Body mass index—percent forced vital capacity—respiratory hospitalization: new staging for idiopathic pulmonary fibrosis patients
Introduction
Idiopathic pulmonary fibrosis (IPF) is relentless progressive interstitial lung disease (ILD). In the diagnosis of IPF, integration of clinical information, pulmonary function test (PFT) results, chest imaging and pathology are required (1,2). In particular, chest high-resolution computed tomography (HRCT) is considered to be of great importance in diagnosis (3,4). Once IPF is diagnosed, both follow-up of the trend of clinical symptoms and prediction of prognosis are crucial for management of these patients (5). From the clinical point of view, an easy, reproducible, less invasive indicator for staging is useful in daily practice (6-8). Recently, meaningful staging such as the composite physiological index (CPI) (9) or the gender, age and physiology (GAP) index (10) have been reported to provide useful information for prediction of mortality in IPF patients (11,12). However, these indices include diffusing capacity for carbon dioxide (DLco). DLco often cannot be performed in patients with more severe disease and may be affected by factors such as anemia and smoking status (13,14). Additionally, du Bois et al. reported that hospitalization predicted mortality of IPF patients (15). Among simple clinical examinations, body mass index (BMI) is easy to measure accurately and repeatedly without stress to the patient (16). Both obesity and body weight loss have a positive relationship with poor prognosis in IPF patients (17). In addition, body weight loss is often associated with dyspnea grade or disease progression in IPF patients, but there are few the clinical studies evaluating body weight change in patients with IPF. We hypothesized that delta BMI, change in the percentage of predicted forced vital capacity (%FVC), and respiratory hospitalization are crucial predictors of mortality in patients with IPF. The aim of this study was to evaluate the serial trend of important indicators of prognosis and create a useful staging method for IPF patients.
Methods
We retrospectively searched medical records, PFT results, and chest HRCT scans from January 1, 2008 through June 30, 2015 at our hospital. The same parameters were evaluated for each patient 1-year later. IPF was diagnosed based on the 2011 International IPF guidelines (1).
Patient clinical characteristics at diagnosis included age, sex, smoking history, BMI. BMI was re-evaluated 1-year later. In laboratory findings, we reviewed white blood cell (WBC), lactate dehydrogenase (LDH) and Krebs von den Lungen-6 (KL-6) values at diagnosis. In terms of physiological findings, we evaluated forced vital capacity (FVC), %FVC, %DLco, total lung capacity (TLC), %TLC, and forced expiratory volume in 1 s (FEV1) and %FEV1. We re-evaluated pulmonary function 1-year later when possible or survive. If patient died within 1-year, only baseline PFT results were analyzed. These values were calculated based on Japanese standardized reference. In addition, we calculated the GAP staging system using %FVC and %DLco values (10). We also calculated CPI according to the following formula (9):
CPI = 91 − (0.65× %DLco) − (0.53× %FVC) + (0.34× %FEV1)
On radiological findings, chest CT was obtained with 1mm-thick axial sections at 1-cm intervals throughout the entire thorax in the inspiratory phase (18). No oral or intravenous contrast material was administered. We chose three levels for imaging scoring; aortic arch, carina, and 1 cm above the right diaphragm (19,20). Reticulation, traction bronchiectasis, honeycombing, and ground glass opacity (GGO) were evaluated. Reticulation was defined as interlacing lines in secondary lobules (21). Fibrosis scores consisted in the combined reticulation, traction bronchiectasis, and honeycombing, and was defined as the 0, none; 1, involvement >25% of each zone; 2, 25–50% of each zone; and 3, >50% of each zone (22). GGO scores were calculated in the same manner.
