
Chylothorax: pathophysiology, diagnosis, and management—a comprehensive review
Introduction
Chylothorax is defined as the accumulation of chyle within the pleural space (1). Chyle is a milky fluid that is produced during fat digestion and has a high content of triglycerides, fat-soluble vitamins, lymphocytes, and immunoglobulins (1). Although chylothorax is relatively uncommon, accounting for around 3% cases of all pleural effusions (2), the 90-day mortality rates associated with this condition may be as high as 82% (3), stemming largely from nutritional losses (owing to the high fat content of chyle), immunosuppression (due to its high lymphocyte and immunoglobulin content) and fluctuating intravascular volumes (4). As such, chylothorax represents a challenging condition for healthcare teams. Management typically involves addressing the underlying cause, careful attention to nutritional support, and selection of appropriate interventions (including judicial drainage of pleural fluid) to achieve the best possible outcome for patients.
This review aims to provide an overview of the relevant anatomy, pathophysiology, diagnosis, and management of chylothorax, highlighting the importance of adopting a multi-disciplinary approach to treatment decisions. An initial PubMed search was conducted using the keywords “chylothorax”, “chylous effusion” and “chyle”, and relevant articles were selected based on abstract screening and review of bibliographies of the selected articles.
Relevant anatomy
A chylothorax results from rupture of, or disruption to, the thoracic duct (5), which is a 35–46 cm long structure that arises at the cisterna chyli in the abdomen (6). The thoracic duct is formed by the coalescence of intestinal lymphatic vessels known as lacteals (6), and enters the thorax though the aortic hiatus of the diaphragm, running to the right side of the midline between the aorta and azygous vein posterior to the oesophagus (2,7-9). At the level of the thoracic plane (T4-T6) it crosses to the left of the midline and continues superiorly before arching and terminating at the junction between the left jugular and subclavian veins (7-9) (see Figure 1). The most common site of termination of the thoracic duct is the internal jugular vein (46% of cases), followed by the jugulosubclavian angle (32% of cases) (10).
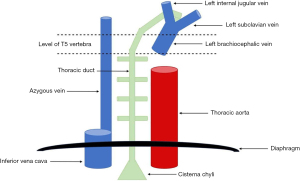
The course of the thoracic duct underpins whether injury to this structure results in a left- or right-sided chylothorax: injury above the thoracic plane usually results in a left-sided chylothorax, whereas injury below the thoracic plane typically gives rise to a right-sided chylothorax (11); bilateral chylothoraces may occur when injury occurs at the level of the thoracic plane. Approximately 83% of chylothoraces are unilateral (50% right-sided, 33% left-sided), while 17% are bilateral (12,13). There is, however, substantial anatomical variation between individuals (2,10), with the thoracic duct running the course described in around 60% of cases. This accounts for the high incidence of iatrogenic chyle leak following surgery despite careful planning and approach (14).
Overview of chyle
Chyle is a milky substance formed in the small intestine. Its primary function is to transport fat and fat-soluble vitamins into the venous circulation following digestion (14). Long-chain triglycerides (LCTs), obtained from dietary sources, coalesce with cholesterol and phospholipids to form chylomicrons in the cells lining the jejunal wall. Lacteals (small lymphatic channels within the villi of the small intestine) take up chylomicrons and transport them as chyle in the thoracic duct. By contrast, small and medium-chain triglycerides (MCTs) are absorbed directly into the portal circulation (15). This underpins the use of MCTs as the primary source of nutrition in the conservative management of chylothorax, as outlined later in this review. Other constituents of chyle include lymphocytes and immunoglobulins, which are derived from the liver and gastrointestinal tract (16).
Pathophysiology
A chylothorax may be broadly classified as either traumatic or non-traumatic in nature (12). Traumatic chylothoraces are most commonly encountered, with iatrogenic injury during surgery the leading cause within this category (17). This may include, for example, lung cancer resection, coronary artery bypass surgery, thoracic aneurysm repair, or cardio-pulmonary transplantation (18-21). The incidence is particularly high (up to 4%) in oesophageal surgeries (22) owing to the close proximity and inconsistent course of the thoracic duct in relation to the oesophagus (10). Other iatrogenic causes include duct blockage due to central venous catheter-related thrombosis, and damage following subclavian vein catheterization (23). Non-iatrogenic traumatic chylothorax, although less common, has been reported in the literature in up to 20% of cases (14), usually in the context of raised intra-abdominal pressure, such as occurs during severe vomiting or childbirth (24,25).
