
Sternal resection and reconstruction: a review
Introduction
Sternal resection and reconstruction is not a common chest wall procedure, but when one of these cases occurs, its management can be challenging. Even after small partial resections of the sternum, both thoracic cavities are at risk of instability, so it is crucial to ensure not only aesthetic but mainly protective and functional restoration to preserve respiratory mechanics. Therefore, any sternal resection must begin with a careful patient selection including evaluation of operability and resectability followed by selection of the most appropriate technique for reconstruction. Unfortunately, a large variety of options are available and the existing data for their evaluation come mainly from retrospective single center studies including few patients. Since randomized trials and even comparative studies between different techniques are lacking, the available knowledge on sternal reconstruction depends largely on expert consensus or more frequently on the simple preference of each surgical team.
In this sea of uncertainty, the objective of this article is to offer an overview of the preoperative evaluation, indications, techniques and results of sternal resection and reconstruction, trying to describe the main advantages and disadvantages of each type of technique in order to facilitate the most appropriate choice in each specific clinical case.
Indications for sternal resection (Table 1)
Table 1
| Primary malignant tumors |
| Chondrosarcoma |
| Osteosarcoma |
| Others |
| Secondary malignant tumors |
| Local involvement |
| Lung carcinoma |
| Mediastinal neoplasms |
| Metastatic carcinoma |
| Breast cancer |
| Plasmacytoma |
| Melanoma |
| Others |
| Benign tumors |
| Chondroma |
| Bone cysts |
| Others |
| Non-tumoral lesions |
| Sternoclavicular joint infection |
| Radiation injuries |
Sternal resection indications (in descending order of frequency).
Sternoclavicular joint infection
The sternoclavicular joint includes the clavicular notch of the manubrium, the sternal head of the clavicle, and the costocartilage of the first rib. In certain circumstances such as intravenous drug use, local trauma and some immune compromised states such as diabetes, chronic hemodialysis or longstanding steroid therapy, this joint can get infected and failure to control infection by conservative means might mandate surgical resection (1,2).
Radiation injuries
Months or even years after radiation therapy, some patients experience a serious local complications known as late radiation tissue injury (1). Due to obliteration of tissue small vessels, a progressive deterioration secondary to reduced vascularity occurs, followed by replacement of normal soft tissue architecture by dense fibrosis that ultimately leads to tissue ulceration. In such cases, wide surgical resection plus coverage with a well vascularized soft tissue flap might be the only chance to avoid progressive necrosis and infection.
Neoplasms
Primary sternal tumors are rarely benign, consisting of chondroma, bone cyst, fibrous dysplasia hemangioma, osteoma or Langerhans cells histiocytosis (3,4). Much more often they are malignant, most of them being chondrosarcomas and osteosarcomas. While osteosarcomas may be treated with neoadjuvant chemotherapy, chondrosarcomas are unresponsive to radiation or chemotherapy, therefore complete surgical resection is their only chance for cure. Other infrequent primary tumors such as squamous cell carcinoma have also been described (5).
Secondary sternal tumors are uncommon, representing about 15% of all sternal tumors and involving mainly the body of the sternum (6). Among these lesions we find both sternal invasion from adjacent diseases such as breast or mediastinal tumors (thymic carcinoma, germ cell tumors and others) as well as purely metastatic lesions. Among the latter, breast cancer is usually the most frequent with up to 50% incidence in some series (7) but other possible metastatic tumors are solitary plasmacytoma (8), renal cell cancer (9), melanoma (10), thyroid carcinoma (11), colorectal cancer, cervical cancer, hemangioma (12) or hepatocellular carcinoma (13). Given their overall low incidence and therefore limited published data, there is no consensus on their treatment (13), though given their bad prognosis and the high rate of incomplete resections performed the trend is towards limited palliative exeresis to avoid pain, infection or pulmonary function impairment, always within a multimodal treatment scheme (7).
Preoperative assessment
As in other thoracic procedures, preoperative diagnostic encompasses the evaluation of operability and resectability, as well as a detailed reconstruction plan. All of them should ideally carried out by multidisciplinary teams including at least thoracic surgeons, plastic surgeons, anaesthesiologists and physiotherapists.
Operability workup
The operability assessment for sternal resection does not differ much from that required for any other major thoracic intervention and must include a thorough clinical history plus physical examination, some laboratory tests and a final cardiopulmonary evaluation. Within the clinical history, the underlying respiratory diseases (especially if they are oxygen-dependent) are relevant, since even a small surgery can produce a high postoperative dysfunction (14). Other important parameters to be recorded are evaluation of daily life activities, nutritional status, sarcopenia (progressive and generalized loss of skeletal muscle and strength) or frailty (unintentional weight loss, fatigue, poor grip strength, inactivity and low walking speed), a variable related to postoperative adverse outcomes (15,16).
After clinical history, a complete physical examination is mandatory, being important again to search for signs and symptoms that may indicate frailty. Operability evaluation is finally completed with the usual laboratory tests and a structured cardiopulmonary assessment according to current guidelines (17).
All this information allows us to classify our patients as low, medium or high risk patients, these latter being those with a score equal or higher than 4 in a frailty scale, severe comorbidity and/or high cardiac and/or pulmonary impairment; in such cases, a possible solution is proceed with a surgical prehabilitation or preoperative intervention on those adverse conditions that can worsen postoperative results (18-22).
Resectability workup
The assessment of resectability differs between benign and malignant lesions. In the first case, it is usually based on less invasive techniques such as the isolation of pathogen cultures in the case of infections and is mainly aimed at distinguishing between the need for medical or surgical treatment.
In the case of a tumor, the evaluation is aimed at evaluating the type of resection necessary, the required surgical margin and, where appropriate, the best reconstruction technique. It should include both a diagnosis by biopsy of the type of tumor and its degree of differentiation as well as a staging as accurate as possible.
Staging is usually performed by image methods such as plain radiograph, axial computed tomography (CT) scan, magnetic resonance image (MRI) or positron emission tomography-CT (PET-CT). CT scan is the most sensitive and commonly used tool but may not be reliable when assessing depth of invasion (23-25), therefore other techniques such as ultrasound (26,27), dynamic CT scan, two-step CT scan (28), MRI (29) or cine MRI (30) have been proposed to increase accuracy with mixed results. PET-CT is considered clearly suboptimal to assess sternal invasion due to blooming artifact, which may overestimate the size of the lesion (31). Finally, to get information about the type of tumor and its differentiation, available resources are fine needle aspiration, core needle biopsy and incisional or excisional biopsies according to the case (32,33).