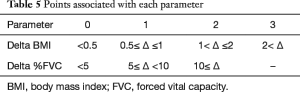
For our new staging method, we chose three crucial parameters that proved significant predictors of mortality in our cohort; delta BMI, delta %FVC, and respiratory hospitalization within a year (BFR staging). First, delta BMI was defined as baseline BMI minus BMI at 1-year. Delta BMI was scored on a four-point scale (0–3) based on delta value; 0, Δ <0.5; 1, 0.5≤ Δ ≤1, 2, 1< Δ ≤2, 3, 2< Δ. Optimum cut-off levels were determined from receiver operating characteristics (ROC) curves. Second, delta %FVC was defined as baseline %FVC minus %FVC at 1-year. Delta %FVC was scored on a three-point scale (0–2) based on delta value; 0, Δ <5, 1, 5≤ Δ <10, 2, 10≤ Δ. These cut-off value are based on a previous study for evaluation of %FVC in IPF patients (23,24). Third, respiratory hospitalization was defined as admission related to bronchitis, pneumonia, pneumothorax and pulmonary embolism, or acute cor pulmonale within 1-year from IPF diagnosis; hospitalization for heart failure and other causes were excluded. These parameters were summed. Finally, patients were grouped as follows: stage I, consisting of 0–2 points; stage II, consisting in 3–5 points; and stage III, consisting in ≥6 points. In making staging, discrimination was measured using the C-statistic Index which ranges from 0 to 1.0. The study was approved by Okinawa Chubu Hospital ethics committee (No. H28-20). They waived informed consent because of the retrospective nature of the medical record review.
Statistical analysis
Continuous variables are presented as mean ± standard deviation. Linear regression was used to study univariate and multivariable associations between potential predictors and mortality based on previous studies of IPF (25,26). The threshold for the candidates of predictor of mortality was P<0.1. Next, Cox proportional hazards regression models were used to evaluate hazard ratios (HRs), 95% confidence intervals (CIs) and P values for each factor, adjusted for confounders such as prednisolone (PSL) and immunosuppressants. Analysis of variance (ANOVA) was conducted in the three patient groups.
Survival time was analyzed using the Kaplan-Meier method and the log-rank test with the end points being death or the last contact. P<0.05 was considered statistically significant.
All analyses were performed using Stata Data Analysis and Statistical Software STATA version 11.0 (Stata Corp., College Station, TX, USA).
Results
Patient characteristics
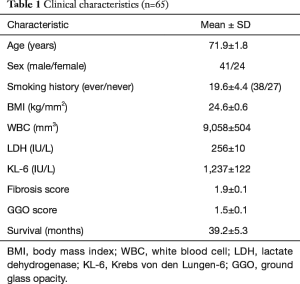
Our cohort of IPF patients consisted in 65 consecutive patients; patient clinical characteristics are shown in Table 1. Mean age was 71.9±1.8 years, and there were 41 men and 24 women. In terms of smoking status, 38 were active or ex-smokers (mean 19.6 pack-years). Mean follow-up period was 32.4 months. Baseline BMI was 24.6 kg/m2. In major comorbidities, diabetes mellitus (DM) and hypertension were 18% and 43%.
Full table
In laboratory findings, mean WBC, LDH and KL-6 were 9,058 mm3, 256 IU/L, and 1,237 IU/L, respectively. Mean survival was 39.2 months (Table 1).
PFTs
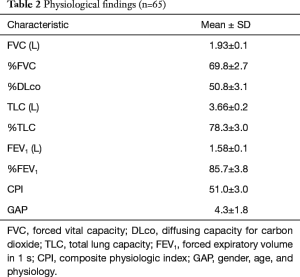
Mean FVC, %FVC, %DLco, TLC and %TLC were 1.93 L, 69.8%, 50.8%, 3.66 L, and 78.3%, respectively. Mean FEV1, and %FEV1 were 1.58 L and 85.7%. In addition, mean CPI and GAP were 51.0 and 4.3, respectively (Table 2). In diagnostic method, 11 patients (17%) were pathologically proven IPF.
