
Chest wall reconstruction after the Clagett procedure and other types of open-window thoracostomy: a narrative review
Introduction
Background
Pleural empyema refers to pus-filled pockets in the pleural cavity, with clinical features of dyspnea, fever, and chest discomfort (1). The American Thoracic Society recognizes three stages of pleural empyema based on the duration and severity of infection, namely: the exudative, fibrinopurulent, and organizational stage (2,3). The acute exudative stage describes parapneumonic effusion, while the intermediate fibrinopurulent stage describes complicated parapneumonic effusion where the pleural fluid becomes purulent. Here, pleural cavity inflammation may result in compromised lung expansion (1). Clinical studies have reported mortality rates of 20% to 30% for patients with pleural empyema (4). When empyema lasts longer than 4 weeks or is recurrent, it is classified as chronic empyema (5). To prevent chronic empyema, the primary treatment of early-stage pleural empyema may involve the administration of antibiotics and tube thoracostomy. Non-surgical therapeutic options include chest cavity irrigation, postural drainage, and administration of intrapleural fibrinolytics, such as tissue plasminogen activator (tPA) (6,7). Nonetheless, surgical evacuation remains the first choice of treatment. Chronic empyema is characterized by the organization and thickening of the pleural fluid. The migration of fibroblasts into the pleural cavity forms a pleural peel, which may result in an entrapped lung (1,5). Empyema may also occur in association with bronchopleural fistula. The morbidity associated with bronchopleural fistula varies between 25% and 71%. Treatment often involves primary closure with the use of flaps. Additionally, the use of airway stents, coils and fibrin glue have been described (8-10).
Rationale
Chronic empyema generally requires surgical intervention (5). Classically, an open approach via thoracotomy was used for adequate drainage and debridement of the pleural cavity. During the last two decades, minimally invasive thoracic surgery made its entrance and is currently widely used for empyema treatment as well. Nowadays, most surgeons use a multiportal video-assisted thoracoscopic surgery (VATS) approach, but even a less invasive technique as uniportal VATS safely allows for adequate treatment via one small incision of just a few centimeters (6,11). Despite these advanced treatment options, which are successful in the vast majority of cases, there are still circumstances where chronic or recurrent empyema necessitates an open-window thoracostomy. For the latter, there are several techniques, such as the Eloesser or Clagett method, that essentially amount to the same principle: a pleural window is created allowing for open drainage and packing of the cavity (5,12).
The Clagett procedure, named after inventor Dr. Oscar Theron ‘Jim’ Clagett, presents two stages. In the first stage, an open chest wall window is made to drain and irrigate the empyema cavity, followed by a period of daily dressing changes, irrigation, and sometimes the use of a vacuum-assisted closure (VAC)-device (13,14). It is expected that the cavity will subsequently become smaller (15). Once the empyema is resolved, the wound may close spontaneously. This is mainly dependent on the surgical technique that was used and the degree of the chest wall defect (15). If spontaneous closure does not occur after several months and there is no recurrent disease, efforts can be made to close the defect, if the patient’s condition allows. This constitutes the second procedure (16).
When primary wound closure is not possible or insufficient to obliterate the pleural cavity, reconstruction of the chest wall is indicated (16). This is typically performed by a reconstructive plastic surgeon, and aims to restore the anatomical integrity, stability, and appearance of the chest wall. Defects greater than five centimeters in diameter or encompassing more than four ribs should be reconstructed. This should be done to avoid lung herniation, paradoxical breathing, and respiratory failure (17,18). Skin, muscle, and bone autografts, homografts, and allografts may be used in addition to metal plates and prostheses to accomplish reconstruction (17). The muscles used are chosen by considering the damage caused by previous operations (16).
Objective
The purpose of this narrative review is to present an overview of the options to reconstruct soft tissue defects of the chest wall after the Clagett procedure and other types of open-window thoracostomy. Since the techniques for open-window thoracostomy are quite similar (12), the presented reconstructive options can be generally applied. This review also includes four articles in which reconstruction was performed after tube thoracostomy, where no open-window was created (19-22). However, these involved complex cases where a large residual pleural space was present, requiring a pedicled or free flap reconstruction to completely obliterate the dead space. Although these cases did not involve a large thoracic wall defect, these cases were nevertheless included, since the reconstruction methods may be applicable to fill large cavities after open-window thoracostomy, possibly in combination with another flap. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-684/rc).
Methods
A literature review was performed on articles in PubMed, Cochrane Library, ClinicalTrials.gov, and Google Scholar in January 2023 to identify published papers on reconstructive options after the Clagett procedure, using the terms stated in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 30/01/2023 |
| Databases and other sources searched | PubMed, Cochrane Library, ClinicalTrials.gov, and Google Scholar |
| Search terms used | “Empyema” [MeSH], “Thoracic wall” [MeSH], “Thoracostomy” [MeSH], “Clagett”, “Clagett procedure”, “Clagett thoracostomy”, “Modified Clagett procedure”, “Clagett open-window thoracostomy”, “Thoracostomy”, “Fenestration of pleura”, “Pleural fenestration”, “Clagett fenestration”, “Clagett fenestration of pleural window”, “Intrathoracic defect”, “Omentum” [MeSH], “Myocutaneous flap” [MeSH], “Chest wall reconstruction”, “Chest-wall reconstruction”, “Thoracic wall reconstruction”, “Loco-regional flaps”, “Pectoralis major flap”, “Thoracoacromial artery muscle perforator flap”, “Rectus abdominis flap”, “Omentum flap”, “Superior epigastric artery perforator flap”, “SEAP flap”, “SEAP”, “Latissimus dorsi flap”, “Latissimus dorsi musculocutaneous flap”, “Deep inferior epigastric perforator flap”, “DIEP flap”, “DIEP”, “Anterolateral thigh flap”, and “ALT flap”. Filters: English, Dutch, humans |
| Timeframe | 1986–2023 |
| Inclusion and exclusion criteria |
Inclusion: all available studies, written in English or Dutch, where patient cases were presented, i.e., randomized controlled trials, prospective, retrospective, and comparative studies, case series, and case reports were considered |
| Exclusion: papers that were not in English or Dutch. Papers of which no full text was available | |
| Selection process | The literature review was performed by two researchers. Differences were discussed and conferred to obtain consensus. When no consensus could be obtained, a third reviewer was consulted |
MeSH, Medical Subject Headings; SEAP, superior epigastric artery perforator; DIEP, deep inferior epigastric perforator; ALT, anterolateral thigh.
