
Role of metformin in treatment of patients with chronic obstructive pulmonary disease: a systematic review
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease with features such as continuous respiratory symptoms and airflow limitations. It is often associated with coexisting chronic diseases that increases its morbidity and mortality and significantly burdens health care systems and families (1).
COPD patients usually have increased inflammation and oxidative stress, and are most commonly treated with corticosteroids, moreover, they tend to have a sedentary lifestyle and obesity. All these conditions contribute to the development of metabolic syndrome and diabetes mellitus (DM) in patients with COPD (2) more frequently than in patients without COPD. Gupta et al. (3) concluded that metabolic syndrome is more common in COPD patients than the general population. Since the presence of diabetes increases the potential risk of cardiovascular disease, it is necessary to employ a comprehensive medical care approach for patients with COPD to adequately evaluate and address the various comorbidities associated with DM (3). However, patients with diabetes were observed to exhibit decreased pulmonary function as well as significantly reduced activity-related quality of life and exercise endurance capacity (4). Therefore, identification of appropriate treatments that address both diabetes and COPD appear to be highly important.
Bronchodilator medications and inhaled glucocorticosteroids are conventional pharmacotherapy agents that can significantly improve health status, reduce symptoms, and minimize exacerbation of symptoms in patients with COPD (1). However, due to a basic lack of understanding regarding the pathogenesis of COPD, current treatments are inadequate and do not decelerate disease progression or reduce mortality. Thus, there is an urgent need to develop novel treatments for COPD (5).
Metformin (N,N-dimethylbiguanide) is an anti-glycemic, biguanide drug widely used in the treatment of DM (6,7). It can reduce all-cause mortality and aging-related diseases independent of its effect on diabetes control (8). Several studies have been extended to evaluate the anti-tumour, antiaging, cardiovascular protective, and neuroprotective effects of metformin, as well as its potential as an optional treatment for polycystic ovarian syndrome (9). Metformin can modify glucose flow across the airway epithelium, which limits hyperglycaemia-induced bacterial growth, and might confer additional benefits to prevent and treat respiratory infections (10). It has been described as a geroprotector with anti-aging or anti-oxidant properties that could serve as a therapeutic strategy for COPD (11,12). In recent years, several studies have been conducted on the clinical effectiveness of metformin in the treatment of COPD (13,14). However, additional studies failed to replicate these results (15). Practitioners have often avoided the use of metformin in patients at risk of hypoxia due to a potential risk of lactic acidosis even though metformin is rarely associated with lactic acidosis (16). Due to its rare association with lactic acidosis, which may be fatal, its safety in COPD is uncertain. Therefore, we performed this systematic review to determine if patients with COPD could clinically profit from metformin administration, and to explore the factors which may potentially influence its clinical effect.
Methods
Search strategies
A comprehensive English language literature search from 1997 to 2018 was independently conducted by two investigators (A Zhu and Y Teng) in PubMed, EMBASE, and the Cochrane Database to identify relevant reports. The following terms were used: Metformin OR melbine OR dimethyldiguanide OR Glucophage OR OAD OR OHA OR (Oral antidiabetic agents) OR (Oral hypoglycemic agents) AND (Chronic Obstructive Pulmonary Disease) OR bronchitis OR (bronchial hyper-reactivity) OR (pulmonary emphysema) OR COPD OR COLD OR emphysema OR (airway obstruction) OR (airway inflammation).
Study selection criteria
Eligible studies: (I) those in which the trial group comprised patients exposed to metformin and the control group included unexposed patients; (II) studies in which the effects of metformin were assessed by comparing with the baseline values. Abstracts, including those providing the relevant information, but without the full text, were included. We included studies of adult patients with or without DM, and diagnosed with COPD based on criteria set forth in the Global Strategy for the Diagnosis, Management, and Prevention of COPD, GOLD (17) or ATS/ERS. We excluded studies which investigated animal models or in vitro cultures.