Strategies for resection
Sternoclavicular joint infection
Surgery is usually performed via an inverted L-shaped incision that extends laterally over the medial half of the clavicle and inferiorly over the manubrium down to the second or third interspace (1). Devitalized soft tissue is widely debrided and if the damage is judged not important, vacuum assisted closure (VAC) dressing can be attempted for closure by secondary intention. When tissue destruction is extensive, half the manubrium is typically resected to preserve stability of the contralateral side and the costal cartilage and medial portion of the first rib can then be divided with rib instruments, along with medial portions of the second or third rib if involved with infection. In such cases, soft tissue coverage for closure is required, most commonly with an ipsilateral pectoralis muscle flap (2). The use of a prosthetic reconstruction is contraindicated due to infection risk, thus biological meshes are preferred.
Radiation injuries
Once underlying malignancy has been ruled out, the general management of these lesions includes debridement of necrotic tissues and reconstruction with well-vascularized flaps (1). Mesh reconstruction should be avoided due to the risk of infection and is generally unnecessary as due to the chronic nature of infection there is always some degree of fibrosis that stabilizes by itself the chest wall (2).
Neoplasms
Resection of the sternum often requires extensive excision of the overlying skin and soft tissues along with the affected part of the bone, sometimes extending to the adjacent pericardium, thymus, large blood vessels, and other major organs (3). Since complete resection with free surgical margins is crucial for the prognosis of these tumors, radicality is usually the norm, as demonstrated by a recent consensus study where up to 41% of surgeons agreed that the skin of the tumor surface should be extensively excised even though imaging and palpation examinations did not indicate invasion (34), thus surgical access is usually made through a vertical, elliptical incision that encompasses not only the tumor but all the affected surrounding structures.
In tumors that involve more than one part of the bone (considering a division of the sternum into manubrium plus medial third of the clavicles, middle sternal body and lower third of the sternal body plus xiphoid) (Figure 1), a subtotal or total sternectomy is performed. Partial sternectomies are reserved for more localized tumors (4). Some authors (35) have even reported more conservative approaches with preservation of the posterior cortex of the sternum, but in our opinion, since an R0 resection is necessary to avoid the high risk of tumor recurrence and since these supposedly limited resections frequently include skin, soft tissue or ribs excision, its advantages are highly questionable.
Regardless of the type of resection, most authors agree that a minimum margin of 3 cm is considered necessary to minimize the risk of local recurrence (34). As these resections are often extensive, they require planning for both skeletal and soft tissue reconstruction and, in cases where reconstructive surgery cannot be performed due to close vicinity of the tumor to vital organs, a positive surgical margin (R1) is allowed, always considering postoperative radiotherapy. As a particular situation, in manubrium sterni tumors, a large percentage of surgeons believe that the capsula articularis sternoclavicularis can be used as a safe margin marker if the tumor does not invade the joint capsule (34).
Reconstruction techniques
The goals of sternal reconstruction are to stabilize the chest wall to minimize the risk of prolonged mechanical ventilation and respiratory complications, to protect the underlying mediastinum, and to minimize the resulting deformity. Sternectomies defects often involve resection of the overlying skin or sacrifice of the internal mammary vessels, and furthermore patients undergoing oncologic resections typically present with an impaired wound healing secondary to adjuvant chemoradiation. Thus, oncologic sternectomy creates heterogeneous clinical scenarios that demand an integrated reconstructive strategy (3,36).
The review of the literature on prosthetic reconstruction is hampered by several limitations, the strongest of which is the absence of prospective trials comparing different techniques and materials with each other because of the low surgical volume, even in specialized centers. Furthermore, most single-institution experiences encompass multiple decades, and therefore do not optimally show the continuous refinements in patient selection, surgical technique, reconstructive materials, and postoperative care. To end, some outcomes, such as patient quality of life and cosmetic considerations, have seldom been measured scientifically but are often postulated (37). Consequently, the choice of technique to use often depends on previous surgical experience and surgeon preference rather than on the demonstrable superiority of one technique over another for that particular defect (Table 2).
Table 2
| Type of technique | Advantages | Disadvantages |
|---|---|---|
| Non-rigid methods | ||
| Soft tissue (omentum) | Protect from friction against other prosthetic material, well vascularized tissue | Available omentum amount highly variable, difficult harvest if previous abdominal surgery |
| Meshes | Available, cheap, easy to handle and store | Low resistance to infection, poor protection of vital organs |
| Synthetic (braided)† | Good tissue ingrowth, thinner | |
| Synthetic (compact)‡ | Bad tissue ingrowth, impermeable, thicker | |
| Biological§ | Rigid, durable, good tissue integration, decreased infection risk | |
| Rigid methods | ||
| Bone allografts¶ | No donor site morbidity; unlimited availability; easily adjusted; cost-effective | Possible graft reabsorption |
| Prosthesis | Immediate stabilization of chest wall (low risk of respiratory complications), acceptable cosmetic results | Little real tissue integration, high risk of migration, fracture or erosion, low resistance to infection |
| 3D printed prosthesis†† | More precise setting of resection margins, minimal intraoperative adjustment, shorter surgery, less dislocation or migration, reduced pain, improved aesthetics | Indications of use not clearly defined, higher cost, no data about functional outcomes |
†, e.g., polyester or polypropylene meshes; ‡, e.g., PTFE meshes; §, refers to dermis crosslinked collagen matrixes; ¶, iliac bone, rib or sternal allograft from tissue bank; ††, theoretical advantages of 3D printed prosthesis over other standard devices. PTFE, polytetrafluoroethylene.
Although the extent of resection that mandates a rigid versus semi-rigid reconstruction (this latter being those composed solely of meshes and soft tissue flaps) remains to be determined, in a recent publication, the majority of patients (85%) undergoing a total or subtotal sternectomy received some rigid prosthetic reconstruction (36), which together with some encouraging results with semi-rigid reconstructions for partial sternectomy defects point to a shift to reserve rigid reconstructions for more extensive sternectomy defects (Figure 2).
Sometimes, some specific situations may demand even more specific technical solutions. The sternoclavicular joint disruption represents a particular technical defy, because the shoulder and upper limb are destabilized, leading to pain and dysfunction as the shoulder internally rotates and medially displaces (38), but authors such as Rocco et al. (10) found a full range of mobilization of the shoulder without reconstruction of the clavicles more than three months after surgery. Sometimes the effort to preserve the upper portion of the sternum to minimize a possible functional alteration carries a high risk of recurrence due to positive surgical margins, so when in doubt a complete resection for oncological benefits is strongly advised (39). Another particular case for reconstruction is children, where complications related to prosthetic materials and their probable growth restriction make bone grafts and biological meshes specially preferred although again no studies reporting mid-long term results are available (12).