Articles on open-window thoracostomy in general were also reviewed, since most articles were written by plastic surgeons and the technique used for open-window thoracostomy was not always specified.
The search resulted in 412 papers that after applying the filters (English, Dutch, humans) resulted in 335 papers. All articles were screened on title and abstract by one author (A.K.), which resulted in 72 articles. An additional 27 articles were added for review, based on expert opinion, following hand-searching on Google Scholar by one author (S.S.Q.). The articles considered relevant were then selected by two authors (A.K. and S.S.Q.) based on the full text. Two articles were excluded because the full text was not available. Finally, after review and selection consensus, 21 papers were included in this narrative review. The search and selection resulted mainly in case reports and case series. We excluded papers that were not in English or Dutch. A single article in French and one in Spanish were included since these were also (partly) available in English, which made understanding the articles possible.
Results: reconstructive options after the Clagett procedure and other types of open-window thoracostomy
Since the first chest wall reconstruction surgery in 1906 was described, a variety of reconstructive techniques has arisen (17). An overview of the patient characteristics, reconstruction methods, and the outcomes of the studies considered in this review are presented. A subdivision was made based on reconstruction type: pedicled flaps, free flaps, and the use of a VAC-device.
The following literature is quite dated, which may be due to the fact that the use of an open-window thoracostomy is required less often nowadays. This may be because of the decline in the number of patients contracting pneumonia, which is the main cause of empyema, as well as an improvement in treatment options (23,24).
Pedicled flaps (Table 2)
Table 2
| Reference | Country/region | Type of study | Population | Etiology of empyema | BPF (n=1) | Type of fenestration | Type of reconstruction | Follow-up time (months) | Outcomes | Complications | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numbers | Age (years), median [range] | Sex ratio, n (male/female) | ||||||||||
| Kamiyoshihara, 2017, (25) | Japan | Case series | 3 | 72 [63–78] | 1/2 | 1 (n=3) | 1 | Open window thoracostomy (n=1) | Paraspinous muscle flap | NR | NR | NR |
| Celik, 2016, (19) | Turkey | Case report | 1 | 50 | 1/0 | 2 | NR | Tube thoracostomy (n=1) | Omental flap with serratus muscle flap | NR | NR | None |
| Takeuchi, 2012, (26) | Japan | Case report | 1 | 64 | 1/0 | 1 | 1 | Open window thoracostomy NOS (n=1) | Paraspinous muscle flap (including erector spinae muscles) | 12 | No reduction in muscle strength (n=1) | None |
| No spinal deformation (n=1) | ||||||||||||
| Belmahi, 2008, (27) | Maroc | Case series | 12 | NR [25–45] | 12/0 | 2 (n=12), 3 (n=8) |
NR | Open window thoracostomy NOS (n=12) | Ipsilateral latissimus dorsi muscle flap (n=6) | 36 (mean) | Recurrence of empyema or BPF (none) | None |
| Ipsilateral latissimus dorsi muscle flap combined with serratus anterior muscle flap (n=2) | ||||||||||||
| Free contralateral latissimus dorsi muscle flap (n=3) | ||||||||||||
| Free contralateral latissimus dorsi muscle flap combined with serratus anterior muscle flap (n=1) | ||||||||||||
| Seify, 2007, (20) | USA | Retrospective study | 55 | 62 [39–77] | 42/13 | 1 (n=13), 2 (n=16), 4 (n=9), 5 (n=17) |
20 | Eloesser window thoracostomy (n=27) | Serratus anterior muscle flap (n=16, 23 flaps) | NR | Recurrence of empyema (n=2) | Death by sepsis POD10 (n=1) |
| Tube thoracostomy with decortication (n=2) | Latissimus dorsi muscle flap (n=16, 18 flaps) | Partial flap necrosis (rectus abdominis flap) (n=1) | ||||||||||
| Pectoralis muscle flap (n=3) | ||||||||||||
| Intercostal muscle flap (n=3) | ||||||||||||
| RAM flap (n=3) | ||||||||||||
| Omental flap (n=1) | ||||||||||||
| Pichler, 2006, (28) | Germany | Case report | 1 | 75 | 1/0 | 3 | 1 | Open window thoracostomy NOS with decortication (n=1) | Ipsilateral latissimus dorsi muscle flap with skin transplant (n=1) |
NR | Patient recovered well and the septic process diminished rapidly | NR |
| Okumura, 2005, (29) | Japan | Retrospective study | 23 | 58 [21–72] | 20/3 | 3 (n=23) | 16 | Open window thoracostomy NOS (n=6) | Omental flap with/without muscle flap (n=11) | 6 | Clinical success (n=19) | Death (muscle flap infection and sepsis) (n=1) |
| Omental flap with partial thoracoplasty (n=12) | Deaths in remote period by respiratory failure (n=5) | Ileus (n=3) | ||||||||||
| No significant change in vital capacity and FEV1 | Gastrointestinal bleeding (n=1) | |||||||||||
| Minor abdominal complications (n=6) | ||||||||||||
| Duan, 1999, (21) | China | Comparative study | 50 | 38 [15–58] | 35/15 | 2 (n=2), 3 (n=35), 6 (n=13) |
32 | Tube thoracostomy (n=50) with modified decortication (n=38) | One-stage omental flap (n=50) | 102 (mean) | Recurrence of empyema (n=2) | Air leak POD1 (n=2) requiring re-thoracotomy (n=1) |