Data extracted
Two reviewers (A Zhu and Y Teng) independently scanned the titles, abstracts, and full texts of all the articles retrieved according to the search criteria. The studies that met the inclusion criteria were finally selected. Any doubts regarding potentially eligible studies were discussed with a third reviewer (X Yao) until any disagreements were resolved. The included data were derived directly from the paper for each research and included demographic data, administration doses and routes, article type, course of treatment, results assessments, and conclusions.
Results
Quality evaluations were conducted for each randomised controlled trial (RCT) using the Jadad criteria (scoring was based on the descriptions of randomization, double blinding, and withdrawals and dropouts, using an overall maximum score of five) (18). If the score was less than two, we identified it as a poor score; three to five was considered a good score. The RCT study received a score of four (randomization: 1, double-blind: 2, description dropouts: 1) (15). Three articles could not be evaluated for their quality because they were published in the form of an abstract and not enough information could be extracted.
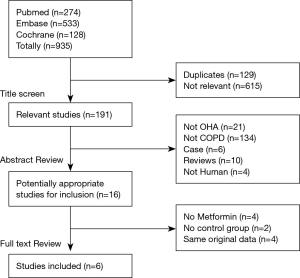
The systematic literature search (Figure 1) revealed the following results. In the primary literature search, 935 studies were identified, of which 615 irrelevant and 129 duplicate studies were excluded after scanning the titles. An additional 175 studies failed to meet the inclusion criteria after screening the abstracts. Of the remaining 16 relevant studies, four included other oral hypoglycemic agents (OHA) and one had no control group (19), and four had duplicate data. As Kim et al. (20) expanded the OHA according to our selection criteria, we eventually excluded their report from this study. Finally, six articles (involving 3,467 participants) were included in our review: one was an RCT (15), four were retrospective studies (13,21-23) (three were published as abstracts) and the last (14) was a prospective, open-label observational study comparing metformin to placebo or baseline (Table 1).

Full table
There were significant differences in the methodology, demographic data, baseline characteristics, and outcome indicators between the RCT and retrospective studies. Moreover, the data were insufficient in the three retrospective studies with relatively strict study designs, based on achieving scores of four or more points in the quality evaluations. Our attempts to obtain the original data by emailing the authors were unsuccessful. Therefore, a meta-analysis would be impractical, and hence a systematic narrative synthesis was carried out.
Effectiveness of metformin in patients with COPD
Health status and symptoms
Sexton et al. (14) demonstrated that there was a significant improvement in the St. George’s Respiratory Questionnaire (SGRQ) total score by a median of five points (P=0.005), which exceeded the minimal clinically significant difference defined as four points. Total transition dyspnea index (TDI), ‘task magnitude’, and ‘effort magnitude’ sub-scores confirmed statistically and clinically significant improvements. Hitchings et al. (15) discovered an unfortunate result in patients with COPD without type 2 diabetes mellitus (T2DM). There was no significant difference in COPD assessment test (CAT) scores or the EXAcerbations of COPD Tool (EXACT) scores between metformin and placebo treatments.
Systemic and airway inflammation
Three articles (14,15,21) reported the outcomes with respect to C-reactive protein (CRP) levels. Turner et al. (21) reported that CRP showed a decreasing trend in the metformin group (18, 5–62 mg/L) compared to controls (47, 16–152 mg/L) (P=0.078), in patients with COPD patients and T2DM, which was similar to the report of Sexton et al. (14) (insufficient data were available). Hitchings et al. reported that metformin use did not decrease in blood sugar in non-diabetic patients admitted for severe exacerbations of COPD when comparing the metformin group and the placebo group (P=0.545) (15). Only one article (14) referred to airway inflammation, and the conclusion was that there was no significant difference in fractional exhaled nitrous oxide (FeNO) levels in patients with COPD with or without metformin treatment.