Full table
Management
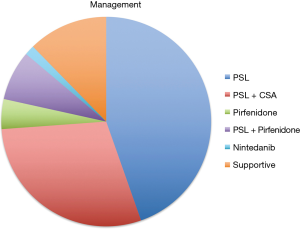
Of the 65 patients, 29 patients received PSL alone and 19 patients took both PSL and cyclosporine A. Three patients received pirfenidone (PFD) and five took both PSL and PFD. One patient received nintedanib. Eight patients were followed up without active medication (Figure 1). We had no transplanted patient.
Cause of death
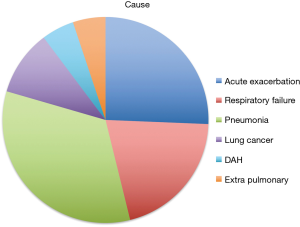
During the follow up period, 39 patients died. The leading cause of death was bacterial pneumonia (n=13). Other causes included acute exacerbation (n=10) and progression of respiratory failure (n=8), lung cancer (n=4), alveolar hemorrhage (n=2), and extra-pulmonary diseases (n=2) (Figure 2).
Predictors of mortality
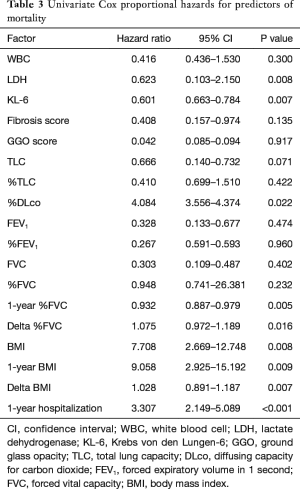
Among the clinical parameters including demographic, laboratory, physiological, radiological and admission history, univariable analysis revealed 16 items as statistically significant predictors of mortality. After adjustment for age, sex, and treatment, multivariate analysis showed LDH, KL-6, %DLco, 1-year %FVC, BMI, 1-year BMI, and 1-year respiratory hospitalization were significant predictors of mortality (Table 3).
Full table
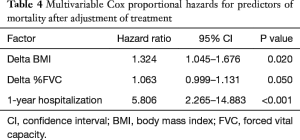
Among these predictor candidates, delta BMI, delta %FVC and respiratory hospitalization within 1-year were strong predictors of mortality in patients with IPF (Table 4).
Full table
New staging
In creating new staging, C-statistic values for univariate survival model of delta BMI, delta %FVC and respiratory hospitalization within 1-year were 0.561, 0.633 and 0.780. And C-statistic values for multivariate survival model of delta BMI + delta %FVC, delta BMI + respiratory hospitalization within 1-year, delta %FVC + respiratory hospitalization within 1-year and delta BMI + delta %FVC + respiratory hospitalization within 1-year were 0.623, 0.692, 0.753 and 0.765. Our new BFR staging system consists in delta BMI, delta %FVC and respiratory hospitalization within 1-year (Table 5). According to these parameters, we divided patients into three stages (Table 6). Patient clinical characteristics according to the new staging system are shown in Table 7. Stage I patients were light smokers with relatively preserved pulmonary function and fewer respiratory hospitalizations within 1-year. Stage II patients were heavy smokers with high BMI, and showed elevated of KL-6 and decreased %DLco. All stage III patients were never smokers and had low baseline BMI. In addition, this group showed large delta BMI and delta %FVC, and were hospitalized more often for respiratory reasons.
Full table

Full table

Full table
Survival
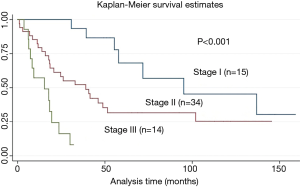
Finally, we created a survival curve based on the new staging system. Stage III patients showed poor survival compared with that of stage I and II patients (14.8 vs. 77.9 and 43.9 months; P<0.001) (Figure 3).
Discussion
We propose a new staging system for patients with IPF at our hospital. Both age and sex proportions of our cohort were consistent with that of previous studies (27-30). However, smoking history in our patients was rather light compared with classical IPF cohorts. In laboratory findings, mean KL-6 was >1,000 IU/L and stage II patients had the most elevated value. Therefore, these patients may have more active proliferation of type II alveolar cells (31-33).