Non-rigid reconstructive methods
Soft tissue coverage
Since sternal defects may require complex soft tissue reconstruction, which is usually performed by an experienced plastic surgeon, a description of the many soft tissue coverage options is well beyond the scope of this article. Generally, pectoralis major muscle advancement flaps without disinsertion are the preferred reconstructive option and if these are not present or diminutive, a distant free flap is selected such as a fasciocutaneous flap from the leg, abdomen, or the thorax. Omental flaps are also a reliable option for sternal reconstruction (38,40,41) as they protect mediastinal organs from friction against any other added prosthetic material and provide a well vascularized tissue with a rich lymphatic network (42).
Meshes
There is a great variety of flexible implants (generically known as “meshes”) classified according to their composition (synthetic or biological) or their properties (knitted of compact, etc.). Well-known advantages of meshes are their availability, cheapness and easiness to be handled and stored but as opposed they are poorly resistant to infections and sometimes do not provide enough protection for vital organs. Despite a formal comparison between different types of meshes has not been performed and their postoperative outcomes are similar to those of autologous flaps (43), meshes seem to be one of the most preferred techniques of reconstruction, as they were used by an 82.5% of surgeons in a recent consensus study (34). The quality of life and patient satisfaction after their implantation is clearly improvable, as stated in the study by Daigeler et al. (44) where only 38% of patients described it as much or slightly better.
Synthetic meshes
Several studies conclude than sternal reconstruction with a synthetic mesh provides a secure base for reconstruction but needs to be covered by a muscle flap (what is called a semi-rigid reconstruction) to avoid paradoxical respiration (45,46).
Braided or reticular meshes (polyester, polypropylene, etc.) are permeable to air and liquid, allowing connective tissue cell ingrowth; not being very thick, some are used for rigid reconstruction in combination with other materials such as methyl methacrylate (Figure 3). Compact meshes such as polytetrafluoroethylene (PTFE) are thicker and more impermeable to air and liquid, but favour tissue growth to a lesser degree. As an intermediate solution between flexible and rigid implants, the use of titanium meshes has been proposed. These devices seem easy to cut and shape, achieving the right rigidity on the chest wall while preserving the elasticity and dynamics of the thorax, resulting well tolerated by the patient (47-49).
Biological meshes
Biological meshes are usually biological crosslinked collagen matrixes derived from porcine dermis in which cells, debris and all genetic material have been removed (50). The final structure combines the rigidity and durability of non-absorbable synthetic materials with the ability for tissue integration and remodelling, which would make it especially interesting in cases of pediatric reconstructions given the possibility of growing with the patient. Due to its capacity for tissue integration, this material is also advocate to decrease the risk of site infection associated to prostheses although there is still an open debate since some authors (51) reported the occurrence of wound healing difficulties (haematoma or infection) in several patients while other recent studies with follow-up periods of about two years report no postoperative complications with good functional outcomes (52,53).
Rigid reconstructive methods
Allografts
Either iliac bone allograft from a tissue bank (54-57), donor cryopreserved rib allografts (58) or cadaveric cryopreserved sternal allografts (59,60) have been proposed as a simple and cost-effective technique for sternal reconstruction.
Autologous iliac graft was one of the first bones to be used for sternal reconstruction in combination with titanium bars. A common drawback for this implant is it limited size, which makes it unsuitable to cover large surfaces. Moreover, the combination of the graft with the fixation bars results in fact in a “rigid plate-effect” with consequences very similar to those of methylmethacrylate sandwiches. In this sense, the largest published series (56) registered up to 66% of postoperative complications, most of them cardiopulmonary.
In our own experience (58), cryopreserved ribs are far better for reconstruction than other tissue bank bones because size and shape of ribs are easily adjusted to the defect, even when it is irregular. Its use seems especially interesting for sternal manubrium reconstruction as stated by Zhang et al. (61). These grafts eliminate possible morbidity at the contralateral hemithorax donor site (pain, instability, lung herniation) and have no limitations regarding the amount of available bone, processed and stored for long periods at a reasonable cost.
Sternal replacement with cadaveric allograft is also considered an effective procedure which provides optimal stability (59). The largest series published is a multicenter study encompassing 58 patients submitted for sternal resection due to primary and secondary tumors and other non-neoplastic conditions; median postoperative follow-up was of 52 months with a 30-day mortality of 5% and a morbidity rate of 31%, mostly secondary to respiratory complications or surgical wound problems. Interestingly, no respiratory deficiency or complications derived from insufficient or altered ventilatory mechanics were recorded and the graft integrated perfectly into the host as recently demonstrated by bone scintigraphy scans (60). As a particular modification of this technique, Rosenberg et al. published the complete resection of the sternum with ex vivo curettage, cryotherapy and posterior reimplantation in a case of breast carcinoma metastasis (62).
Finally, some other strategies have been explored such as fascia lata grafts (63), free vascularized iliac osteocutaneous flaps (64) or even regenerative approaches with strategies aimed at promoting tissue regeneration with bone remodelling using cell therapy based on mesenchymal stem cells (6).
Prostheses
The term “prosthesis” encompasses a wide variety of devices that range from the relatively simple systems such as methylmethacrylate sandwiches to the more complex titanium devices. Most of them allow an immediate stabilization of the chest wall with a possibly lowered risk of respiratory complications and good cosmetic results but at the same time tend to have little tissue integration and a high risk of migration, fracture and erosion as well as low resistance to infection. However, whether these complications primarily relate to the kind of prosthesis or to confounders, such as the size of the chest wall defects or the type of soft tissue transposition for coverage, is impossible to determine within the context of the available retrospective studies (37).
One of the first synthetic rigid implants were methylmethacrylate plates, also known as “sandwich meshes” (Figure 2). In our opinion, this type of reconstruction is slightly outdated by many other techniques as it can produce ventilatory restriction, has an increased risk of migration and erosion and causes discomfort to the patient (if not directly pain) because of an excessive stiffness of the chest wall. Wound complications such as seroma or infection are reported in 10% to 20% of patients at 90 days, which requires removal extraction of the prosthesis in approximately 5% of cases (65).