Hospitalization and survival
Bishwakarma et al. (22) showed that patients with COPD that were on metformin had a lower odds ratio (OR) for all-cause hospitalisations [OR: 0.50; 95% confidence interval (CI): 0.42–0.60] and COPD-related hospitalisations (OR: 0.74; 95% CI: 0.56-0.98) compared to those on insulin (22). Survival was improved in patients that were on metformin (median: 5.2 years; 95% CI: 4.5–5.8) compared to those not on metformin (median: 1.9 years; 95% CI: 1.1–2.6) (P=0.011). This difference remained significant after the analysis was adjusted for age, sex, body weight, year of admission, co-morbidity burdens, illness severity, hemoglobin A1c (HbA1c), and blood glucose concentration. Lactate concentrations were irrelevant for survival (13). Turner et al. demonstrated that 13% of patients on metformin and 23% of patients not on metformin died during admission (P=0.275) and the lengths of hospital stays were similar (P=0.977) (21).
Safety of metformin in patients with COPD
Lactate concentration
Four articles (13,15,21,23) focused on the lactate concentrations in patients with COPD with or without T2DM. Two of the articles reported that the median plasma lactate concentrations were slightly higher in patients on metformin [P=0.012 (13), P=0.11 (23)]. Hitchings et al. (15) demonstrated that lactate concentrations were 2.0 mmol/L in the two groups on/off metformin (mean difference: 0.0; 95% CI: −0.3 to 0.3 mmol/L; P=0.879). The remaining article (21) reported that admission lactate levels exhibited no difference between the groups [lactate levels were 1.4 (1.1–2.0) mm in the metformin-exposed group, and 1.2 (0.7–1.7) mm in the metformin-unexposed group; P=0.191]. Lactic acidosis, defined as a lactate concentration of ≥5 mmol/L was not reported. All of the above indicated that there were no significant differences between the two groups.
Blood glucose and HbA1c
Hitchings et al. (15) reported that the mean inpatient blood glucose concentrations in patients with COPD without T2DM was 7.1±0.9 mmol/L in the metformin group and 8.0±3.3 mmol/L in the placebo group (95% CI: −2.1 to 0.3; P=0.273). Twelve patients (23%) experienced at least one hypoglycemic event, but no significant difference was seen between the two groups (P=0.202) (15). The serum fructosamine concentration was not significantly different between the two groups. Sexton et al. (14) reported that HbA1c decreased by a median value of 3 mmol/mol after metformin treatment in patients with COPD and T2DM.
Adverse events
No serious metformin-related adverse effects were reported besides gastrointestinal effects. Sexton et al. (14) reported that five patients (28%) experienced moderately troublesome gastrointestinal side effects, which were resolved in four of the patients by lowering their metformin dose (the last patient dropped out of the study). Hitchings et al. (15) reported 69 adverse reactions in the metformin group and 25 in the placebo group (event rates of 2.0 and 1.4 per patient, respectively; P=0.149).
Discussion
Summary of main findings
A total of six studies were included in this research. Of these, five studies demonstrated a beneficial role for metformin treatment in patients with COPD and T2DM. The benefits of metformin use in these patients included improvements in health status, decreased dyspnea, and improvements in physiological parameters and prognosis. However, one of the studies failed to support the potential benefits of metformin during severe exacerbations of COPD in patients without T2DM. All studies showed that metformin use was safe in patients with COPD alone, or in combination with T2DM, with little effect on lactate and glucose concentration.
SGRQ or CAT is an established self-administered health status questionnaire that is often used to assess patients with COPD (24,25). A retrospective study showed that the SGRQ total score was strongly associated with clinically and statistically significant improvements in COPD symptoms. These equate to the improvements observed in Randomised trials of long-term bronchodilators for the treatment of COPD. That study intimated that the SGRQ, similar to the TDI, identifies the meaningful symptomatic benefits achieved by metformin use in patients with COPD and T2DM (14). However, the RCT study which designed only one-month time period did not arrive at the same conclusion for patients with COPD with no T2DM. A longer treatment period lasted months to years, may be necessary, given the positive studies.