Non-traumatic chylothoraces are less commonly encountered but encompass a wide range of differential diagnoses (see Table 1). Four pathophysiological mechanisms have been proposed for the development of non-traumatic chylothorax, including: direct invasion of the thoracic duct by malignant cells; increased hydrostatic pressure within the lymphatic vessels; hyper-permeability of the lymphatic vessels secondary to lymphatic dysfunction; and transdiaphragmatic passage of chylous ascites into the pleural space (2). These are diagrammatically represented in Figure 2.
Table 1
| Traumatic |
| 1. Iatrogenic lymphatic injury |
| 1.1 Surgery (especially cardiothoracic, oesophageal) |
| 1.2 Central venous access (neck lines, pacemaker insertion) |
| 2. Non-iatrogenic lymphatic injury |
| 2.1 High-energy trauma (e.g., road traffic collision) |
| 2.2 Penetrating chest trauma |
| 2.3 Vertebral fracture |
| 2.4 Raised intrabdominal pressure (e.g., coughing, childbirth, sneezing) |
| Non-traumatic |
| 1. Direct tumour invasion into lymphatic wall |
| 1.1 Lymphoma |
| 1.2 Carcinoma |
| 2. Increased hydrostatic pressure within lymphatics |
| 2.1 Obstruction by tumour/lymphoma/thromboembolism |
| 2.2 Increased central venous pressure (heart failure, congenital cardiac abnormality) |
| 3. Hyperpermeability of lymphatics |
| 3.1 Conditions causing primary lymphatic dysfunction |
| 3.1.1 Lymphangioleiomyomatosis |
| 3.1.2 Generalised lymphatic anomalies |
| 3.1.3 Lymphatic aplasia |
| 3.1.4 Yellow nail syndrome |
| 3.1.5 Congenital chylothorax |
| 3.2 Conditions causing secondary lymphatic dysfunction |
| 3.2.1 Rarely |
| 3.2.1.1 Previous mediastinal radiotherapy |
| 3.2.2 Very rarely |
| 3.2.2.1 Tuberculosis/non-tuberculous mycobacterium |
| 3.2.2.2 Sarcoidosis |
| 3.2.2.3 Filariasis |
| 3.2.2.4 Histoplasmosis |
| 4. Passage of chylous ascites into the pleural space via transdiaphragmatic lymphatic anastomoses |
| 4.1 Liver cirrhosis |
| 4.2 Pancreatitis |
| 4.3 Nephrotic syndrome |
| 5. Idiopathic |
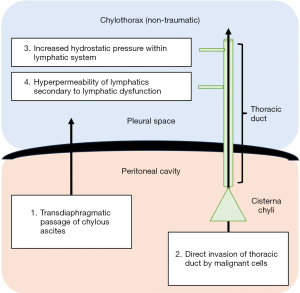
Malignancy represents the most common cause of non-traumatic chylothorax (14,17,26), with lymphoma (most prominently non-Hodgkin lymphoma) accounting for approximately 70% of cases (12,14,22,27). Other haematological malignancies such as multiple myeloma, solid organ tumours, and metastatic epithelial tumours have also been described (17,27-29). The pathophysiology is somewhat controversial but is thought to relate to chyle leak following direct invasion of the thoracic duct, and/or extrinsic compression of lymphatics by enlarged lymph nodes (30). Additionally, it has been proposed that the upstream movement of chyle into the thoracic space through transdiaphragmatic defects secondary to a negative intrathoracic pressure gradient may also contribute to development of malignant chylothorax (31).
Primary lymphatic disorders, such as lymphangioleiomyomatosis (LAM) and lymphangiectasia syndromes, are rare. LAM affects women of childbearing age and is characterized by the abnormal proliferation of smooth muscle cells in the lymphatics of the peribronchial, perivascular and perilymphatic spaces of the lungs (32-34). Perilymphatic proliferation leads to lymphatic dysfunction which, in-turn, leads to hyperpermeability of lymphatic vessels and lymph leakage (2,35,36). Akin to malignant obstruction, a chyle leak in the retroperitoneum stemming from lymphatic dysfunction can migrate preferentially into the chest through transdiaphragmatic connections (31). This is also the mechanism observed in chylothoraces that develop in the setting of chylous ascites. Moreover, it is possible that raised intra-abdominal pressure in the presence of ascites contributes to thoracic duct obstruction and subsequently, chyle leak (2,12,26).