From these first prostheses many other different techniques have been proposed for rigid chest wall reconstruction with variable results such as the use of other mesh-bone cement sandwiches (66), Kirschner wires (67), steel sutures (68), ceratite prosthesis (69), porous alumina (70), Ley prosthesis (71), rib-like technique (72) or customized sternal plates (73,74) but titanium-based devices are by far the most commonly used nowadays. They have clear advantages over other systems (biocompatibility, osseointegration, resistance to infection, a high strength/weight ratio and low optical density), although they are not free of complications similar to other devices such as rupture, displacement, thoracic pain or infection (47,50). Common osteosynthesis systems are Stratos® (Strasbourg Thorax Osteosyntheses System), Sternalock® (Walter Lorenz Surgical Inc., Jacksonville, FL, USA) or the MatrixRIB Fixation® (DePuy Synthes, West Chester, PA, USA) while some other companies also offer made to measure titanium implants. Although widely available and highly customizable in different surgical settings, 3D printed prostheses could be the next generation of these devices.
3D printed prostheses
Additive manufacturing (also known as 3D printing) is a technology that allows to manufacture all sort of pre-designed objects by depositing layer upon layer of different materials such as plastic, metal, ceramics and others (75). Versus other types of rigid implants, 3D printed prosthesis offers some theoretical advantages such as a more precise setting of resection margins, minimal need for intraoperative adjustment and better fixation systems to prevent dislocation or migration. As a consequence, the operative time is shortened, pain is reduced and aesthetics are improved (76).
Many different materials have been proposed as the ideal for sternal 3D printed prostheses yet titanium being still the most widely used (34,77). However, from a functional point of view, new bone-like materials such as polyether-ether-ketone (PEEK) has an elastic modulus closer to that of cortical bone and are promising alternatives to titanium as it improves integration into the host with less functional impairment (78).
Despite all the possible advantages, these devices are far away of being considered as a day-to-day technology in our specialty. Against a large number of experimental studies, there are few clinical studies, mostly heterogeneous and limited to clinical cases without information about mid- and long-term outcomes, thus making impossible to address fundamental questions such as the precise use indications of these devices, their real outcomes compared to other well-known reconstructive techniques or even their supposed unaffordable cost (79). There is a clear need for collaborative studies in order to standardize this device as much as possible, this meaning to agree on some general designs and materials for the sternal prostheses, so that only a few minor adjustments in each particular case are made. This would allow mass-production which would reduce costs, manufacturing time and simplify some regulatory hurdles. Our group is precisely in this line of work through the development of modular sternal 3D printed prostheses with a common central axis and modifiable “lateral combs” according to the needs of each patient (80) (Figure 4).
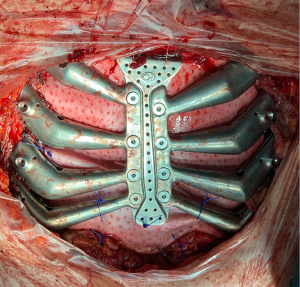
Resection and reconstruction outcomes (Table 3)
Table 3
| Author | Patients | Indications† | Resection† | R0 percentage | Reconstruction‡ | Complications§ |
|---|---|---|---|---|---|---|
| Dudek et al. (7) | 8 | SM | TS [2], PS [6] |
50% | Methylmethacrylate, synthetic meshes, titanium plates | Empyema, hemothorax, subclavian vein thrombosis |
| Butterworth et al. (36) | 49 | PM [12], SM [31], OT [6] |
TS [13], PS [36] |
Non stated | Rigid reconstruction [18], semi-rigid reconstruction [22], no reconstruction [9] | Prolonged mechanical ventilation, pulmonary embolus, wound complications, mesh removal, in-hospital mortality [1] |
| Fabre et al. (39) | 24 | PM [9], SM [15] |
TS [24] | Non stated | Titanium-rib bridge | Pulmonary infection, seroma |
| Gritsiuta et al. (42) | 4 | PM [4] | TS [3], PS [1] |
Non stated | Methylmethacrylate, prolene mesh, titanium plates, biological mesh | “Complicated hospital course” [1] |
| Gonfiotti et al. (52) | 27 | PM [18], SM [9] |
TS [5], PS [22] |
Non stated | Biological mesh | None |
| Xu et al. (56) | 12 | PM [3], SM [4], BT [2], OT [3] |
TS [10], PS [2] |
Non stated | Iliac graft + titanium plates | Pleural effusion, pulmonary infection, atelectasis, atrial fibrillation, tissue flap necrosis [8] |
| Marulli et al. (59) | 14 | PM [8], SM [5], OT [1] |
TS [2], PS [12] |
Non stated | Sternal allograft | Displaced implant, wound dehiscence |
| Dell’Amore et al. (60) | 58 | PM [15], SM [13], OT [30] |
TS [42], PS [16] |
Non stated | Sternal allograft | Respiratory complications, wound complications [18], in-hospital mortality [3] |
| Zhang et al. (61) | 12 | PM [5], SM [3], OT [4] |
TS [7], PS [5] |
Non stated | Rib allografts | Paradoxical movement of the chest wall |
| Puviani et al. (63) | 8 | PM and SM | TS [8] | Non stated | Fascia lata graft | Wound complications |
| Girotti et al. (72) | 101 | PM [42], SM [52], OT [7] |
TS [24], PS [77] |
93% | Synthetic mesh, rigid prosthesis, rib-like technique | Cardiorespiratory complications [7], wound infections, graft necrosis [15], prosthetic removal [7] |
| Marulli et al. (81) | 23 | PM [23] | TS [18], PS [5] |
85,4% | Rigid reconstruction, semi-rigid reconstruction | Cardiorespiratory complications [5], wound complications [2], others [4] |
| Bongiolatti et al. (82) | 36 | PM [23], SM [13] | TS [6], PS [30] |
100% | Rigid reconstruction, semi-rigid reconstruction | Cardiorespiratory complications [7] |
| Ahmad et al. (83) | 78 | PM [28], SM [45], OT [5] |
TS [73], PS [5] |
77% TS, 72% PS |
Synthetic mesh, biological mesh | Cardiorespiratory complications [17] |
| Novoa et al. (84) | 6 | SM [6] | TS [2], PS [4] |
Non stated | Methylmethacrylate, PTFE mesh | Respiratory insufficiency with mechanical ventilation [1] |
| Elahi et al. (85) | 93 | PM [45], SM [48] |
Non stated | 69% | Methylmethacrylate, synthetic mesh, osteosynthesis | Cardiorespiratory complications [36], wound complications [7], other [5] |
| Leuzzi et al. (86) | 35 | PM and SM | Non stated | 71% | Synthetic mesh, no reconstruction | Anemia, seroma, atelectasis, respiratory failure |
†, number for each subset in square brackets. ‡, all the reconstructions added a soft-tissue flap coverage. Number of cases in square brackets. §, number of patients for subsets in square brackets. SM, secondary malignant tumors; TS, total/subtotal sternectomy; PS, partial sternectomy; PM, primary malignant tumors; OT, other non-neoplastic diseases (radionecrosis, sternoclavicular joint infection); BT, benign tumors; PTFE, polytetrafluoroethylene.
