PubMed]
- Simone G, Papalia R, Guaglianone S, et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol 2009;56:520-6. [Crossref] [PubMed]
- Favaretto RL, Shariat SF, Chade DC, et al. Comparison between laparoscopic and open radical nephroureterectomy in a contemporary group of patients: are recurrence and disease-specific survival associated with surgical technique? Eur Urol 2010;58:645-51. [Crossref] [PubMed]
- Walton TJ, Novara G, Matsumoto K, et al. Oncological outcomes after laparoscopic and open radical nephroureterectomy: results from an international cohort. BJU Int 2011;108:406-12. [Crossref] [PubMed]
- Stewart GD, Humphries KJ, Cutress ML, et al. Long-term comparative outcomes of open versus laparoscopic nephroureterectomy for upper urinary tract urothelial-cell carcinoma after a median follow-up of 13 years. J Endourol 2011;25:1329-35. [Crossref] [PubMed]
- Ariane MM, Colin P, Ouzzane A, et al. Assessment of oncologic control obtained after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinomas (UUT-UCs): results from a large French multicenter collaborative study. Ann Surg Oncol 2012;19:301-8. [Crossref] [PubMed]
- Fairey AS, Kassouf W, Estey E, et al. Comparison of oncological outcomes for open and laparoscopic radical nephroureterectomy: results from the Canadian Upper Tract Collaboration. BJU Int 2013;112:791-7. [Crossref] [PubMed]
- Lee JN, Kim BS, Kim HT, et al. Oncologic outcomes of laparoscopic nephroureterectomy for pT3 upper urinary tract urothelial carcinoma. Minerva Urol Nefrol 2014;66:157-64. [PubMed]
- Zou L, Zhang L, Zhang H, et al. Comparison of post-operative intravesical recurrence and oncological outcomes after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinoma. World J Urol 2014;32:565-70. [Crossref] [PubMed]
- Liu JY, Dai YB, Zhou FJ, et al. Laparoscopic versus open nephroureterectomy to treat localized and/or locally advanced upper tract urothelial carcinoma: oncological outcomes from a multicenter study. BMC Surg 2017;17:8. [Crossref] [PubMed]
- Kido K, Hatakeyama S, Fujita N, et al. Oncologic outcomes for open and laparoscopic radical nephroureterectomy in patients with upper tract urothelial carcinoma. Int J Clin Oncol 2018;23:726-33. [Crossref] [PubMed]
- Moschini M, Zamboni S, Afferi L, et al. Comparing oncological outcomes of laparoscopic vs open radical nephroureterectomy for the treatment of upper tract urothelial carcinoma: A propensity score-matched analysis. Arab J Urol 2020;19:31-6. [Crossref] [PubMed]
- Alothman KI, Mehmood S, Alzahrani HM, et al. Surgical and oncological outcome after laparoscopic versus open nephroureterectomy for non-metastatic, upper-tract urothelial carcinoma. A single-centre experience. Saudi Med J 2020;41:25-33. [Crossref] [PubMed]
- Correia J, Mendes G, Texeira B, et al. Perioperative and oncological outcomes of laparoscopic and open radical nephroureterectomy for locally advanced upper tract urothelial carcinoma: a single-center cohort study. Cent European J Urol 2022;75:257-64. [PubMed]
- Veeratterapillay R, Geraghty R, Pandian R, et al. Ten-year survival outcomes after radical nephroureterectomy with a risk-stratified approach using prior diagnostic ureteroscopy: a single-institution observational retrospective cohort study. BJU Int 2022;129:744-51. [Crossref] [PubMed]
- Liu G, Yao Z, Chen G, et al. Laparoscopic compared with open nephroureterectomy in upper urinary tract urothelial carcinoma: A systemic review and a meta-analysis. Int J Clin Pract 2021;75:e14639. [Crossref] [PubMed]
