Epithelial Dclk1+ cells are not neural crest derived
Crest-derived progenitors as adult progenitor and cancer initiating cells?
We have read with great interest the editorial by Koga and colleagues in this journal. In their publication, the authors bring forward a fascinating hypothesis as to why Doublecortin-like kinase 1 (Dclk1) might be expressed in some neuroendocrine tumors. Dclk1 is known to be a marker for radial glial cells, which are precursors for neural stem cells. In addition, Dclk1 also labels rare epithelial cells outside of the central nervous system (e.g., tuft cells), some of which may possess progenitor or cancer stem and initiating properties. Based on the fact that Dclk1 is expressed in melanoma, neuroblastoma and some neuroendocrine tumor cells, the authors suggest that the Dclk1+ lineage might share a common origin, namely, the neural crest (1). As neural crest cells are known to undergo epithelial to mesenchymal transition leading them to exit the neural tube, migrate and invade the developing embryo (2,3), this hypothesis of a neural crest origin could in theory explain the high degree of plasticity and tumorigenicity found in Dclk1 cells in the pancreas and the colon, as well as in pancreatic cancer cell lines (4-7). While the degree of plasticity of the crest-derived cells is reduced once they migrate, some cells have been postulated to be multipotent when they arrive in the bowel (8,9) and could therefore be candidates for quiescent stem or progenitor cells in the adult individual.
The notion of a neural crest origin for enteroendocrine (APUD) cells and tumors has been around for decades, and was likely first proposed by Pearse and Pokak (10). A few other studies of migrating neural crest cells were suggestive of a potential contribution of crest cells to the gut epithelium (11). However, in later years, as pointed out by Andrew and colleagues, the evidence that gut and pancreatic endocrine cells are not derived from the neural crest was overwhelming (12). Nevertheless, based on preliminary results from our group, we initially proposed the concept that crest-derived Dclk1+ cells can indeed be found in the adult gastrointestinal tract where they function as reserved stem cells (13), and then set out to test the notion.
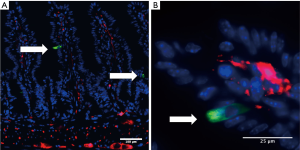
Wnt1 is a bona fide marker of the neural crest (14). To investigate a potential contribution of crest derived cells to the gastrointestinal epithelium in general, and to the Dclk1+ tuft cell lineage specifically, we took advantage of Wnt1-Cre mice that label all neural crest-derived cells (3,15). When crossed to the R26-tdTomato (tdTom) reporter mouse strain, all cells that expressed Wnt1 at any given point in time, or were derived from a Wnt1+ progenitor population, will express the tdTom reporter. We took small intestinal and colonic sections from adult Wnt1-Cre tdTomato mice and stained for Dclk1+ to label the tuft cell lineage. In these experiments, we were not able to identify tdTomato+/Dclk1+ double positive cells, arguing strongly against the neural crest as the predominant origin of intestinal Dclk1+ cells (Figure 1A,B). This is in line with the finding that gastrointestinal tuft cells are largely derived from intestinal progenitors, such as Lgr5+ stem cells (7). Moreover, we did not find any evidence for crest-derived epithelial cells in general. Finally, we did not find any pancreatic exocrine cells (ductal/acinar) within the Wnt1 lineage (not shown). Based on these results, we would argue at this point that, while there are overlapping marker profiles and similarities between neural crest progenitors and epithelial and cancerous Dclk1+ cells, the epithelial Dclk1+ (tuft) cells found in the adult intestine are of epithelial origin, rather than neural crest-derived.
Plasticity as a prerequisite for cancer repair and tumorigenesis?