Mortality, morbidity and functional outcomes
Despite his apparent aggressiveness, sternal resection seems to be a safe surgery. Overall complication rates for reconstructions of partial and total or subtotal sternectomies were equivalent which could justify the trend towards more aggressive resections (36).
Mortality rates ranged from zero to 7% in most published series (81-84). Deaths were usually secondary to systemic respiratory complications that with an incidence of 1.1% to 24.4% are the main source of morbidity after chest wall resections due to flail chest and paradoxical breathing resulting in sputum retention, atelectasis, pneumonia and respiratory failure (36,42,81-85).
Local morbidity was commonly related to wound complications (pain, seroma, infection, dehiscence) or device-related problems such as prosthesis erosion, fracture, displacement or migration (3).
After sternal resection and reconstruction, many authors have focused on the in-hospital period only, while in other studies the data mainly refer to survival, recurrence, and metastasis rates. Only sporadic reports focusing on quality of life and postoperative pulmonary function exist.
Because of immediate stabilization, the use of prostheses is especially attractive for sternal reconstruction, although this topic remains subject to controversy. On the one hand, several works support the idea of that the repercussion on respiratory mechanics of sternal resection may not be as severe as previously thought (86); thus, when part of the distal sternal third or a small part of the manubrium with the sternoclavicular joint is preserved, rigid materials might not be essential and primary closure or a semi-rigid reconstruction could be enough as no differences were observed between the pre and postoperative respiratory function (87-90). On the other hand, authors like Scarnecchia (91) systematically recommend rigid reconstruction in the so-called critical areas of the chest such as the anterior chest wall because its stabilization showed an inverse correlation with acute respiratory complications, flail chest and deformities (100% occurrence in the non-reconstructed subgroup versus only 5.7% after reconstruction). Moreover, not even the most rigid prostheses such as methylmethacrylate sandwiches seem to pose a high restrictive effect on lung function, since evidence up to 92% concordant movement of the wall and the prosthesis 6 months after surgery, with no relevant differences between preoperative and postoperative lung function (92). Since neither of the two types of prosthesis has a great impact on lung mechanics, it seems reasonable, to reserve rigid reconstructions for more extensive sternectomy defects to reduce the risk of infections, patient discomfort and the cost of the procedure.
Data about in vivo functional evaluation of 3D printed prostheses are scarce since randomized controlled trials are not possible and only indirect data obtained in patients with previously implanted prosthesis substituted by 3D printed devices are available. Nonetheless, these preliminary reports in which pulmonary function tests, cardiopulmonary exercise tests or motion range capture studies are performed with reflective surface markers (optoelectronic plethysmography or photogrammetry) seem to reveal that 3D prostheses increase forced expiratory volume in the first second (FEV1), abolish paradoxical movement in upper rib cage and increases synchrony between thoracic and abdominal movement compared to the preoperative settings, what seems to be promising outcomes (93-95).
Oncological outcomes
As previously said, the most common primary sternal tumor is chondrosarcoma and radical resection without adjuvant therapy seems to be associated with a good overall survival. After sternal resection in 89 patients, Marulli et al. (81) found a 5- and 10-year overall survival of 67% and 58% respectively, with a disease-free survival of 70% and 52%. Another recent series including a 64% of sternectomies performed for primary tumors recorded a 61% overall survival with a median follow-up of 24 months (82). The main prognostic factor for survival is radicality of resection with adequate R0 surgical margins (at least 3 cm) which translates into a rate of total and subtotal sternectomies reaching over 80% in some publications (42,83). Other prognostic factors such as histological low grading, younger age, diameter equal or less than 6 cm and no adjuvant treatment have also been pointed in other studies although these data should be interpreted with caution because the study population is small due to the low incidence of these type of tumors.
Survival is clearly worse for patients undergoing resection of purely metastatic disease compared to primary tumors or sternal involvement secondary to neighborhood diseases. Breast cancer is the most frequent secondary sternal tumor and surgery can offer a 5-year overall survival ranging 20% to 50% provided an R0 resection is achieved, although radical surgery does not appear to decrease recurrence rates. Metastasis from a source other than the breast result in the worst outcomes (less than 40% at 36 months and 0% at 5 years) (7,36,82) and although limited data are available on these cases, the radicality of the resection neither seem to modify global survival nor the recurrence rates, so it is likely that a conservative approach will be more appropriate (83).