- Peyronnet B, Seisen T, Dominguez-Escrig JL, et al. Oncological Outcomes of Laparoscopic Nephroureterectomy Versus Open Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: An European Association of Urology Guidelines Systematic Review. Eur Urol Focus 2019;5:205-23. [Crossref] [PubMed]
- Shigeta K, Matsumoto K, Takeda T, et al. Evaluating the Oncological Outcomes of Pure Laparoscopic Radical Nephroureterectomy Performed for Upper-Tract Urothelial Carcinoma Patients: A Multicenter Cohort Study Adjusted by Propensity Score Matching. Ann Surg Oncol 2021;28:465-73. [Crossref] [PubMed]
- Shigeta K, Kikuchi E, Abe T, et al. Long-Term Oncologic Outcomes of Laparoscopic Versus Open Radical Nephroureterectomy for Patients with T3N0M0 Upper Tract Urothelial Carcinoma: A Multicenter Cohort Study with Adjustment by Propensity Score Matching. Ann Surg Oncol 2019;26:3774-81. [Crossref] [PubMed]
- Lim SK, Shin TY, Kim KH, et al. Intermediate-term outcomes of robot-assisted laparoscopic nephroureterectomy in upper urinary tract urothelial carcinoma. Clin Genitourin Cancer 2013;11:515-21. [Crossref] [PubMed]
- Aboumohamed AA, Krane LS, Hemal AK. Oncologic Outcomes Following Robot-Assisted Laparoscopic Nephroureterectomy with Bladder Cuff Excision for Upper Tract Urothelial Carcinoma. J Urol 2015;194:1561-6. [Crossref] [PubMed]
- Lee H, Kim HJ, Lee SE, et al. Comparison of oncological and perioperative outcomes of open, laparoscopic, and robotic nephroureterectomy approaches in patients with non-metastatic upper-tract urothelial carcinoma. PLoS One 2019;14:e0210401. [Crossref] [PubMed]
- De Groote R, Decaestecker K, Larcher A, et al. Robot-assisted nephroureterectomy for upper tract urothelial carcinoma: results from three high-volume robotic surgery institutions. J Robot Surg 2020;14:211-9. [Crossref] [PubMed]
- Zeuschner P, Vollmer SG, Linxweiler J, et al. Robot-assisted versus open radical nephroureterectomy for urothelial carcinoma of the upper urinary tract: A retrospective cohort study across ten years. Surg Oncol 2021;38:101607. [Crossref] [PubMed]
- Kenigsberg AP, Smith W, Meng X, et al. Robotic Nephroureterectomy vs Laparoscopic Nephroureterectomy: Increased Utilization, Rates of Lymphadenectomy, Decreased Morbidity Robotically. J Endourol 2021;35:312-8. [Crossref] [PubMed]
- Soria F, Pradere B, Hurle R, et al. Radical Nephroureterectomy Tetrafecta: A Proposal Reporting Surgical Strategy Quality at Surgery. Eur Urol Open Sci 2022;42:1-8. [Crossref] [PubMed]
- Veccia A, Carbonara U, Djaladat H, et al. Robotic vs Laparoscopic Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Multicenter Propensity-Score Matched Pair "tetrafecta" Analysis (ROBUUST Collaborative Group). J Endourol 2022;36:752-9. [Crossref] [PubMed]
- Marchioni M, Primiceri G, Cindolo L, et al. Impact of diagnostic ureteroscopy on intravesical recurrence in patients undergoing radical nephroureterectomy for upper tract urothelial cancer: a systematic review and meta-analysis. BJU Int 2017;120:313-9. [Crossref] [PubMed]
- Guo RQ, Hong P, Xiong GY, et al. Impact of ureteroscopy before radical nephroureterectomy for upper tract urothelial carcinomas on oncological outcomes: a meta-analysis. BJU Int 2018;121:184-93. [Crossref] [PubMed]
- Katims AB, Say R, Derweesh I, et al. Risk Factors for Intravesical Recurrence after Minimally Invasive Nephroureterectomy for Upper Tract Urothelial Cancer (ROBUUST Collaboration). J Urol 2021;206:568-76. [Crossref] [PubMed]
- Nowak Ł, Krajewski W, Łaszkiewicz J, et al. The Impact of Surgical Waiting Time on Oncological Outcomes in Patients with Upper Tract Urothelial Carcinoma Undergoing Radical Nephroureterectomy: A Systematic Review. J Clin Med 2022;11:4007. [Crossref] [PubMed]