| Good control of septic focus (n=47) | Coma (7 days) due to accidental diazepam overdose (n=1) | |||||||||||
| Operation successful in 93.1% | Abdominal wound dehiscence (n=1) | |||||||||||
| Closure rate of BPF 89.5% | ||||||||||||
| Serletti, 1996, (30) | USA | Case series | 4 | 68 [58–72] | 2/2 | 2 (n=4) | NR | Clagett window thoracostomy (n=1) | De-epithelialized TRAM flap (n=4) | 35 (median) | Recurrence of empyema (n=1) | Recurrent loculation requiring a second Clagett procedure (n=1) |
| Eloesser window thoracostomy (n=1) | Healing of abdominal donor site without significant functional deficits (n=4) | Incarcerated hernia requiring a small bowel resection (n=1) | ||||||||||
| Open-window thoracostomy NOS (n=1) | Obliteration of previous tract (n=4) | |||||||||||
| No open window thoracostomy before reconstruction, Clagett window thoracostomy after reconstruction (n=1) | ||||||||||||
| Shirakusa, 1990, (31) | Japan | Case series | 11 | 65 [32–76] | 9/3 | 7 (n=6), 8 (n=5) |
7 | Open window thoracostomy NOS (n=6) | One-stage omental flap (n=1) | NR | Recurrence of empyema (n=1) | Subacute ileus (n=1) |
| Two-stage omental flap (n=6) | Partial recurrence of empyema (n=2) | |||||||||||
Etiology of empyema: 1, post-lobectomy; 2, post-pneumonectomy; 3, post-tuberculosis/tuberculous; 4, prophylactic post-pneumonectomy or post-lobectomy; 5, no surgical resection; 6, bacterial/abscess; 7, aspergillus; 8, post-operative acute empyema. BPF, bronchopleural fistula; NR, not reported; NOS, not otherwise specified; POD, postoperative day; RAM, rectus abdominis muscle; FEV1, forced expiratory volume in 1 second; TRAM, transverse rectus abdominis myocutaneus.
The results of the articles in this review on pedicled flaps are as follow: ten studies with a total of 161 patients used pedicled flaps to reconstruct the chest wall. Most patients (n=72) suffered from post-tuberculosis empyema, followed by post-pneumonectomy (n=35) and post-lobectomy (n=17) empyema.
The studies reported that pedicled flaps can be a reliable option due to their excellent blood supply and are therefore often the first choice of reconstruction (20,21,25-27,31).
In the included articles, 134 patients recovered without notable complications. The most common complication was abdominal donor-site related (n=13), followed by (partial) recurrence of empyema (n=8), requiring a second Clagett procedure in one patient. Then significant air-leakage (n=2), requiring re-thoracotomy in one patient, followed by death by sepsis (n=2), and partial flap necrosis (n=1) (Table 2). The omental flap was applied in most patients (n= 82) in the selection of studies in this review (19-21,25-31).
In a long-term follow-up study by Okumura et al. [2005] clinical success was achieved in 82.6% (n=23), using a pedicled omental flap. Clinical success was defined as an empyema space that was cured without dead space or infection 6 months postoperatively (29). One of the larger studies in this review was by Duan et al. [1999]. In this study, 50 patients underwent one-stage pedicled omentum transplantation to treat chronic empyema after initial tube thoracostomy. The intervention was successful in 93.1% and the closure rate of the bronchopleural fistula was 89.5%. These high percentages may be attributed to the fact that the patients had previously undergone tube thoracostomy and not an open-window thoracostomy. According to Duan et al., the high success rate was due to the formation of highly vascularized adhesions when using the omentum, providing a rich blood supply, which aids in clearing the infection. In general, it also offers sufficient volume to obliterate the dead space (21). However, the volume of the omentum may be insufficient in completely obliterating the cavity in malnourished patients and in patients who underwent previous abdominal surgery (29). Furthermore, a risk of herniation and the formation of larger hematomas of the abdominal organs must be considered before choosing for this procedure (31).
Other reconstructive options using pedicled flaps based on the muscles present in the thoracic wall such as the latissimus dorsi flap (n=23), the serratus anterior flap (n=17), and in two other cases, a combination of the two, were described. According to Belmahi et al. [2008], these flaps can be utilized to close bronchopleural fistulae due to their anatomical proximity. A prerequisite for using the ipsilateral latissimus dorsi and serratus anterior muscles is that the muscles and their pedicles are intact and not damaged. When damaged, the contralateral latissimus dorsi muscle can be harvested as a free flap (27). In a study by Seify et al. [2007] the serratus and latissimus dorsi muscle flaps were the most frequently used flaps, among other things because of their ability to reach any place in the pleural cavity. The rectus abdominis muscle (RAM) flap was used in three cases. In one of these three cases, partial flap necrosis occurred, requiring local flap closure. This was the only flap loss reported in this series (n=55) (20).