Regarding physiological findings, mean %FVC and %DLco were consistent with that of IPF patients in a large study (34-37). Mean CPI and GAP scores were approximately 50 and 4, respectively. Therefore, our cohort showed moderate severity of restrictive disorder. Furthermore, mean %FEV1 was 85.7% in our study; therefore, the obstructive component contributes little.
With respect to medical management, approximately three-quarters of our patients received PSL. This might be associated with the leading cause of death of pneumonia found in this study.
With respect to death in our cohort, both acute exacerbation and progression of respiratory failure were important, consistent with previous studies (38-41). In addition, we sometimes see extra-pulmonary cause including cardiovascular disease. Therefore, we should monitor crucial comorbidity carefully in daily practice (42-45).
On physiological prediction of mortality in IPF patients, both FVC and DLco have been reported as useful parameters (46-50). DLco was a strong predictor of mortality of our cohort on Cox proportional hazard analysis. However, DLco is often variable and is affected by respiratory infection or anemia (51,52). In addition, when the vital capacity of a patient is <1.5 L, it cannot be measured with the single breath method. Therefore, it showed less consistency or reproducibility compared with FVC; accordingly, we omitted DLco from our new staging system, and instead included delta BMI, delta %FVC, and respiratory hospitalization.
For creating new staging system of IPF cohort, we chose delta BMI, delta %FVC and respiratory hospitalization.
First, BMI is a simple and reproducible item, and less invasive for the patient. Among IPF patients, we previously reported that 1-year modified medical research council breathlessness scale is a useful predictor of mortality (53). In IPF patients, severe dyspnea is associated with greater consumption of body energy. Therefore, our hypothesis is that body weight loss has a positive relationship with dyspnea severity and mortality.
Second, we chose delta %FVC for the new staging system. %FVC is a classic robust marker of prognosis of IPF patients (23,47). And, more decreased %FVC within six months or 1-year have been reported to predict mortality of IPF patients (23,24,52). We considered the trend of %FVC is more robust than a single measurement of %FVC.
Third, we included respiratory hospitalization within 1-year. du Bois et al. reported hospitalization within 1-year as a crucial predictor of mortality with composite index (15). We considered that respiratory hospitalization such as bronchitis, pneumonia, pneumothorax and pulmonary embolism would contribute more to prognosis of IPF patients. IPF patients often develop respiratory deterioration or pulmonary dysfunction within 1-year of diagnosis (53). Therefore, we set 1-year as the cut-off for evaluating the trend of significant parameters or hospitalization history. Accurate cause of all stage III patients was never smoker remains unknown. According to our previous report, never smoker IPF patients had poor prognosis (53), therefore one possibility is never smoker IPF patients tend to have pure active fibrosis compared to smoker IPF patients. Detailed evaluation of pathological findings is interesting topic.
Finally, we stratified our cohort into three groups according to our new staging. According to our study, stage I patients remained fairly clinically and functionally stable. However, stage II patients had more severe pulmonary dysfunction and elevation of KL-6. Therefore, monitoring IPF activity such as change of pulmonary function or frequent evaluation with chest imaging is required. Stage III patients showed remarkable decreases in BMI and %FVC within 1-year. In addition, stage III patients required respiratory hospitalization more often. Based on these information, we should pay attention to not only physiological trends but also to general medical status, including body weight, nutrition and infection in stage III patients. We require comprehensive management for stage III patients.