- Lee JN, Kwon SY, Choi GS, et al. Impact of surgical wait time on oncologic outcomes in upper urinary tract urothelial carcinoma. J Surg Oncol 2014;110:468-75. [Crossref] [PubMed]
- Rink M, Soave A, Dahlem R, et al. Impact of Perioperative Allogenic Blood Transfusion on Survival After Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. Clin Genitourin Cancer 2016;14:96-104. [Crossref] [PubMed]
- Rieken M, Schubert T, Xylinas E, et al. Association of perioperative blood transfusion with oncologic outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur J Surg Oncol 2014;40:1693-9. [Crossref] [PubMed]
- Bagrodia A, Kaffenberger S, Winer A, et al. Timing of blood transfusion and oncologic outcomes in patients treated with radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 2018;36:645-53. [Crossref] [PubMed]
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet 2020;395:1268-77. [Crossref] [PubMed]
- Matsunaga T, Komura K, Hashimoto T, et al. Adjuvant chemotherapy improves overall survival in patients with localized upper tract urothelial carcinoma harboring pathologic vascular invasion: a propensity score-matched analysis of multi-institutional cohort. World J Urol 2020;38:3183-90. [Crossref] [PubMed]
- Zhai TS, Jin L, Feng LM, et al. Perioperative Chemotherapy on Survival in Patients With Upper Urinary Tract Urothelial Carcinoma Undergoing Nephroureterectomy: A Population-Based Study. Front Oncol 2020;10:481. [Crossref] [PubMed]
- Nazzani S, Preisser F, Mazzone E, et al. Survival effect of perioperative systemic chemotherapy on overall mortality in locally advanced and/or positive regional lymph node non-metastatic urothelial carcinoma of the upper urinary tract. World J Urol 2019;37:1329-37. [Crossref] [PubMed]
- Koll FJ, Meisenzahl E, Haller B, et al. Evaluation of Pre-operative Biopsy, Surgical Procedures and Oncologic Outcomes in Upper Tract Urothelial Carcinoma (UTUC). Front Surg 2021;8:790738. [Crossref] [PubMed]
- Mori K, Katayama S, Laukhtina E, et al. Discordance Between Clinical and Pathological Staging and Grading in Upper Tract Urothelial Carcinoma. Clin Genitourin Cancer 2022;20:95.e1-6. [Crossref] [PubMed]
- Pinar U, Calleris G, Grobet-Jeandin E, et al. The role of perioperative chemotherapy for upper tract urothelial carcinoma patients treated with radical nephroureterectomy. World J Urol 2023; Epub ahead of print. [Crossref] [PubMed]
- Kim MH, Yuk HD, Jeong CW, et al. Estimated Glomerular Filtration Rate as a Prognostic Factor in Urothelial Carcinoma of the Upper Urinary Tract: A Systematic Review and Meta-Analysis. J Clin Med 2021;10:4155. [Crossref] [PubMed]
- Kim TH, Sung HH, Oh JJ, et al. Clinical Implication of Preoperative Renal Function on Oncological Outcomes in Patients with Upper Tract Urothelial Carcinoma after Radical Nephroureterectomy. Biomedicines 2022;10:1340. [Crossref] [PubMed]
- Li S, Chen X, Zheng J, et al. Reduced Preoperative Glomerular Filtration Rate Is Associated With Adverse Postoperative Oncological Prognosis in Patients Undergoing Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Retrospective Cohort Study. Front Surg 2022;9:872273. [Crossref] [PubMed]
- Rieken M, Xylinas E, Kluth L, et al. Diabetes mellitus without metformin intake is associated with worse oncologic outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur J Surg Oncol 2014;40:113-20. [Crossref] [PubMed]
- Gao X, Zhou L, Ai J, et al. The Impact of Diabetes on the Prognosis of Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy: A Systematic Review and Meta-Analysis. Front Oncol 2021;11:741145. [Crossref] [PubMed]