Conclusions
Sternal resection and reconstruction is a rare but frequently extensive surgical procedure with important anatomical and functional implications. Therefore, an adequate preoperative evaluation followed by an adequate planning of the reconstruction is essential to ensure good oncological and functional results. In a clinical setting with many available reconstructive techniques and in the absence of high-quality data comparing them, specific recommendations for particular cases are difficult to make, making clear the need for more multicenter, comparative studies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Erik de Loos, José Ribas de Campos and Jean Daemen) for the series “Chest Wall Resections and Reconstructions” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-450/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-450/coif). The series “Chest Wall Resections and Reconstructions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blasberg JD, Donington JS. Infections and radiation injuries involving the chest wall. Thorac Surg Clin 2010;20:487-94. [Crossref] [PubMed]
- Bergeron EJ, Meguid RA, Mitchell JD. Chronic Infections of the Chest Wall. Thorac Surg Clin 2017;27:87-97. [Crossref] [PubMed]
- Chapelier A. Resection and reconstruction for primary sternal tumors. Thorac Surg Clin 2010;20:529-34. [Crossref] [PubMed]
- Smith SE, Keshavjee S. Primary chest wall tumors. Thorac Surg Clin 2010;20:495-507. [Crossref] [PubMed]
- Garach NR, Kammar PS, Deshpande A, et al. Primary Squamous Cell Carcinoma of the Sternum. Ann Thorac Surg 2021;111:e37-9. [Crossref] [PubMed]
- Aramini B, Masciale V, Radaelli LFZ, et al. The sternum reconstruction: Present and future perspectives. Front Oncol 2022;12:975603. [Crossref] [PubMed]
- Dudek W, Schreiner W, Horch RE, et al. Sternal resection and reconstruction for secondary malignancies. J Thorac Dis 2018;10:4230-5. [Crossref] [PubMed]
- Choi CW, Park YK, Shin HK, et al. Sternal Resection and Reconstruction for Solitary Plasmacytoma of the Sternum: Case Report. J Chest Surg 2021;54:400-3. [Crossref] [PubMed]
- Lee SY, Lee SJ, Lee CS. Sternum resection and reconstruction for metastatic renal cell cancer. Int J Surg Case Rep 2011;2:45-6. [Crossref] [PubMed]
- Rocco G, de Chiara AR, Fazioli F, et al. Primary giant clear cell sarcoma (soft tissue malignant melanoma) of the sternum. Ann Thorac Surg 2009;87:1927-8. [Crossref] [PubMed]
- Sabih Q, Spafford MF, Dietl CA. Poorly differentiated thyroid carcinoma with sternal invasion. A case report and review of the literature. Int J Surg Case Rep 2014;5:816-20. [Crossref] [PubMed]
- Sadegh Beigee F, Sheikhy A, Sheikhy K. Reconstruction of Chest Wall by Cryopreserved Sternum Allograft After Resection of Sternal Hemangioma: A Case Report. Front Surg 2022;9:796806. [Crossref] [PubMed]
- Yoon YC, Lee J, Jeong JY. Radical resection and reconstruction of the sternum for metastasis of hepatocellular carcinoma. J Cardiothorac Surg 2020;15:202. [Crossref] [PubMed]
- Pennathur A, Brunelli A, Criner GJ, et al. Definition and assessment of high risk in patients considered for lobectomy for stage I non-small cell lung cancer: The American Association for Thoracic Surgery expert panel consensus document. J Thorac Cardiovasc Surg 2021;162:1605-1618.e6. [Crossref] [PubMed]
- Starke H, von Dossow V, Karsten J. Preoperative evaluation in thoracic surgery: limits of the patient's functional operability and consequence for perioperative anaesthesiologic management. Curr Opin Anaesthesiol 2022;35:61-8. [Crossref] [PubMed]
- Lee JA, Yanagawa B, An KR, et al. Frailty and pre-frailty in cardiac surgery: a systematic review and meta-analysis of 66,448 patients. J Cardiothorac Surg 2021;16:184. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Dezube AR, Cooper L, Jaklitsch MT. Prehabilitation of the Thoracic Surgery Patient. Thorac Surg Clin 2020;30:249-58. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Sanchez-Lorente D, Navarro-Ripoll R, Guzman R, et al. Prehabilitation in thoracic surgery. J Thorac Dis 2018;10:S2593-600. [Crossref] [PubMed]
- Ferreira V, Minnella EM, Awasthi R, et al. Multimodal Prehabilitation for Lung Cancer Surgery: A Randomized Controlled Trial. Ann Thorac Surg 2021;112:1600-8. [Crossref] [PubMed]
- Gravier FE, Smondack P, Prieur G, et al. Effects of exercise training in people with non-small cell lung cancer before lung resection: a systematic review and meta-analysis. Thorax 2022;77:486-96. [Crossref] [PubMed]
- Kawaguchi K, Mori S, Usami N, et al. Preoperative evaluation of the depth of chest wall invasion and the extent of combined resections in lung cancer patients. Lung Cancer 2009;64:41-4. [Crossref] [PubMed]
- Maconachie R, Mercer T, Navani N, et al. Lung cancer: diagnosis and management: summary of updated NICE guidance. BMJ 2019;364:l1049. [Crossref] [PubMed]
- Bai JH, Hsieh MS, Liao HC, et al. Prediction of pleural invasion using different imaging tools in non-small cell lung cancer. Ann Transl Med 2019;7:33. [Crossref] [PubMed]
- Tahiri M, Khereba M, Thiffault V, et al. Preoperative assessment of chest wall invasion in non-small cell lung cancer using surgeon-performed ultrasound. Ann Thorac Surg 2014;98:984-9. [Crossref] [PubMed]
- Briccoli A, Galletti S, Salone M, et al. Ultrasonography is superior to computed tomography and magnetic resonance imaging in determining superficial resection margins of malignant chest wall tumors. J Ultrasound Med 2007;26:157-62. [Crossref] [PubMed]
- Tsubamoto M, Nishida T, Higaki N, et al. Separation between the chest wall and subpleural lung lesions: A two-step method to preoperatively exclude invasion or focal pleural adhesion by multidetector computed tomography. Eur J Radiol 2019;112:180-5. [Crossref] [PubMed]
- Carter BW, Benveniste MF, Betancourt SL, et al. Imaging Evaluation of Malignant Chest Wall Neoplasms. Radiographics 2016;36:1285-306. [Crossref] [PubMed]