There are several limitations. First, this was a retrospective fashion single-center small sample study. A prospective study is required in the future. Second, this study focuses mainly on clinical information. Therefore, we did not evaluate radiological and pathological findings in detail. However, not all patients undergo surgical lung biopsy, owing to advanced age, physical limitations, severe pulmonary dysfunction and patient refusal. Owing to inconsistencies among thoracic radiologists, radiological scoring is often inaccurate compared with body weight or pulmonary function. Our simple staging is easy to use and reproducible. Third, we could not review all clinical parameters 1-year later. However, we could choose important parameters and re-evaluate strong predictors of mortality 1-year later. Fourth, comorbidities such as DM and hypertension may lead to increase of BMI, and steroid therapy may affect BMI change. Last, the majority of these patients were treated with PSL. We can provide two anti-fibrotic agents such as PFD and nintedanib in clinical practice. We should plan a prospective study with modern therapy for IPF patients. Based on these limitations, we could not over-interpret our result. However, our novel findings and staging are easy to apply. And our findings provide important clinical trend of IPF patients.
In conclusion, we propose a simple new staging system for IPF patients. This staging consists in delta BMI, delta %FVC and respiratory hospitalization within 1-year. We showed a clear difference in long-term survival of IPF patients based on this new staging. A multi-center prospective study is warranted in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Okinawa Chubu Hospital ethics committee (No. H28-20). They waived informed consent because of the retrospective nature of the medical record review.
References
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199-203. [Crossref] [PubMed]
- Nishimura K, Kitaichi M, Izumi T, et al. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology 1992;182:337-42. [Crossref] [PubMed]
- Lynch DA, Godwin JD, Safrin S, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 2005;172:488-93. [Crossref] [PubMed]
- Martinez FJ, Safrin S, Weycker D, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 2005;142:963-7. [Crossref] [PubMed]
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431-40. [Crossref] [PubMed]
- King TE Jr, Tooze JA, Schwarz MI, et al. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171-81. [Crossref] [PubMed]
- Kim JH, Lee JH, Ryu YJ, et al. Clinical predictors of survival in idiopathic pulmonary fibrosis. Tuberc Respir Dis (Seoul) 2012;73:162-8. [Crossref] [PubMed]
- Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003;167:962-9. [Crossref] [PubMed]
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [Crossref] [PubMed]
- Kim ES, Choi SM, Lee J, et al. Validation of the GAP score in Korean patients with idiopathic pulmonary fibrosis. Chest 2015;147:430-7. [Crossref] [PubMed]
- Kishaba T, Shimaoka Y, Fukuyama H, et al. Clinical characteristics of idiopathic pulmonary fibrosis patients with gender, age, and physiology staging at Okinawa Chubu Hospital. J Thorac Dis 2015;7:843-9. [PubMed]
- Guo J, Zheng C, Xiao Q, et al. Impact of anaemia on lung function and exercise capacity in patients with stable severe chronic obstructive pulmonary disease. BMJ Open 2015;5:e008295. [Crossref] [PubMed]
- Sansores RH, Pare PD, Abboud RT. Acute effect of cigarette smoking on the carbon monoxide diffusing capacity of the lung. Am Rev Respir Dis 1992;146:951-8. [Crossref] [PubMed]
- du Bois RM, Weycker D, Albera C, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:459-66. [Crossref] [PubMed]
- Pihtili A, Bingol Z, Kiyan E, et al. Obstructive sleep apnea is common in patients with interstitial lung disease. Sleep Breath 2013;17:1281-8. [Crossref] [PubMed]
- Gries CJ, Bhadriraju S, Edelman JD, et al. Obese patients with idiopathic pulmonary fibrosis have a higher 90-day mortality risk with bilateral lung transplantation. J Heart Lung Transplant 2015;34:241-6. [Crossref] [PubMed]
- Murata K, Khan A, Herman PG. Pulmonary parenchymal disease: evaluation with high-resolution CT. Radiology 1989;170:629-35. [Crossref] [PubMed]
- Goh NS, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008;177:1248-54. [Crossref] [PubMed]