Yellow nail syndrome is a clinical entity characterized by brittle yellow nails, lower limb lymphoedema, and respiratory manifestations, most commonly bronchiectasis and bilateral pleural effusions (37,38). Although only 12.1–30% of effusions in this syndrome are chylous, the underlying mechanism is considered to be that of lymphatic dysfunction secondary to lymphatic transport failure (37-39). Lymphatic dysfunction may also arise in the context of tuberculosis (40), atypical mycobacterial infections (41), Kaposi’s sarcoma (42), and following radiation therapy (22,43,44), all of which may rarely lead to chylothorax formation. It is estimated that the incidence of congenital chylothorax is between 1:10,000 and 1:24,000 (45,46).
Occasionally, a chylothorax may develop in the setting of a transudative pleural effusion. Examples include decompensated liver cirrhosis with chylous ascites, and chronic heart failure (47,48). The proposed mechanism for chyle leak in heart failure is that of increased central venous pressure, raising flow through the thoracic duct. However, owing to the stiffness of the veno-lymphatic junction in the neck, maximal lymphatic flow is limited, leading to increased hydrostatic pressure and lymph extravasation (48). Additionally, increased pressure in the left subclavian vein results in restricted lymphatic drainage and development of lymphatic venous collaterals, ultimately causing a chyle leak (2,48). In up to 9% of cases, the cause of non-traumatic chylothorax remains unknown and is termed idiopathic (49).
Clinical features
The clinical symptomatology in chylothorax is similar to that of any pleural effusion and is influenced by the aetiology and rate of accumulation of chyle in the pleural space (12,14,15). Most patients are asymptomatic in the initial stages when the volume of chyle is low, which is classically observed in non-traumatic aetiologies (15,27). As chyle insidiously accumulates in the pleural cavity in these cases, patients may develop breathlessness progressively over time. By contrast, in traumatic cases, sudden damage to the thoracic duct causes rapid fluid accumulation in the pleural space, which may lead to respiratory and haemodynamic compromise (14,50). Occasionally, for instance following pneumonectomy, patients can present with a tension chylothorax and mediastinal shift secondary to large volumes of chyle accumulating over several weeks (51,52). However, in the post-surgery setting, chylothorax most commonly presents incidentally as a pleural effusion on chest radiography, or by persistent drainage of chylous fluid in pre-existing drains (12).
While cough is a frequently reported symptom, patients may rarely also experience chyloptysis (the expectoration of chylous fluid in sputum), secondary to reflux of chyle into the bronchial tree (53). As chyle is not an irritant to the pleural surface, chest pain and fever are uncommon (54,55). Other symptoms, such as weight loss, asthenia and night sweats may occur in the context of malignancy-associated chylothorax (15). In chronic cases, chyle leak may lead to marked nutritional deficits, weight loss and muscle wasting, as well as electrolyte imbalances (14,50). Loss of immunoglobulins and T lymphocytes can also lead to secondary immunosuppression, thereby predisposing the individual to opportunistic infections (56-58). Notably, however, as chyle is bacteriostatic, infection of the pleural space is rare (14).
Chyle leak may also affect the bioavailability of certain medications, such as amiodarone (59), digoxin (60) and ciclosporin (61), since these agents are sequestered in chyle and can be lost during its drainage. This can cause a subsequent reduction in therapeutic levels and may lead to symptoms relating to poor control of the conditions they are used to treat.
Diagnosis
Pleural fluid analysis remains central to the diagnosis of chylothorax, supported by various thoracic imaging modalities to help establish the underlying cause. Of note, the classic description of milky or opalescent fluid in the pleural space is thought to occur in only 22–44% of patients meeting the diagnostic criteria for chylothorax (26,62), highlighting the importance of performing pleural fluid analysis and maintaining a high index of suspicion even in the absence of classical macroscopic features.
