Single lung retrieval from a donor supported by a left ventricular assist device
Introduction
A donor organ shortage remains a serious issue for lung transplantation, particularly in Japan (1). In Japan, a donor management system has been established to maximize donor lung usage. The rate of donor lung usage is more than that in other developing countries (2). On the other hand, the number of patients who need cardiac support with a left ventricular assist device (LVAD) has increased over the last decade (3), even in Japan. As reported previously, the chest cavity after implantation of an LVAD tends to become highly conglutinated between the pericardium and heart, lung and parietal pleura, and the device and surrounding tissue (4). The risks of tissue injury during organ retrieval, especially lungs, remain high and may result in a surgeon refusing such lungs for transplantation. A case of successful lung retrieval for a single lung transplantation that was performed from a donor on LVAD support is described. In this report, the potential risks to safe organ retrieval are outlined based on this experience.
Case presentation
A 48-year-old man, a brain-dead donor, was assigned for organ donation by the Japan Organ Transplant Network (JOT). The patient had undergone implantation of an LVAD HEART MATE II® (Thoratec Corp, Pleasanton, CA, USA) as a bridge to heart transplantation seven months earlier because of severe dilated cardiomyopathy and also a cardiac resynchronization therapy-defibrillator (CRT-D) was implanted prior to implantation of the LVAD. However, sudden brain death was caused by cerebral hemorrhage.
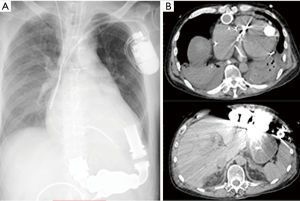
The donor was transferred to a regional hospital after he developed a serious headache and right hemi-paralysis. On arrival at the hospital, he was unconscious and underwent craniotomy for removal of a hematoma three days prior because of intracortical bleeding. The partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio (P/F ratio) was 690.7. The chest X-ray and computed tomography (CT) showed signs of bilateral pulmonary vascular congestion, bilateral pleural effusion, and bilateral lower lobe atelectasis (Figure 1). Bronchoscopy showed some serous secretions in both inferior lobes with a small amount of Gram-positive and -negative bacteria. The P/F ratio decreased gradually to 218.4 by the time of organ retrieval. However, only small amount of bacteria was detected which responded to antibiotics. Secretion in the bronchus was not purulent but serous. Therefore, we judged the inflammation in the donor was caused by atelectasis compressed by pleural effusion. These were the reasons for which we decided to use this lung for transplantation. Eight other institutes had declined organ retrieval for lung transplantation before our institution accepted the offer from JOT, while other institutes had accepted organs for liver, pancreas, and kidney transplantation. However, transplantation of the liver was declined after final confirmation during organ retrieval on account of its poor condition. The lung transplantation recipient was a candidate for single lung transplantation based on the JOT guideline. Thus, it was decided that only the right lung would be used for this recipient.
Organ retrieval was started by sternotomy using an oscillatory bone saw as in re-do sternotomy. There were few adhesions between the sternum and mediastinal tissue, which included the outflow conduit of the LVAD. As in safe management for re-sternotomy, the outside of the outflow conduit of the LVAD was covered by a prosthesis, and the cardiac surgeons moved the outflow conduit far from the sternotomy line and placed a GoreTex® sheet between the outflow conduit and the sternum at the initial surgery to implant the LVAD. However, this step required special care not to injure the heart, great vessels, and device, in particular the outflow conduit. The device was encapsulated by tissue but was gradually exposed. There were some adhesions in the right thoracic cavity and significant adhesions in the left thoracic cavity. Following heparinization and annulation of all organs removed, aortic cross-clamping was done after injection of prostaglandin E1 (500 µg) into the pulmonary artery. Just after aortic clamping, the LVAD was deactivated and removed with the driveline from the left ventricle and aorta. After perfusion of extracellular phosphate-buffered solution (EP-TU), the pacing lead was removed from the heart, and the lung and heart block was explanted following standard protocols. It took 175 minutes from the initial thoracotomy to removal of the lungs. Three surgeons performed the lung retrieval, who were one cardiac surgeon and two general thoracic/transplant surgeons.
The recipient was a 61-year-old man who had stage IV chronic obstructive pulmonary disease (COPD). Right single lung transplantation was performed successfully through a posterolateral thoracotomy with 225 minutes of ischemic time. Four thoracic surgeons performed this lung transplantation using this donor lung. The patient’s postoperative course was good, and the patient was discharged.
Discussion
The number of candidates for LVADs has recently increased significantly (3,5). However, the number of reports describing organ retrieval from donors with LVADs is still small. This is the first case of lung retrieval from an adult donor with an LVAD in Japan.
Most patients with LVADs are candidates for cardiac transplantation (3). However, there is often a long wait for a cadaveric organ because of a donor shortage, particularly in Japan. During the long waiting time, patients with LVADs are exposed to risks of complications. The most frequent late-phase complications are bleeding, infection, pump thrombosis, right heart failure, device malfunction, and stroke (6). In particular, there is a 10–25% risk of cerebrovascular events such as hemorrhage and infarction (6,7). As such patients appreciate the need for donor organs at the time of their LVAD implantation, they themselves are usually willing to be donors should they themselves become brain dead.
The suitability of patients with LVADs for organ donation, in particular, lungs, has not been addressed in the guideline (8). Considering prior cardiac failure and cardiac surgery, it is a relative contraindication. However, after LVAD implantation, the lungs and other organs could be kept stable. Thus, if organ damage from severe heart failure can be avoided, patients with an LVAD can be organ donors (4). However, lung retrieval in such cases requires a high level of technical skill.