Accordingly, the overarching question as to why Dclk1+ labels reserve progenitors and potent cancer initiating cells in a variety of organs remains unanswered. While it seems unlikely that NETs are derived from neural crest cells, the expression of Dclk1 in these and other tumors could certainly contribute to self-renewal and plasticity. In various malignancies, an increased level of plasticity has been shown to render cells more prone to malignant transformation (16). Therefore, it is tempting to speculate that Dclk1-expressing cells in different organs share a common regulatory and signaling network that could support both a progenitor phenotype and help sustain early tumor growth. To date, we have shown that Dclk1+ cells in the gut/pancreas are susceptible to Wnt- or Kras-mediated transformation generating adenocarcinomas, respectively, but whether Dclk1+ cells could be the origin of NET-type tumors remains unaddressed. Nevertheless, understanding the potential plasticity of Dclk1+ cells, and the underlying program of Dclk1 gene expression will be important to better understand the role Dclk1+ cells in health and disease.
Acknowledgements
None
Footnote
Provenance: This is a Guest Correspondence commissioned by Editor-in-Chief Zhizhuang Joe Zhao (Pathology Graduate Program, University of Oklahoma Health Sciences Center, Oklahoma City, USA).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Koga H, Ikezono Y, Torimura T. Pancreatic DCLK1 marks quiescent but oncogenic progenitors: a possible link to neuroendocrine tumors. Stem Cell Investig 2016;3:37. [Crossref] [PubMed]
- Dupin E, Calloni GW, Le Douarin NM. The cephalic neural crest of amniote vertebrates is composed of a large majority of precursors endowed with neural, melanocytic, chondrogenic and osteogenic potentialities. Cell Cycle 2010;9:238-49. [Crossref] [PubMed]
- Danielian PS, Muccino D, Rowitch DH, et al. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 1998;8:1323-6. [Crossref] [PubMed]
- Westphalen CB, Takemoto Y, Tanaka T, et al. Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis. Cell Stem Cell 2016;18:441-55. [Crossref] [PubMed]
- Bailey JM, Alsina J, Rasheed ZA, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology 2014;146:245-56. [Crossref] [PubMed]
- Nakanishi Y, Seno H, Fukuoka A, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 2013;45:98-103. [Crossref] [PubMed]
- Westphalen CB, Asfaha S, Hayakawa Y, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest 2014;124:1283-95. [Crossref] [PubMed]
- Dupin E, Creuzet S, Le Douarin NM. The contribution of the neural crest to the vertebrate body. Adv Exp Med Biol 2006;589:96-119. [Crossref] [PubMed]
- Gershon MD, Chalazonitis A, Rothman TP. From neural crest to bowel: development of the enteric nervous system. J Neurobiol 1993;24:199-214. [Crossref] [PubMed]
- Pearse AG, Polak JM. Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut 1971;12:783-8. [Crossref] [PubMed]
- Pomeranz HD, Rothman TP, Gershon MD. Colonization of the post-umbilical bowel by cells derived from the sacral neural crest: direct tracing of cell migration using an intercalating probe and a replication-deficient retrovirus. Development 1991;111:647-55. [PubMed]
- Andrew A, Kramer B, Rawdon BB. The origin of gut and pancreatic neuroendocrine (APUD) cells--the last word? J Pathol 1998;186:117-8. [Crossref] [PubMed]
- Quante M, Westphalen CB, Asfaha S, et al. 515a DCLK1 Labels a Multipotent and Residual Neural Crest-Derived Stem Cell in the Gut Epithelium and Enteric Nervous System. Gastroenterology 2012;142:S-108. [Crossref]
- Jiang X, Rowitch DH, Soriano P, et al. Fate of the mammalian cardiac neural crest. Development 2000;127:1607-16. [PubMed]
- Rowitch DH. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci 1999;19:8954-65. [PubMed]
- Roy N, Hebrok M. Regulation of Cellular Identity in Cancer. Dev Cell 2015;35:674-84. [Crossref] [PubMed]
Cite this article as: Westphalen CB, Middelhoff M, Quante M, Wang TC. Epithelial Dclk1+ cells are not neural crest derived. Stem Cell Investig 2016;3:60.