Another case series by Serletti et al. [1996] described the use of the de-epithelialized transverse rectus abdominis myocutaneus (TRAM) flap based on the superior epigastric vessels in four cases. This reconstruction method was appropriate in patients with inadequate local musculocutaneous flaps for reconstruction due to previous surgeries. The advantages of this flap were its volume and long vascular pedicle that reached to the thoracic wall. Furthermore, obliteration of the dead space in their patients could not have been achieved using only a single local flap. However, it should be considered that this operation requires maintaining a flexed position of the waist several days postoperatively, with high risk of atelectasis and pneumonia development. Due to the severity of such a procedure, the prognosis of the patient should be carefully considered (30).
Other local flaps that have been used to a lesser extent were the paraspinous (n=4), pectoralis (n=3), and intercostal muscle flaps (n=3), the latter two were mainly used in addition to other flaps. Kamiyoshihara et al. [2017] described three cases using a pedicled paraspinous muscle flap. They reported volume maintenance even in malnourished patients. This flap could be used bilaterally whenever a larger volume was needed and it was particularly indicated to fill posterior pleural cavities, though also limited in its ability to solely fill this region due to its arc of rotation. Moreover, little is known about the long-term effects on posture, especially with unilateral cases. They concluded that this muscle flap was best regarded as a supportive factor as a plombage material (25). Takeuchi et al. [2012] also presented a case report in which a paraspinous muscle flap including erector spinae muscles was utilized. They reported that flap elevation was easy and less invasive, but that the arc of rotation was limited and that there was a risk of spinal deformation when too much of the erector spinae muscles was harvested (26).
In summary, the primary advantage of pedicled flaps is their reliable vascularization (20,21,25-27,31). Additional advantages include superior tissue match, reduced scarring, and shorter operation time in comparison to free-flap reconstruction (32). In general, a pedicled flap should be preferred whenever possible, this is also shown in the flowchart (Figure 1) that was made based on the reviewed literature. To maintain the possibility of using a pedicled flap, preservation of the vascular pedicle and knowledge of the anatomy is mandatory (27). An overview of the vascularization of each flap is shown in Table 3.
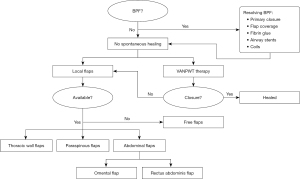
Table 3
| Flap | Main vascularization | Location of the source vessels |
|---|---|---|
| Paraspinal muscle | Dorsal branches of the intercostal arteries | Lie deep to the thoracic and lumbar fascia |
| Trapezius muscle | Transverse cervical artery | Posterior inferior neck and superior anterior margin of the muscle |
| LD muscle | Thoracodorsal artery | Enters the deep surface of the muscle in the posterior axilla 10 cm inferior to the muscle insertion into the humerus |
| Serratus anterior muscle | Lateral thoracic artery | Direct branch of the axillary artery that runs along the anterolateral surface of the muscle and descends to the level of the fifth intercostal space |
| Intercostal muscle | (I) Posterior intercostal arteries (T3–T11) | (I) Superior aspect of intercostal space |
| (II) Anterior intercostal arteries | (II) Inferior aspect of intercostal space and anterior abdominal wall | |
| Pectoralis major muscle | Thoraco-acromial artery | Emerges below the clavicle and enters the deep surface of the upper border of the muscle at its midpoint |
| RAM | (I) Superior epigastric artery | (I) Lies beneath the muscle insertion at the costal margin. The pedicle enters the medial to mid-posterior third of the muscle |
| (II) Inferior epigastric artery | (II) Lies beneath the muscle origin at the groin. The pedicle enters the lateral muscle 4 cm superior to the fibers of origin | |
| Vastus lateralis | Descending branch of the lateral circumflex femoral artery | Superior one-third of the muscle extending inferiorly along the medial border of the muscle belly |
| ALT | Perforators of the descending branch of the lateral circumflex femoral artery | The perforators of the pedicle enter the deep fascia between the rectus femoris muscle and the vastus lateralis muscle |
| DIEP | Perforators of the deep inferior epigastric artery | The perforators enter the deep fascia of the RAM |
LD, latissimus dorsi; RAM, rectus abdominis muscle; ALT, anterolateral thigh; DIEP, deep inferior epigastric perforator.