Inflammation is the main pathophysiological characteristic observed in COPD patients. Biomarkers of systemic inflammation, especially CRP, increase during COPD exacerbations (26). It has been confirmed that metformin is effective in reducing CRP levels. Measuring CRP levels may be an effective way to evaluate the efficacy of metformin therapy in patients with T2DM (27). Recent preclinical and clinical studies have demonstrated that metformin can suppress the inflammatory response and minimize the rate of aging by inhibiting nuclear factor κB via 5’-AMP-activated protein kinase (28,29). Additionally, metformin modifies glucose flux across the airway epithelium to limit hyperglycaemia-induced bacterial growth and prevent infection (10). This concept is consistent with the findings of another in vivo study (30). Metformin has been associated with reduced systemic inflammation. However, there is a lack of robust evidence confirming that metformin can reduce CRP level during severe exacerbations of COPD in patients without coexisting T2DM (15). The negative results in these studies may due to potential selection bias and confounding factors. The FeNO did not change significantly with metformin treatment. This suggests that metformin does not affect allergic airway inflammation in COPD. Dias et al. also showed that there were no effects on NOx and that metformin did not resolve bronchoconstriction in obese mice (31). More studies are needed to delineate the effects of metformin on inflammation.
Diabetes coexisting with exacerbations of obstructive lung disease impacts long-term mortality (32). Our study showed that diabetic patients hospitalized for COPD exacerbations who were prescribed metformin not only survived longer, but they were less likely to be re-admitted for all-cause and COPD related hospitalisations than those who were not prescribed metformin (13,22). However, this finding must be interpreted with caution due to the possible effects of unpredictable confounding factors. Mortality is associated with age, lung function, body mass index (BMI), and many other factors. The presence of these other factors should be considered when making clinical decisions for patients with COPD exacerbations (33). Further studies on metformin treatment in COPD are needed to confirm this.
Metformin directly acts on mitochondria to limit citric acid cycle activity and oxidative phosphorylation, leading cells to accept less glucose carbon, which favours lactic acid production (34). However, the elevations of plasma lactate levels were limited in our study. The possible pathophysiology may lie in the fact that metformin is not metabolized by the liver. It is excreted mainly by the kidneys through the multidrug and toxin extrusion-1 protein that is encoded by the SLC47A1 gene (7). The incidence of lactic acidosis is much lower in patients with normal kidney function (35). In non-COPD cohorts, similar results indicated that patients with DM had a higher risk of developing hyperlactaemia without lactate acidosis (36,37). These data support the expansion of metformin use (38).
Combing with T2DM of COPD participants may benefit from the clinical effectiveness of metformin. As a first-line treatment for most patients with T2DM (39), metformin acts through reducing intestinal glucose absorption, promoting peripheral glucose uptake, decreasing fasting insulin levels, improving insulin sensitivity, and inhibiting gluconeogenesis, which altogether results in a reduction of blood glucose concentration (7). Hyperglycaemia is very common among hospitalized patients experiencing COPD exacerbations (40). In non-diabetics, metformin has a relatively small effect on blood sugar, which extends the application of metformin.
Adverse effects, especially gastrointestinal reactions, were more common in metformin-treated patients. However, no statistically significant difference was observed between the metformin group and the placebo group. Further studies with larger samples are needed to determine the safety of metformin treatment in patients with COPD.
Rinne et al. demonstrated that another OHA (thiazolidinediones) was associated with a significant reduction in the expected number of COPD exacerbations, and provided a new therapeutic strategy to the prevention of COPD exacerbations (41). Kim et al. showed that treatment with insulin sensitizers, such as metformin and thiazolidinediones, was independently associated with improvements in FVC in patients with both COPD and DM (20). RCTs will be required to further evaluate the benefit of OHAs in patients with COPD.
Strengths and limitations
To the best of our knowledge, our study is the first systematic review to focus on the safety and efficacy of metformin in patients with COPD. However, it has certain limitations. We used relatively broad selection criteria. For example, due to insufficient studies in this field, we selected abstracts and studies with baseline comparisons-only in addition to the RCT and case-control studies. Additionally, we could not collect enough information and failed to perform a meta-analysis. Therefore, we could not provide statistically significant evidence to assess the effectiveness of metformin. Studies focusing on the pathophysiologic model of COPD as a disease of advanced aging of the lung are rare, and thus more future research in this area is clearly required.