- Kajiwara N, Akata S, Uchida O, et al. Cine MRI enables better therapeutic planning than CT in cases of possible lung cancer chest wall invasion. Lung Cancer 2010;69:203-8. [Crossref] [PubMed]
- Jaykel TJ, Clark MS, Adamo DA, et al. Thoracic positron emission tomography: (18)F-fluorodeoxyglucose and beyond. J Thorac Dis 2020;12:6978-91. [Crossref] [PubMed]
- Shah AA, D'Amico TA. Primary chest wall tumors. J Am Coll Surg 2010;210:360-6. [Crossref] [PubMed]
- Gonfiotti A, Salvicchi A, Voltolini L. Chest-Wall Tumors and Surgical Techniques: State-of-the-Art and Our Institutional Experience. J Clin Med 2022;11:5516. [Crossref] [PubMed]
- Wang L, Yan X, Zhao J, et al. Expert consensus on resection of chest wall tumors and chest wall reconstruction. Transl Lung Cancer Res 2021;10:4057-83. [Crossref] [PubMed]
- Sakamoto A, Tsuge I, Noguchi T, et al. Preserving the posterior cortex of the sternum during resection of a superficial anterior chest wall sarcoma. J Surg Case Rep 2021;2021:rjab450. [Crossref] [PubMed]
- Butterworth JA, Garvey PB, Baumann DP, et al. Optimizing reconstruction of oncologic sternectomy defects based on surgical outcomes. J Am Coll Surg 2013;217:306-16. [Crossref] [PubMed]
- Thomas PA, Brouchet L. Prosthetic reconstruction of the chest wall. Thorac Surg Clin 2010;20:551-8. [Crossref] [PubMed]
- Isaac KV, Elzinga K, Buchel EW. The Best of Chest Wall Reconstruction: Principles and Clinical Application for Complex Oncologic and Sternal Defects. Plast Reconstr Surg 2022;149:547e-62e. [Crossref] [PubMed]
- Fabre D, El Batti S, Singhal S, et al. A paradigm shift for sternal reconstruction using a novel titanium rib bridge system following oncological resections. Eur J Cardiothorac Surg 2012;42:965-70. [Crossref] [PubMed]
- Hann MC, Pettiford B, Babycos C. Novel Chest Wall Reconstruction Following Excision of an Xiphisternal Chondrosarcoma. Ochsner J 2018;18:180-2. [Crossref] [PubMed]
- Rocco G, Fazioli F, La Manna C, et al. Omental flap and titanium plates provide structural stability and protection of the mediastinum after extensive sternocostal resection. Ann Thorac Surg 2010;90:e14-6. [Crossref] [PubMed]
- Gritsiuta AI, Bracken A, Abbas AE, et al. Complex anterior chest wall reconstruction after extensive oncologic resections: a narrative review. Shanghai Chest 2021;5:41. [Crossref] [PubMed]
- Hanna WC, Ferri LE, McKendy KM, et al. Reconstruction after major chest wall resection: can rigid fixation be avoided? Surgery 2011;150:590-7. [Crossref] [PubMed]
- Daigeler A, Druecke D, Hakimi M, et al. Reconstruction of the thoracic wall-long-term follow-up including pulmonary function tests. Langenbecks Arch Surg 2009;394:705-15. [Crossref] [PubMed]
- Akiba T, Marushima H, Nogi H, et al. Chest wall reconstruction using Gore-Tex® dual mesh. Ann Thorac Cardiovasc Surg 2012;18:166-9. [Crossref] [PubMed]
- Chiappetta M, Facciolo F. Sternum reconstruction using titanium plates matched with "sandwich" Gore-Tex meshes. J Vis Surg 2018;4:47. [Crossref] [PubMed]
- Sanna S, Brandolini J, Pardolesi A, et al. Materials and techniques in chest wall reconstruction: a review. J Vis Surg 2017;3:95. [Crossref] [PubMed]
- Divisi D, Tosi D, Zaccagna G, et al. Case Report: A New Tool for Anterior Chest Wall Reconstruction After Sternal Resection for Primary Or Secondary Tumors. Front Surg 2021;8:691945. [Crossref] [PubMed]
- Ersöz E, Evman S, Alpay L, et al. Chondrosarcoma of the anterior chest wall: surgical resection and reconstruction with titanium mesh. J Thorac Dis 2014;6:E230-3. [Crossref] [PubMed]
- Novoa NM, Aranda Alcaide JL, Gomez Hernández MT, et al. Chest wall—reconstruction: yesterday, today and the future. Shanghai Chest 2019;3:15.
- D'Amico G, Manfredi R, Nita G, et al. Reconstruction of the Thoracic Wall With Biologic Mesh After Resection for Chest Wall Tumors: A Presentation of a Case Series and Original Technique. Surg Innov 2018;25:28-36. [Crossref] [PubMed]
- Gonfiotti A, Viggiano D, Vokrri E, et al. Chest wall reconstruction with implantable cross-linked porcine dermal collagen matrix: Evaluation of clinical outcomes. JTCVS Tech 2022;13:250-60. [Crossref] [PubMed]
- Ely S, Gologorsky RC, Hornik BM, et al. Sternal Reconstruction With Non-Rigid Biologic Mesh Overlay. Ann Thorac Surg 2020;109:e357-9. [Crossref] [PubMed]
- Cara JA, Laclériga AF, Cañadell J. Iliac allograft used for sternal reconstruction after resection of a chondrosarcoma. Int Orthop 1993;17:297-9. [Crossref] [PubMed]
- Rocco G, Fazioli F, Scognamiglio F, et al. The combination of multiple materials in the creation of an artificial anterior chest cage after extensive demolition for recurrent chondrosarcoma. J Thorac Cardiovasc Surg 2007;133:1112-4. [Crossref] [PubMed]
- Xu S, Dou Y, Zhao G, et al. Autologous ilium graft combination with Y-shaped titanium plate fixation for chest wall reconstruction after resection of primary sternal tumors-a clinical study from three institutions. Transl Cancer Res 2020;9:930-6. [Crossref] [PubMed]
- Drinnon KD, Sherali S, Cox CT, et al. Sternal Tumor Resection and Reconstruction Using Iliac Crest Autograft. Plast Reconstr Surg Glob Open 2020;8:e3002. [Crossref] [PubMed]
- Aranda JL, Varela G, Benito P, et al. Donor cryopreserved rib allografts for chest wall reconstruction. Interact Cardiovasc Thorac Surg 2008;7:858-60. [Crossref] [PubMed]
- Marulli G, De Iaco G, Ferrigno P, et al. Sternochondral replacement: use of cadaveric allograft for the reconstruction of anterior chest wall. J Thorac Dis 2020;12:3-9. [Crossref] [PubMed]
- Dell'Amore A, Kalab M, Miller AS 3rd, et al. Indications and Results of Sternal Allograft Transplantation: Learning From a Worldwide Experience. Ann Thorac Surg 2021;112:238-47. [Crossref] [PubMed]
- Zhang G, Liang C, Shen G, et al. Autogenous rib grafts for reconstruction of the manubrium after resection: technical refinements and outcomes. J Thorac Cardiovasc Surg 2014;148:2667-72. [Crossref] [PubMed]
- Rosenberg M, Castagno A, Nadal J, et al. Sternal metastasis of breast cancer: ex vivo hypothermia and reimplantation. Ann Thorac Surg 2011;91:584-6. [Crossref] [PubMed]