Free flaps (Table 4)
Table 4
| Reference | Country/region | Type of study | Population | Etiology of empyema | BPF (n=1) | Type of fenestration | Type of reconstruction | Follow-up time (months), median [range] | Outcomes | Complications | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numbers | Age (years), median [range] | Sex ratio, n (male/female) | ||||||||||
| Allen, 2022, (13) | UK | Retrospective observational cohort study | 8 | 56 [22–76] | 6/2 | 1 (n=3), 2 (n=1), 3 (n=2), 4 (n=2) |
4 | Open window thoracostomy NOS (n=8) | Free contralateral latissimus dorsi muscle flap with skin paddle divided into two (n=8) | 12 | Recurrence of empyema (none) | Donor site infection requiring drainage (n=2) |
| QoL improvement (n=7) | HAP (n=2) | |||||||||||
| Hematoma (n=1) | ||||||||||||
| Tan, 2016, (33) | UK | Case report | 1 | 60 | 1/0 | 2 | NR | Clagett window thoracostomy (n=1) | Free contralateral latissimus dorsi muscle flap combined with serratus anterior muscle flap | NR | Respiratory problems solved (n=1) | NR |
| Manley, 2013, (34) | UK | Case report | 1 | 65 | 1/0 | 2 | 1 | Open window thoracostomy NOS (n=1) | Free ipsilateral DIEP flap | 20 | Recurrence of BPF or empyema (none) | Respiratory failure requiring non-invasive ventilation (n=1) |
| Functional capacity: 1–2 L/min ambulatory oxygen | Cellulitis surrounding the flap POD27 requiring AB (n=1) | |||||||||||
| Walsh, 2011, (35) | USA | Case series | 6 | 63 [34–69] | NR | 2 (n=3), 3 (n=1), 5 (n=1), 6 (n=2) |
6 | Eloesser window thoracostomy (n=NR) | Free RAM flap (n=4) | 84 [24–168] | Recurrence of empyema or BPF (none) | Donor site seroma (n=1) |
| Free gracilis muscle flap (n=2) | ||||||||||||
| Takanari, 2010, (36) | Japan | Case series | 4 | NR | NR | 2 (n=4) | 3 | Open window thoracostomy NOS (n=4) | Free RAM flap combined with pedicled pectoralis major muscle flap (n=4) | 17 [11–24] | Recurrence of empyema (none) | NR |
| Flap survival (n=4) | ||||||||||||
| Closure of BPF (n=3) | ||||||||||||
| Tsai, 2010, (22) | Taiwan | Case report | 2 | 66, 70 | 2/0 | 2 (n=2), 7 (n=1) | 1 | Resection of the third rib and evacuation of the empyema (n=1) | Free anterolateral thigh combined flap: vastus lateralis and rectus femoris muscles | 6 | Recurrence of empyema (n=1) | Mild extension weakness of the left donor thigh (n=2) |
| Tube thoracostomy with decortication (n=1) | Obliteration of pleural space defect (n=1) | |||||||||||
| Rand, 2000, (37) | USA | Case report | 1 | 55 | 0/1 | 2 | NR | Clagett window thoracostomy (n=1) | Free omental flap combined with free TRAM flap | 18 | Recurrence of empyema (none) | None |
Etiology of empyema: 1, post-lobectomy; 2, post-pneumonectomy; 3, post-pneumonic; 4, spontaneous pleural effusion; 5, after chest wall resection and radiation; 6, stab wound; 7, post-tuberculosis-tuberculous. BPF, bronchopleural fistula; NOS, not otherwise specified; QoL, quality of life; HAP, hospital-acquired pneumonia; NR, not reported; DIEP, deep inferior epigastric perforator; POD, postoperative day; AB, antibiotics; RAM, rectus abdominis muscle; TRAM, transverse rectus abdominis myocutaneus.
Eight studies described using free flaps for the reconstruction of the chest wall in 27 patients. The patients had post-pneumonectomy (n=17), post-lobectomy (n=3), or post-pneumonic (n=3) empyema. The advantage of using free flaps over pedicled flaps was that they provided a larger amount of donor tissue with sufficient bulk and without pedicle length constraint. However, compared to pedicled flaps, free flap surgeries were longer and more complex procedures, potentially bearing a greater risk of postoperative complications. The reported complications were donor site infection (n=3), hospital-acquired pneumonia (n=2), donor site muscle weakness (n=2), recurrence of empyema (n=1), respiratory failure (n=1), and hematoma (n=1) (Table 4) (13,22,27,33-37).
Most studies described a reconstruction using a contralateral free latissimus dorsi flap (n=13). In a recent retrospective observational cohort study by Allen et al. [2022], eight consecutive patients underwent chest wall reconstruction using a single contralateral latissimus dorsi flap that was divided in half. The surgeons preferred the use of free flaps instead of pedicled flaps as it contributed to optimal dead space obliteration and closure of bronchopleural fistulae.
They reported that reconstruction with a free flap was cost-effective compared to lifelong nonsurgical management of residual recalcitrant empyema (13). Tan et al. [2016] performed a combined contralateral latissimus dorsi-serratus anterior free flap, when pedicled flaps were unavailable, had insufficient pedicle length, or the omentum was not deemed voluminous enough. The use of a TRAM or deep inferior epigastric perforator (DIEP) flap was unfavorable because of the potential risk of further diminishing respiratory function. The flap combination provides a pedicle with sufficient length to allow for a deep intrathoracic flap inset. Drawbacks of this approach are the need for intraoperative repositioning of the patient and the risk of scapula alata. The latter, however, can be prevented by sparing the upper digitations of the serratus anterior muscle (33).
Walsh et al. [2011] performed four free RAM flaps and two free gracilis muscle flaps, with satisfactory results. They proposed to use free flaps for large defects, beyond reach for local pedicled flaps, or when previous pedicled flaps have failed (35).
Takanari et al. [2010] reported that single-free flap reconstructions may not be suitable for patients with cachexia. Therefore, the combination of a RAM flap with a pedicled pectoralis major flap was used in four patients with favorable results. The RAM flap can include a large skin paddle of which the excess skin surrounding the paddle can be de-epithelialized and used to fill the dead space (36). Another case report by Rand et al. [2000] combined a free TRAM flap with a pedicled omental flap. The choice for this type of reconstruction was made based on the fact that the patient had redundant abdominal tissue, while local muscles were unavailable or provided insufficient volume (37).