Implications for future research or clinical practice
We found that metformin is an effective and safe treatment for patients with COPD and T2DM, although there is insufficient evidence to conclude that metformin would benefit patients with COPD without T2DM. However, these findings open the door for possible future COPD therapeutic options. A subpopulation of patients with COPD may respond preferentially to metformin, specifically those with hyperglycaemia and obese individuals. The appropriate dosing and duration of metformin therapy also need to be confirmed. Large samples and multicentre clinical trials in selected subpopulations are urgently required to better address the potential effects of metformin in the treatment of COPD.
Acknowledgments
We thank Rongbin Yu, Hui Qin for their suggestions and comments during the systemic review process. We would like to thank Editage (www.editage.cn) for English language editing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Wells CE, Baker EH. Metabolic syndrome and diabetes mellitus in COPD. Vol 592013:117-34.
- Gupta KK, Singh J, Gupta P, et al. Uncovering metabolic syndrome among chronic obstructive pulmonary disease patients in a tertiary care hospital, India. J Clin Diagn Res 2017;11:OC08-11. [PubMed]
- Kinney GL, Black-Shinn JL, Wan ES, et al. Pulmonary function reduction in diabetes with and without chronic obstructive pulmonary disease. Diabetes Care 2014;37:389-95. [Crossref] [PubMed]
- Ito K, Mercado N. STOP accelerating lung aging for the treatment of COPD. Exp Gerontol 2014;59:21-7. [Crossref] [PubMed]
- Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diab Vasc Dis Res 2008;5:157-67. [Crossref] [PubMed]
- Grzybowska M, Bober J, Olszewska M. Metformin - mechanisms of action and use for the treatment of type 2 diabetes mellitus. Postepy Hig Med Dosw (Online) 2011;65:277-85. [Crossref] [PubMed]
- Campbell JM, Bellman SM, Stephenson MD, et al. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res Rev 2017;40:31-44. [Crossref] [PubMed]
- Wang YW, He SJ, Feng X, et al. Metformin: a review of its potential indications. Drug Des Devel Ther 2017;11:2421-9. [Crossref] [PubMed]
- Garnett JP, Baker EH, Naik S, et al. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax 2013;68:835-45. [Crossref] [PubMed]
- Miłkowska-Dymanowska J, Bialas AJ, Makowska J, et al. Geroprotectors as a therapeutic strategy for COPD - where are we now? Clin Interv Aging 2017;12:1811-7. [Crossref] [PubMed]
- Ito K, Colley T, Mercado N. Geroprotectors as a novel therapeutic strategy for COPD, an accelerating aging disease. Int J Chron Obstruct Pulmon Dis 2012;7:641-52. [Crossref] [PubMed]
- Hitchings AW, Archer JRH, Srivastava SA, et al. Safety of metformin in patients with chronic obstructive pulmonary disease and type 2 diabetes mellitus. COPD 2015;12:126-31. [Crossref]
- Sexton P, Metcalf P, Kolbe J. Respiratory effects of insulin sensitisation with metformin: a prospective observational study. COPD 2014;11:133-42. [Crossref] [PubMed]
- Hitchings AW, Lai D, Jones PW, et al. Metformin in severe exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2016;71:587-93. [Crossref] [PubMed]
- Salpeter S, Greyber E, Pasternak GA, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2002.CD002967. [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J 2017;49:557-82. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Caughey GE, Preiss AK, Vitry AI, et al. Comorbid diabetes and COPD: impact of corticosteroid use on diabetes complications. Diabetes Care 2013;36:3009-14. [Crossref] [PubMed]
- Kim HJ, Lee JY, Jung HS, et al. The impact of insulin sensitisers on lung function in patients with chronic obstructive pulmonary disease and diabetes. Int J Tuberc Lung Dis 2010;14:362-7. [PubMed]
- Turner L, Cull R, Archer JRH, et al. Safety of metformin use in COPD patients with type 2 diabetes requiring hospital admission for exacerbations. Br J Clin Pharmacol 2010;70:290-1.