- Dabi Y, El Mrini M, Duquesnes I, et al. Impact of body mass index on the oncological outcomes of patients treated with radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 2018;36:65-71. [Crossref] [PubMed]
- Murakami Y, Matsumoto K, Ikeda M, et al. Impact of body mass index on the oncological outcomes of patients with upper and lower urinary tract cancers treated with radical surgery: A multi-institutional retrospective study. Asia Pac J Clin Oncol 2018;14:310-7. [Crossref] [PubMed]
- Inamoto T, Sassa N, Hattori R, et al. Influence of the Body Mass Index and its Effect on Tumor Characteristics and Survival among a Population with Access to Surgical Management of Upper Tract Urothelial Carcinoma. Curr Urol 2019;12:201-9. [Crossref] [PubMed]
- Yang Z, Bai Y, Hu X, et al. The Prognostic Value of Body Mass Index in Patients With Urothelial Carcinoma After Surgery: A Systematic Review and Meta-Analysis. Dose Response 2020;18:1559325820979247. [Crossref] [PubMed]
- Kang HW, Jung HD, Ha YS, et al. Preoperative Underweight Patients with Upper Tract Urothelial Carcinoma Survive Less after Radical Nephroureterectomy. J Korean Med Sci 2015;30:1483-9. [Crossref] [PubMed]
- Inamoto T, Komura K, Watsuji T, et al. Specific body mass index cut-off value in relation to survival of patients with upper urinary tract urothelial carcinomas. Int J Clin Oncol 2012;17:256-62. [Crossref] [PubMed]
- Cantiello F, Russo GI, Vartolomei MD, et al. Systemic Inflammatory Markers and Oncologic Outcomes in Patients with High-risk Non-muscle-invasive Urothelial Bladder Cancer. Eur Urol Oncol 2018;1:403-10. [Crossref] [PubMed]
- Marchioni M, Cindolo L, Autorino R, et al. High Neutrophil-to-lymphocyte Ratio as Prognostic Factor in Patients Affected by Upper Tract Urothelial Cancer: A Systematic Review and Meta-analysis. Clin Genitourin Cancer 2017;15:343-349.e1. [Crossref] [PubMed]
- Luo HL, Chen YT, Chuang YC, et al. Subclassification of upper urinary tract urothelial carcinoma by the neutrophil-to-lymphocyte ratio (NLR) improves prediction of oncological outcome. BJU Int 2014;113:E144-9. [Crossref] [PubMed]
- Vartolomei MD, Mathieu R, Margulis V, et al. Promising role of preoperative neutrophil-to-lymphocyte ratio in patients treated with radical nephroureterectomy. World J Urol 2017;35:121-30. [Crossref] [PubMed]
- Tan P, Xu H, Liu L, et al. The prognostic value of preoperative neutrophil-to-lymphocyte ratio in patients with upper tract urothelial carcinoma. Clin Chim Acta 2018;485:26-32. [Crossref] [PubMed]
- Vartolomei MD, Kimura S, Ferro M, et al. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta-analysis of 4385 patients. World J Urol 2018;36:1019-29. [Crossref] [PubMed]
- Shao Y, Li W, Wang D, et al. Prognostic value of preoperative lymphocyte-related systemic inflammatory biomarkers in upper tract urothelial carcinoma patients treated with radical nephroureterectomy: a systematic review and meta-analysis. World J Surg Oncol 2020;18:273. [Crossref] [PubMed]
- Obata J, Kikuchi E, Tanaka N, et al. C-reactive protein: a biomarker of survival in patients with localized upper tract urothelial carcinoma treated with radical nephroureterectomy. Urol Oncol 2013;31:1725-30. [Crossref] [PubMed]
- Aziz A, Rink M, Gakis G, et al. Preoperative C-reactive protein in the serum: a prognostic biomarker for upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. Urol Int 2014;93:352-60. [Crossref] [PubMed]
- Tanaka N, Kikuchi E, Shirotake S, et al. The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: a multi-institutional study. Eur Urol 2014;65:227-34. [Crossref] [PubMed]