Tsai et al. [2010] utilized the anterolateral thigh combined flap, consisting of vastus lateralis and rectus femoris muscles, in two patients after a tube thoracostomy. It provided sufficient volume for large cavities. No major complications were reported. However, both patients experienced mild knee extension weakness (22). Lastly, in a case report by Manley et al. [2013] a free ipsilateral DIEP flap was used to fill the space and close the pleural window. To prevent further impairment of ventilatory mechanics, a DIEP flap was preferred over the contralateral chest, abdominal muscle, and omental flaps. The TRAM flap was avoided, as it could further diminish ventilatory function, as abdominal muscles would have to be sacrificed. The DIEP flap provided sufficient bulk, had a large skin paddle, and did not cause further functional impairment (34).
Pedicled flaps vs. free flaps
Based on this review, it is difficult to give overall success rates. According to a review by Chen et al. [2011] the overall success rates are similar, with pedicled flaps reported at 73% and free flaps at 83–100% (38). However, the success of the reconstruction is heavily contingent on the local status and the patient’s general condition. The studies reviewed in this paper collectively suggest that it is essential to evaluate each patient individually to determine the optimal approach based on a multitude of factors. Pedicled and free flaps can be applied if the necessary resources are available, provided that the (reconstructive) surgeon is familiar with the surgical techniques.
Vacuum-assisted treatment options (Table 5)
Table 5
| Reference | Country/region | Type of study | Population | Etiology of empyema | BPF (n=1) | Type of fenestration | Type of reconstruction | Follow-up time (months) | Outcomes | Complications | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numbers | Age (years), median [range] | Sex ratio, n (male/female) | ||||||||||
| Nishii, 2021, (39) | Japan | Retrospective study | 10 | 71 [53–77] | 7/3 | 1 (n=5), 2 (n=1), 3 (n=1), 4 (n=1), 5 (n=1), 6 (n=1) |
8 | Open window thoracostomy NOS | Negative pressure wound therapy {mean duration 71.1 [4–190] days, change of materials every 3–4 days} | NR | Successful closure of open window thoracostomy with surrounding muscles alone (n=9) | Sepsis (n=2), respiratory failure (n=1), increase of air leakage (n=2) |
| Morodomi, 2014, (40) | Japan | Case report | 1 | 70 | 1/0 | 7, 8 | 1 | Open window thoracostomy NOS | Continuous negative pressure irrigation and negative pressure fixation with intercostal muscle flap | 2.5 | BPF completely closed | None |
| Badreldin, 2013, (41) | Germany | Case report | 1 | 75 | 1/0 | 9 | 0 | Open window thoracostomy NOS | Vacuum-assisted instillation therapy (5 weeks) with free contralateral latissimus dorsi flap | 6 | Good wound closure | None |
| 3D CT-imaging showing satisfactory obliteration of residual space | ||||||||||||
| Munguía-Canales, 2013, (42) | México | Case report | 1 | 21 | 0/1 | 10 | 1 | Open window thoracostomy NOS | Portable VAC device (92 days in total, change of materials every 4 days) | 8 | Obliteration of cavity in 92 days | None |
Etiology of empyema: 1, post-pneumonic; 2, postoperative bronchial stump fistula; 3, postoperative prolonged air leakage; 4, chest-tube related infection; 5, secondary pneumothorax; 6, liver abscess; 7, post-lobectomy; 8, post-tuberculosis/tuberculous; 9, post-pneumonectomy; 10, post-pneumonic in patient with cryptogenic cirrhosis. BPF, bronchopleural fistula; NOS, not otherwise specified; NR, not reported; 3D, three-dimensional; CT, computed tomography; VAC, vacuum-assisted closure.
Vacuum-assisted negative pressure wound therapy (VANPWT), also known as VAC therapy, is a technique to facilitate wound healing and reduce the need for extensive reconstruction. The foam dressings are generally changed every 3 to 4 days (43).
One retrospective cohort study and three case reports presented VANPWT as a method for chest reconstruction. Nishii et al. [2021] documented the use of VANPWT in ten patients with thoracic empyema. After an average VANPWT duration of 71.1 days, the cavity could be reconstructed by only using the surrounding muscles, without the necessity for free flaps. Successful closure of the open-window thoracostomy was achieved in nine out of ten patients (39).
Morodomi et al. [2014] described continuous negative pressure irrigation and negative pressure fixation to restore thoracic integrity in a 70-year-old patient who underwent fenestration surgery due to thoracic empyema, after a lobectomy due to tuberculosis (40). Similarly, Munguía-Canales et al. [2013] reported the use of VAC therapy in a 21-year-old female patient who underwent an open-window thoracostomy because of post-pneumonic empyema (42). Badreldin et al. [2013] reported the use of VAC therapy in addition to a free flap reconstruction to reconstruct the chest wall after multiple failed attempts of open-window thoracostomy (41).
Compared to pedicled or free flaps, VANPWT is a novel technique that might yield promising results. However, validation through large, prospective studies is required. Potential advantages of VANPWT, based on the aforementioned studies, include reduced need for invasive surgery, reduced mortality, and less post-operative complications. In addition, when materials are available, VANPWT might be easier to apply than surgical reconstruction or when surgical reconstruction is not possible. However, the time to recovery might be longer and it is questionable which option is more cost-effective. On the other hand, using VANPWT as an adjunct therapy might shorten the overall treatment duration. Nonetheless, the use of VANPWT is contraindicated in patients with prolonged and/or severe infection (39-42). However, severe infection could be mediated via irrigation treatment as proposed by Morodomi et al. (40).