- Bishwakarma R, Lin YL, Kuo YF, et al. Metformin and health care utilization in patients with coexisting COPD and diabetes. Chest 2016;150:896A. [Crossref]
- Hitchings AW, Aslam S, Picton G, et al. The effect of metformin therapy on serum lactate concentration in patients hospitalised for COPD: A retrospective study. COPD 2011;8:40.
- Canavan JL, Dilaver D, Clark AL, et al. Clinical COPD questionnaire in patients with chronic respiratory disease. Respirology (Carlton, Vic.) 2014;19:1006-12. [Crossref] [PubMed]
- Weldam SW, Schuurmans MJ, Liu R, et al. Evaluation of Quality of Life instruments for use in COPD care and research: a systematic review. Int J Nurs Stud 2013;50:688-707. [Crossref] [PubMed]
- Saldías PF, Diaz PO, Dreyse DJ, et al. Etiology and biomarkers of systemic inflammation in mild to moderate COPD exacerbations. Rev Med Chil 2012;140:10-8. [PubMed]
- Shi L, Tan GS, Zhang K. Relationship of the serum CRP level with the efficacy of metformin in the treatment of type 2 diabetes mellitus: a meta-analysis. J Clin Lab Anal 2016;30:13-22. [Crossref] [PubMed]
- Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets 2015;15:196-205. [Crossref] [PubMed]
- Bulterijs S. Metformin as a geroprotector. Rejuvenation Res 2011;14:469-82. [Crossref] [PubMed]
- Baker EH, Baines DL. Airway glucose homeostasis: a new target in the prevention and treatment of pulmonary infection. Chest 2018;153:507-14. [Crossref] [PubMed]
- Dias MD, Goulart M, Dalecio C, et al. Metformin influences on respiratory system in obese mice induced by postnatal overnutrition. Respir Physiol Neurobiol 2018;247:96-102. [Crossref] [PubMed]
- Koskela HO, Salonen PH, Romppanen J, et al. A history of diabetes but not hyperglycaemia during exacerbation of obstructive lung disease has impact on long-term mortality: a prospective, observational cohort study. BMJ Open. 2015;5:e006794. [Crossref] [PubMed]
- Gudmundsson G, Ulrik CS, Gislason T, et al. Long-term survival in patients hospitalized for chronic obstructive pulmonary disease: a prospective observational study in the Nordic countries. Int J Chron Obstruct Pulmon Dis 2012;7:571-6. [PubMed]
- Andrzejewski S, Gravel SP, Pollak M, et al. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab 2014;2:12. [Crossref] [PubMed]
- Eppenga WL, Lalmohamed A, Geerts AF, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: A population-based cohort study. Diabetes Care 2014;37:2218-24. [Crossref] [PubMed]
- Lin YC, Lin LY, Wang HF, et al. Fasting plasma lactate concentrations in ambulatory elderly patients with type 2 diabetes receiving metformin therapy: a retrospective cross-sectional study. J Chin Med Assoc 2010;73:617-22. [Crossref] [PubMed]
- Koren S, Zilberman-Itskovich S, Koren R, et al. Metformin does not induce hyperlactatemia in patients admitted to internal medicine ward. Isr Med Assoc J 2017;19:300-3. [PubMed]
- Trinkley KE, Anderson H, Malone D, et al. Defining the incidence of lactic acidosis in patients prescribed metformin with and without risk factors for lactic acidosis. J Gen Intern Med 2015;30:S133-4.
- Crawford K. Review of 2017 Diabetes Standards of Care. Nurs Clin North Am 2017;52:621-63. [Crossref] [PubMed]
- Koskela HO, Salonen PH, Niskanen L. Hyperglycaemia during exacerbations of asthma and chronic obstructive pulmonary disease. Clin Respir J 2013;7:382-9. [Crossref] [PubMed]
- Rinne ST, Liu CF, Feemster LC, et al. Thiazolidinediones are associated with a reduced risk of COPD exacerbations. Int J Chron Obstruct Pulmon Dis 2015;10:1591-7. [Crossref] [PubMed]