Technically, exposure of the device and organs in the thoracic cavity is still risky. In particular, thoracotomy with sternotomy to find the device is the most challenging part of organ retrieval. To address this issue, other approaches have been reported for re-do after LVAD implantation (9). One is a thoracoscopic approach, which allows surgeons to dissect the LVAD and sternum safely, and another is the subxiphoid approach using separate right and left minithoracotomies. These are options to avoid injury during sternotomy. However, because organ donation often occurs with little or no notice, surgeons have little time to prepare for organ retrieval. Furthermore, cardiac surgeons are not usually present at the donor operation, and general thoracic surgeons need to do everything from thoracotomy to lung and device removal. In this case, cardiac surgeons in the hospital knew how the LVAD had been implanted and where the driveline and outflow graft were because they had implanted the LVAD for this donor, and they performed the dissection and exposure of the device.
Temporary cardiopulmonary support could reduce the risk of unpredictable cardiac arrest (10,11) as hemodynamic instability could occur easily during donor organ retrieval. Kidneys and livers procured from brain death donors on ECMO perform similarly to non-ECMO organs with regard to one-year graft survival and functionality even though livers from ECMO donors have a higher discard rate than non-ECMO donors (12). However, with regard to lungs, only a few cases have been reported (13). In our case, we didn’t prepare ECMO during organ retrieval because cardio-pulmonary bypass would be necessary instead of ECMO if the LVAD was injured during the operation. Furthermore, as ECMO is currently not used for organ retrieval in Japan, we performed the organ retrieval with extreme care. On the other hand, the donor with the LVAD already had a CRT-D prior to implantation of the LVAD. Use of an intravascular cardioverter defibrillator (ICD) is already known to be feasible for long-term placement and ICDs have similar defibrillation thresholds (DFTs) to those of conventional ICDs in animals and humans (14,15). This would be helpful to maintain donor hemodynamic stability as hemodynamic instability could occur easily during donor organ retrieval. For organ retrieval from donors with LVADs, more time is needed to expose the organ than in the standard procedure, and sufficient time must be provided for thoracic surgeons to complete this more complex procedure.
This report described lung retrieval from a brain-dead patient with an LVAD. Such a lung transplantation is possible although is technically difficult. This may be a feasible option in light of donor shortage, however general thoracic surgeons require a high level of technical expertise to perform lung retrieval in such cases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Fukushima N, Ono M, Saito S, et al. Japanese strategies to maximize heart and lung availabilities: experience from 100 consecutive brain-dead donors. Transplant Proc 2013;45:2871-4. [Crossref] [PubMed]
- Nakagiri T, Inoue M, Minami M, et al. Interim report of the Japanese original donor evaluation and management system: the medical consultant system. Surg Today 2014;44:1227-31. [Crossref] [PubMed]
- Khazanie P, Hammill BG, Patel CB, et al. Use of Heart Failure Medical Therapies Among Patients With Left Ventricular Assist Devices: Insights From INTERMACS. J Card Fail 2016;22:672-9. [Crossref] [PubMed]
- Schmidt J, Redwan B, Martens S, et al. Double lung procurement from a donor supported by a left ventricular assist device. Interact Cardiovasc Thorac Surg 2014;19:169-70. [Crossref] [PubMed]
- McCarthy FH, Kobrin D, Rame JE, et al. Increasing Frequency of Left Ventricular Assist Device Exchanges in the United States. Ann Thorac Surg 2015;100:1660-4; discussion 1665.
- Kilic A, Acker MA, Atluri P. Dealing with surgical left ventricular assist device complications. J Thorac Dis 2015;7:2158-64. [PubMed]
- Tsukui H, Abla A, Teuteberg JJ, et al. Cerebrovascular accidents in patients with a ventricular assist device. J Thorac Cardiovasc Surg 2007;134:114-23. [Crossref] [PubMed]
- Aigner C, Winkler G, Jaksch P, et al. Extended donor criteria for lung transplantation--a clinical reality. Eur J Cardiothorac Surg 2005;27:757-61. [Crossref] [PubMed]
- Selzman CH, Madden JL, Healy AH, et al. Bridge to removal: a paradigm shift for left ventricular assist device therapy. Ann Thorac Surg 2015;99:360-7. [Crossref] [PubMed]
- Hsieh CE, Lin HC, Tsui YC, et al. Extracorporeal membrane oxygenation support in potential organ donors for brain death determination. Transplant Proc 2011;43:2495-8. [Crossref] [PubMed]
- Konstam MA, Czerska B, Böhm M, et al. Continuous aortic flow augmentation: a pilot study of hemodynamic and renal responses to a novel percutaneous intervention in decompensated heart failure. Circulation 2005;112:3107-14. [Crossref] [PubMed]
- Carter T, Bodzin AS, Hirose H, et al. Outcome of organs procured from donors on extracorporeal membrane oxygenation support: an analysis of kidney and liver allograft data. Clin Transplant 2014;28:816-20. [Crossref] [PubMed]
- Lee H, Cho YH, Sung K, et al. The Use of Extracorporeal Circulation in Suspected Brain Dead Organ Donors with Cardiopulmonary Collapse. J Korean Med Sci 2015;30:1911-4. [Crossref] [PubMed]
- Neuzil P, Reddy VY, Merkely B, et al. Implantable intravascular defibrillator: defibrillation thresholds of an intravascular cardioverter-defibrillator compared with those of a conventional ICD in humans. Heart Rhythm 2014;11:210-5. [Crossref] [PubMed]
- Merkely B, Molnar L, Geller L, et al. Chronic implantation of intravascular cardioverter defibrillator in a canine model: device stability, vascular patency, and anchor histology. Pacing Clin Electrophysiol 2013;36:1251-8. [PubMed]