Possible disadvantages of VANPWT include pain syndrome and sponge adherence to the cavity. The risk of hemodynamic complications, bleeding and injury of mediastinal structures can be minimized by applying low negative pressure (up to 125 mmHg) and ensuring viable lung parenchyma serves as a buffer, as is often the case in post-lobectomy empyema (44). The suitability of VANPWT should be evaluated on a case-by-case basis.
Limitations
The included studies are mainly case series and reports based on surgeons’ preferences, leading to potential bias. No large-scale prospective or retrospective data was available. Other outcomes such as complication rates, time to discharge, and follow-up were not consistently reported.
Conclusions
To date, the primary method used for surgical chest wall reconstruction after open-window thoracostomy, such as the Clagett procedure, is a pedicled flap. Pedicled flaps provide reliable vascularization and a lower risk of complications. When pedicled flaps are no longer possible due to damage due to previous surgeries or when a larger volume is needed to obliterate the cavity, free flaps may be a solution. Lastly, though VANPWT techniques are novel, they may offer similar results to pedicled or free flaps, possibly adjunct to surgical reconstruction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “Chest Wall Resections and Reconstructions”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-684/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-684/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-684/coif). The series “Chest Wall Resections and Reconstructions” was commissioned by the editorial office without any funding or sponsorship. E.R.d.L. and J.H.T.D. served as the unpaid Guest Editors of the series. E.R.d.L. gets consulting fees from Johnson & Johnson for training in uniportal VATS lobectomy. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iguina MM, Danckers M. Thoracic Empyema. In: StatPearls. Treasure Island: StatPearls Publishing; 2023.
- Andrews NC. Management of nontuberculous empyema: a statement of the subcommittee on surgery. Am Rev Respir Dis 1962;83:935-6.
- Ricciardi S, Giovanniello D, Carleo F, et al. Which Surgery for Stage II-III Empyema Patients? Observational Single-Center Cohort Study of 719 Consecutive Patients. J Clin Med 2022;12:136. [Crossref] [PubMed]
- Markatis E, Perlepe G, Afthinos A, et al. Mortality Among Hospitalized Patients With Pleural Effusions. A Multicenter, Observational, Prospective Study. Front Med (Lausanne) 2022;9:828783. [Crossref] [PubMed]
- Biswas A, Jantz MA, Penley AM, et al. Management of chronic empyema with unexpandable lung in poor surgical risk patients using an empyema tube. Lung India 2016;33:267-71. [Crossref] [PubMed]
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017;3:CD010651. [Crossref] [PubMed]
- Cameron R, Davies HR. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev 2008;CD002312. [Crossref] [PubMed]
- Salik I, Vashisht R, Abramowicz AE. Bronchopleural Fistula. In: StatPearls. Treasure Island: StatPearls Publishing; 2023.
- Kang B, Myung Y. Treatment of chronic bronchopleural fistula and recurrent empyema using a latissimus dorsi myocutaneous flap: a case report and literature review. Arch Plast Surg 2021;48:494-7. [Crossref] [PubMed]
- Shrestha P, Safdar SA, Jawad SA, et al. Successful closure of a bronchopleural fistula by intrapleural administration of fibrin sealant: a case report with review of literature. N Am J Med Sci 2014;6:487-90. [Crossref] [PubMed]
- van Middendorp LB, Franssen S, Gillissen S, et al. Uniportal video-assisted thoracoscopy is a safe approach in patients with empyema requiring surgery. J Thorac Dis 2020;12:1460-6. [Crossref] [PubMed]
- Reyes KG, Mason DP, Murthy SC, et al. Open window thoracostomy: modern update of an ancient operation. Thorac Cardiovasc Surg 2010;58:220-4. [Crossref] [PubMed]
- Allen LC, Milton R, Bourke G. Multidisciplinary reconstructive management of residual recalcitrant empyema cavity: A retrospective observational cohort study. J Plast Reconstr Aesthet Surg 2022;75:1057-63. [Crossref] [PubMed]
- Clagett OT, Geraci JE. A procedure for the management of postpneumonectomy empyema. J Thorac Cardiovasc Surg 1963;45:141-5.
- Nasreen S, Ali N, Ahmad T, et al. Effect of Circumference of Open Window Thoracostomy on Chest Wall Closure, Pleural Cavity Clearance, and Lung Expansion. Cureus 2021;13:e18781. [Crossref] [PubMed]
- Massera F, Robustellini M, Pona CD, et al. Predictors of successful closure of open window thoracostomy for postpneumonectomy empyema. Ann Thorac Surg 2006;82:288-92. [Crossref] [PubMed]
- Sanna S, Brandolini J, Pardolesi A, et al. Materials and techniques in chest wall reconstruction: a review. J Vis Surg 2017;3:95. [Crossref] [PubMed]
- Ferraro P, Cugno S, Liberman M, et al. Principles of chest wall resection and reconstruction. Thorac Surg Clin 2010;20:465-73. [Crossref] [PubMed]
- Celik A, Sayan M, Tastepe AI. Omento-myoplasty for postpneumonectomy empyema. Asian Cardiovasc Thorac Ann 2016;24:222. [Crossref] [PubMed]
- Seify H, Mansour K, Miller J, et al. Single-stage muscle flap reconstruction of the postpneumonectomy empyema space: the Emory experience. Plast Reconstr Surg 2007;120:1886-91. [Crossref] [PubMed]
- Duan M, Chen G, Wang T, et al. One-stage pedicled omentum majus transplantation into thoracic cavity for treatment of chronic persistent empyema with or without bronchopleural fistula. Eur J Cardiothorac Surg 1999;16:636-8. [Crossref] [PubMed]
- Tsai YT, Chen CC, Lu HI, et al. Free anterolateral thigh combined flap for chronic postpneumonectomy empyema. Ann Thorac Surg 2010;90:651-4. [Crossref] [PubMed]
- Garvia V, Paul M. Empyema. In: StatPearls. Treasure Island: StatPearls Publishing; 2023.