- Puviani L, Fazio N, Boriani L, et al. Reconstruction with fascia lata after extensive chest wall resection: results. Eur J Cardiothorac Surg 2013;44:125-9. [Crossref] [PubMed]
- Chang SH, Tung KY, Hsiao HT, et al. Combined free vascularized iliac osteocutaneous flap and pedicled pectoralis major myocutaneous flap for reconstruction of anterior chest wall full-thickness defect. Ann Thorac Surg 2011;91:586-8. [Crossref] [PubMed]
- Ng CS. Recent and Future Developments in Chest Wall Reconstruction. Semin Thorac Cardiovasc Surg 2015;27:234-9. [Crossref] [PubMed]
- Collaud S, Pfofe D, Decurtins M, et al. Mesh-bone cement sandwich for sternal and sternoclavicular joint reconstruction. Eur J Cardiothorac Surg 2013;43:643-5. [Crossref] [PubMed]
- Dahan M, Broucher L, Berjaud J, et al. Chirurgie des tumeurs de la paroi thoracique. Ann Chir Plast Esthet 2003;48:93-8. [Crossref] [PubMed]
- Iqbal S, Ali S, Fatimi SH. Novel Reconstruction of Neosternum With Steel Wires for Recurrent Chondrosarcoma. Ann Thorac Surg 2022;113:e371-4. [Crossref] [PubMed]
- Watanabe A, Watanabe T, Obama T, et al. New material for reconstruction of the anterior chest wall, including the sternum. J Thorac Cardiovasc Surg 2003;126:1212-4. [Crossref] [PubMed]
- Mainard N, Sharma D, Fron D, et al. Porous Ceramic Sternal Prosthesis Implantation in a 13-Year-Old Patient Presenting with Metastatic Ewing's Sarcoma. European J Pediatr Surg Rep 2022;10:e1-5. [Crossref] [PubMed]
- Pedersen TA, Pilegaard HK. Reconstruction of the thorax with Ley prosthesis after resection of the sternum. Ann Thorac Surg 2009;87:e31-3. [Crossref] [PubMed]
- Girotti P, Leo F, Bravi F, et al. The "rib-like" technique for surgical treatment of sternal tumors: lessons learned from 101 consecutive cases. Ann Thorac Surg 2011;92:1208-15; discussion 1215-6. [Crossref] [PubMed]
- Turna A, Kavakli K, Sapmaz E, et al. Reconstruction with a patient-specific titanium implant after a wide anterior chest wall resection. Interact Cardiovasc Thorac Surg 2014;18:234-6. [Crossref] [PubMed]
- Demondion P, Mercier O, Kolb F, et al. Sternal replacement with a custom-made titanium plate after resection of a solitary breast cancer metastasis. Interact Cardiovasc Thorac Surg 2014;18:145-7. [Crossref] [PubMed]
- Aranda JL, Espiago AM. Custom-made prosthesis in thoracic surgery. Precis Cancer Med 2019;2:15.
- Aranda JL, Jiménez MF, Rodríguez M, et al. Tridimensional titanium-printed custom-made prosthesis for sternocostal reconstruction. Eur J Cardiothorac Surg 2015;48:e92-4. [Crossref] [PubMed]
- Kamel MK, Cheng A, Vaughan B, et al. Sternal Reconstruction Using Customized 3D-Printed Titanium Implants. Ann Thorac Surg 2020;109:e411-4. [Crossref] [PubMed]
- Wang L, Huang L, Li X, et al. Three-Dimensional Printing PEEK Implant: A Novel Choice for the Reconstruction of Chest Wall Defect. Ann Thorac Surg 2019;107:921-8. [Crossref] [PubMed]
- Okereke IC. Is 3-dimensional printing the right fit for your reconstruction? J Thorac Cardiovasc Surg 2018;155:e61-2. [Crossref] [PubMed]
- Aranda JL, Novoa N, Jiménez MF. Thoracic customized modular titanium-printed prosthesis. AME Case Rep 2019;3:35. [Crossref] [PubMed]
- Marulli G, Duranti L, Cardillo G, et al. Primary chest wall chondrosarcomas: results of surgical resection and analysis of prognostic factors. Eur J Cardiothorac Surg 2014;45:e194-201. [Crossref] [PubMed]
- Bongiolatti S, Voltolini L, Borgianni S, et al. Short and long-term results of sternectomy for sternal tumours. J Thorac Dis 2017;9:4336-46. [Crossref] [PubMed]
- Ahmad U, Yang H, Sima C, et al. Resection of Primary and Secondary Tumors of the Sternum: An Analysis of Prognostic Variables. Ann Thorac Surg 2015;100:215-21; discussion 221-2. [Crossref] [PubMed]
- Novoa N, Benito P, Jiménez MF, et al. Reconstruction of chest wall defects after resection of large neoplasms: ten-year experience. Interact Cardiovasc Thorac Surg 2005;4:250-5. [Crossref] [PubMed]
- Elahi L, Zellweger M, Abdelnour-Berchtold E, et al. The size and sternal involvement of chest wall resections for malignant disease predict postoperative morbidity. Transl Cancer Res 2022;11:1162-72. [Crossref] [PubMed]
- Leuzzi G, Nachira D, Cesario A, et al. Chest wall tumors and prosthetic reconstruction: A comparative analysis on functional outcome. Thorac Cancer 2015;6:247-54. [Crossref] [PubMed]
- Gonfiotti A, Santini PF, Campanacci D, et al. Malignant primary chest-wall tumours: techniques of reconstruction and survival. Eur J Cardiothorac Surg 2010;38:39-45. [Crossref] [PubMed]
- Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg 1996;98:804-10. [Crossref] [PubMed]
- Meadows JA 3rd, Staats BA, Pairolero PC, et al. Effect of resection of the sternum and manubrium in conjunction with muscle transposition on pulmonary function. Mayo Clin Proc 1985;60:604-9. [Crossref] [PubMed]
- Kohman LJ, Auchincloss JH, Gilbert R, et al. Functional results of muscle flap closure for sternal infection. Ann Thorac Surg 1991;52:102-6. [Crossref] [PubMed]
- Scarnecchia E, Liparulo V, Capozzi R, et al. Chest wall resection and reconstruction for tumors: analysis of oncological and functional outcome. J Thorac Dis 2018;10:S1855-63. [Crossref] [PubMed]
- Lardinois D, Müller M, Furrer M, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg 2000;69:919-23. [Crossref] [PubMed]
- Oswald N, Senanayake E, Naidu B, et al. Chest Wall Mechanics In Vivo With a New Custom-Made Three-Dimensional-Printed Sternal Prosthesis. Ann Thorac Surg 2018;105:1272-6. [Crossref] [PubMed]
- Smelt J, Pontiki A, Jahangiri M, et al. Three-Dimensional Printing for Chest Wall Reconstruction in Thoracic Surgery: Building on Experience. Thorac Cardiovasc Surg 2020;68:352-6. [Crossref] [PubMed]
- Layton AM, Swinarski D, Port JL, et al. Exercise motion analysis demonstrating correction of paradoxical chest wall motion following 3D printed sternal implant for sternal chondrosarcoma resection. J Case Rep Images Surg 2019;5:100063Z12AL2019.