- Soria F, Giordano A, D'Andrea D, et al. Prognostic value of the systemic inflammation modified Glasgow prognostic score in patients with upper tract urothelial carcinoma (UTUC) treated with radical nephroureterectomy: Results from a large multicenter international collaboration. Urol Oncol 2020;38:602.e11-9. [Crossref] [PubMed]
- Inamoto T, Matsuyama H, Sakano S, et al. The systemic inflammation-based Glasgow Prognostic Score as a powerful prognostic factor in patients with upper tract urothelial carcinoma. Oncotarget 2017;8:113248-57. [Crossref] [PubMed]
- Cha EK, Shariat SF, Kormaksson M, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol 2012;61:818-25. [Crossref] [PubMed]
- Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009;115:1224-33. [Crossref] [PubMed]
- Ehdaie B, Shariat SF, Savage C, et al. Postoperative nomogram for disease recurrence and cancer-specific death for upper tract urothelial carcinoma: comparison to American Joint Committee on Cancer staging classification. Urol J 2014;11:1435-41. [PubMed]
- Krajewski W, Nowak Ł, Małkiewicz B, et al. The Impact of Primary Tumor Location on Long-Term Oncological Outcomes in Patients with Upper Tract Urothelial Carcinoma Treated with Radical Nephroureterectomy: A Systematic Review and Meta-Analysis. J Pers Med 2021;11:1363. [Crossref] [PubMed]
- Wu Y, Dong Q, Liu L, et al. The impact of tumor location and multifocality on prognosis for patients with upper tract urothelial carcinoma: a meta-analysis. Sci Rep 2014;4:6361. [Crossref] [PubMed]
- Tai YS, Chen CH, Huang CY, et al. The effect of tumor location on oncologic outcomes in patients with upper urinary tract urothelial carcinoma stratified by pathologic stage. Urol Oncol 2016;34:4.e19-25. [Crossref] [PubMed]
- Inamoto T, Matsuyama H, Komura K, et al. Tumor Location Based Segmentation in Upper-Tract Urothelial Carcinoma Impacts on the Urothelial Recurrence-Free Survival: A Multi-Institutional Database Study. Curr Urol 2020;14:183-90. [Crossref] [PubMed]
- Chromecki TF, Cha EK, Fajkovic H, et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur Urol 2012;61:245-53. [Crossref] [PubMed]
- Milojevic B, Bumbasirevic U, Santric V, et al. Prognostic significance of tumor multifocality on outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy: A cohort study. Curr Probl Cancer 2021;45:100747. [Crossref] [PubMed]
- Stangl-Kremser J, Muto G, Grosso AA, et al. The impact of lymphovascular invasion in patients treated with radical nephroureterectomy for upper tract urothelial carcinoma: An extensive updated systematic review and meta-analysis. Urol Oncol 2022;40:243-61. [Crossref] [PubMed]
- Song SH, Ye CH, Lee S, et al. Association between lymphovascular invasion and oncologic outcomes among upper urinary tract urothelial carcinoma patients who underwent radical nephroureterectomy. J Cancer Res Clin Oncol 2019;145:2863-70. [Crossref] [PubMed]
- Hurel S, Rouprêt M, Ouzzane A, et al. Impact of lymphovascular invasion on oncological outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int 2013;111:1199-207. [Crossref] [PubMed]
- Chung HS, Hwang EC, Kim MS, et al. Effects of Variant Histology on the Oncologic Outcomes of Patients With Upper Urinary Tract Carcinoma After Radical Nephroureterectomy: A Propensity Score-Matched Analysis. Clin Genitourin Cancer 2019;17:e394-407. [Crossref] [PubMed]
- Tang Q, Xiong G, Li X, et al. The prognostic impact of squamous and glandular differentiation for upper tract urothelial carcinoma patients after radical nephroureterectomy. World J Urol 2016;34:871-7. [Crossref] [PubMed]