- Dadonaite B, Roser M. Pneumonia. Pneumonia is the leading cause of death for children younger than 5 years. 2019. Available online: https://ourworldindata.org/pneumonia
- Kamiyoshihara M, Ibe T, Igai H, et al. Paraspinous muscle flap for the treatment of an empyema cavity: three case reports. Gen Thorac Cardiovasc Surg 2017;65:297-301. [Crossref] [PubMed]
- Takeuchi M, Sakurai H. Paraspinous muscle flap for the treatment of an empyema cavity. J Plast Reconstr Aesthet Surg 2012;65:824-6. [Crossref] [PubMed]
- Belmahi A, Ouezzani S, El Aziz S. Muscular flaps and reconstructive surgery of empyema: about 12 cases. Ann Chir Plast Esthet 2008;53:1-8. [Crossref] [PubMed]
- Pichler M, Albrecht J, Padberg W. Chest wall defect and chronic pleural infection: surgical treatment with thoracomyoplasty and open window thoracostomy. Jpn J Thorac Cardiovasc Surg 2006;54:402-4. [Crossref] [PubMed]
- Okumura Y, Takeda S, Asada H, et al. Surgical results for chronic empyema using omental pedicled flap: long-term follow-up study. Ann Thorac Surg 2005;79:1857-61. [Crossref] [PubMed]
- Serletti JM, Feins RH, Carras AJ, et al. Obliteration of empyema tract with deepithelialized unipedicle transverse rectus abdominis myocutaneous flap. J Thorac Cardiovasc Surg 1996;112:631-6. [Crossref] [PubMed]
- Shirakusa T, Ueda H, Takata S, et al. Use of pedicled omental flap in treatment of empyema. Ann Thorac Surg 1990;50:420-4. [Crossref] [PubMed]
- Saber AY, Hohman MH, Dreyer MA. Basic Flap Design. In: StatPearls. Treasure Island: StatPearls Publishing; 2022.
- Tan HB, Mohan AT, Coonar AS, et al. Creative Use of Contralateral Combined Myocutaneous Free Flap for Empyema Cavity. Ann Thorac Surg 2016;101:e1-3. [Crossref] [PubMed]
- Manley K, Gelvez S, Meldon CJ, et al. Free deep inferior epigastric perforator flap used for management of post-pneumonectomy space empyema. Ann Thorac Surg 2013;95:e83-5. [Crossref] [PubMed]
- Walsh MD, Bruno AD, Onaitis MW, et al. The role of intrathoracic free flaps for chronic empyema. Ann Thorac Surg 2011;91:865-8. [Crossref] [PubMed]
- Takanari K, Kamei Y, Toriyama K, et al. Management of postpneumonectomy empyema using free flap and pedicled flap. Ann Thorac Surg 2010;89:321-3. [Crossref] [PubMed]
- Rand RP, Maser B, Dry G, et al. Reconstruction of irradiated postpneumonectomy empyema cavity with chain-link coupled microsurgical omental and TRAM flaps. Plast Reconstr Surg 2000;105:183-6; discussion 187. [Crossref] [PubMed]
- Chen HC, Lo SJ, Kim JH. Management of intrathoracic defects. Semin Plast Surg 2011;25:70-7. [Crossref] [PubMed]
- Nishii K, Nakajima T, Yamamoto T, et al. Management of thoracic empyema with broncho-pulmonary fistula in combination with negative-pressure wound therapy. Gen Thorac Cardiovasc Surg 2021;69:843-9. [Crossref] [PubMed]
- Morodomi Y, Takenoyama M, Yamaguchi M, et al. Application of continuous negative pressure irrigation and negative pressure fixation to treat a bronchopleural fistula with thoracic empyema. J Am Coll Surg 2014;218:e87-90. [Crossref] [PubMed]
- Badreldin AM, Bader RD, Hekmat K. Successful management of a massive residual space empyema using intrathoracic vacuum-assisted instillation. Thorac Cardiovasc Surg 2013;61:642-5. [Crossref] [PubMed]
- Munguía-Canales DA, Vargas-Mendoza GK, Alvarez-Bestoff G, et al. Management of pleural empyema with a vacuum-assisted closure device and reconstruction of open thoracic window in a patient with liver cirrhosis. Arch Bronconeumol 2013;49:447-9. [Crossref] [PubMed]
- Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: A review. J Clin Orthop Trauma 2019;10:845-8. [Crossref] [PubMed]
- Gritsiuta AY, Eguchi T, Jones DR, et al. A Stepwise Approach for Postlobectomy Bronchopleural Fistula. Oper Tech Thorac Cardiovasc Surg 2020;25:85-104. [Crossref] [PubMed]