- Sumikawa H, Johkoh T, Colby TV, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med 2008;177:433-9. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol 1997;169:977-83. [Crossref] [PubMed]
- du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011;184:1382-9. [Crossref] [PubMed]
- Taniguchi H, Kondoh Y, Ebina M, et al. The clinical significance of 5% change in vital capacity in patients with idiopathic pulmonary fibrosis: extended analysis of the pirfenidone trial. Respir Res 2011;12:93. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Fell CD, Martinez FJ, Liu LX, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2010;181:832-7. [Crossref] [PubMed]
- Navaratnam V, Fleming KM, West J, et al. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax 2011;66:462-7. [Crossref] [PubMed]
- Collard HR, Chen SY, Yeh WS, et al. Health care utilization and costs of idiopathic pulmonary fibrosis in U.S. Medicare beneficiaries aged 65 years and older. Ann Am Thorac Soc 2015;12:981-7. [Crossref] [PubMed]
- Thomeer M, Demedts M, Vandeurzen K, et al. Registration of interstitial lung diseases by 20 centres of respiratory medicine in Flanders. Acta Clin Belg 2001;56:163-72. [Crossref] [PubMed]
- Tinelli C, De Silvestri A, Richeldi L, et al. The Italian register for diffuse infiltrative lung disorders (RIPID): a four-year report. Sarcoidosis Vasc Diffuse Lung Dis 2005;22 Suppl 1:S4-8. [PubMed]
- Kohno N, Kyoizumi S, Awaya Y, et al. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest 1989;96:68-73. [Crossref] [PubMed]
- Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J Med Invest 1999;46:151-8. [PubMed]
- Ishikawa N, Hattori N, Yokoyama A, et al. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 2012;50:3-13. [Crossref] [PubMed]
- Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040-7. [Crossref] [PubMed]
- Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:821-9. [Crossref] [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760-9. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- Okamoto T, Ichiyasu H, Ichikado K, et al. Clinical analysis of the acute exacerbation in patients with idiopathic pulmonary fibrosis. Nihon Kokyuki Gakkai Zasshi 2006;44:359-67. [PubMed]
- Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636-43. [Crossref] [PubMed]
- Johannson K, Collard HR. Acute Exacerbation of Idiopathic Pulmonary Fibrosis: A Proposal. Curr Respir Care Rep 2013.2. [PubMed]
- Collard HR, Yow E, Richeldi L, et al. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res 2013;14:73. [Crossref] [PubMed]
- Behr J, Kreuter M, Hoeper MM, et al. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J 2015;46:186-96. [Crossref] [PubMed]
- Nathan SD, Basavaraj A, Reichner C, et al. Prevalence and impact of coronary artery disease in idiopathic pulmonary fibrosis. Respir Med 2010;104:1035-41. [Crossref] [PubMed]
- Kizer JR, Zisman DA, Blumenthal NP, et al. Association between pulmonary fibrosis and coronary artery disease. Arch Intern Med 2004;164:551-6. [Crossref] [PubMed]
- Kreuter M, Ehlers-Tenenbaum S, Palmowski K, et al. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS One 2016;11:e0151425. [Crossref] [PubMed]
- Collard HR, King TE Jr, Bartelson BB, et al. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:538-42. [Crossref] [PubMed]
- Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med 2006;174:803-9. [Crossref] [PubMed]
- Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:543-8. [Crossref] [PubMed]
- Hanson D, Winterbauer RH, Kirtland SH, et al. Changes in pulmonary function test results after 1 year of therapy as predictors of survival in patients with idiopathic pulmonary fibrosis. Chest 1995;108:305-10. [Crossref] [PubMed]
- Salisbury ML, Xia M, Zhou Y, et al. Idiopathic Pulmonary Fibrosis: Gender-Age-Physiology Index Stage for Predicting Future Lung Function Decline. Chest 2016;149:491-8. [Crossref] [PubMed]
- Jegal Y, Kim DS, Shim TS, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:639-44. [Crossref] [PubMed]
- Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003;168:531-7. [Crossref] [PubMed]
- Kishaba T, Nagano H, Nei Y, et al. Clinical characteristics of idiopathic pulmonary fibrosis patients according to their smoking status. J Thorac Dis 2016;8:1112-20. [Crossref] [PubMed]