Pleural fluid analysis
The macroscopic appearance of chylothorax is typically described as milky, though may occasionally appear serous in nature (particularly if patients are fasting, secondary to reduced lipid ingestion, e.g., in the post-operative setting), or blood-stained (especially after trauma) (12). Other reported descriptions include serosanguineous, yellow, and green coloured fluid (12,63). Important differentials, based on gross appearance, are pseudochylothorax and pleural infection, both of which may cause an opalescent appearance of pleural fluid similar to chylothorax (27). Pseudochylothoraces usually occur in the context of longstanding exudative effusions that have become enriched with cholesterol; common causes include tuberculosis, rheumatoid-related effusions, chronic pneumothorax or haemothorax, and poorly drained empyema (12). Pleural fluid centrifugation can help to distinguish chylothorax and infected pleural fluid with a milky appearance, with separation of the constituent components (cell debris and clear supernatant) characteristic of pleural space infection/empyema (64). Nonetheless, it is not possible to diagnose chylothorax based on appearances alone.
A definitive diagnosis of chylothorax is based on demonstrating the presence of chylomicrons within the pleural fluid (27,62,65). Ideally, detection of chylomicrons is achieved via lipoprotein electrophoresis, yet this remains an expensive and often inaccessible technique. Therefore, in clinical practice, measurement of pleural fluid triglyceride and cholesterol levels is the most widely adopted method for diagnosis of chylothorax (14). Specifically, a pleural fluid triglyceride level greater than 110 mg/dL (1.24 mmol/L), and a cholesterol level less than 200 mg/dL (5.18 mmol/L) are diagnostic for chylothorax (65). Conversely, a pseudochylothorax may be diagnosed when pleural fluid triglyceride levels are less than 50 mg/dL (0.56 mmol/L) and cholesterol levels greater than 200 mg/dL (5.18 mmol/L), particularly in the presence of cholesterol crystals (14). Table 2 provides a summary of these diagnostic criteria.
Table 2
| Condition | Chylothorax | Pseudochylothorax |
|---|---|---|
| Diagnostic criteria | ||
| Pleural fluid triglyceride level | >110 mg/dL (>1.24 mmol/L) | <50 mg/dL (<0.56 mmol/L) |
| Pleural fluid cholesterol level | <200 mg/dL (<5.18 mmol/L) | >200 mg/dL (>5.18 mmol/L) |
| Pleural fluid to serum triglyceride level | >1 | <1 |
| Pleural fluid to serum cholesterol level | <1 | >1 |
| Additional confirmatory diagnostic criteria | ||
| Presence of chylomicrons in pleural fluid | Yes | No |
| Presence of cholesterol crystals in pleural fluid | No | Yes |
| Classic appearance of pleural fluid | Milky white or opalescent (Note: the absence of this appearance does not exclude chylothorax) |
Milky white or opalescent |
It is important to note that nutritional status has a direct impact on pleural fluid triglyceride levels; as demonstrated by Maldonado et al. (26), poor nutritional status can cause low triglyceride levels, making the diagnosis of chylothorax challenging. In these circumstances, lipoprotein electrophoresis may be a useful clarifying investigation. Where pleural fluid triglyceride levels are greater than 110 mg/dL, there is a small (1%) chance that the effusion is non-chylous. In contrast, the chance of a chylous effusion occurring with triglyceride levels less than 50 mg/dL is roughly 5% (64,65), though this could be impacted by secondary pathology (e.g., when chylothorax is complicated by an additional transudative cause of pleural effusion) (66). Therefore, in situations where pleural fluid triglyceride values are between 55 and 110 mg/dL, or when there is suspicion of dual pathology (such as cirrhosis, or heart failure), lipoprotein electrophoresis may help to clarify the diagnosis by direct detection of chylomicrons within the pleural fluid (67). Other reported diagnostic criteria include a pleural fluid to serum triglyceride ratio of greater than 1, and a pleural fluid to serum cholesterol ratio of less than 1, respectively (14); such criteria may be particularly relevant in patients who have high baseline serum triglyceride levels or, rarely, in patients with pseudochylothorax who have both high fluid triglyceride and cholesterol levels.
The majority of chylous effusions can be termed ‘protein-discordant exudates’—characterised by high protein content but not elevated lactate dehydrogenase (LDH) (57)—despite the relatively low protein content of chyle (2–3 g/dL) (68). The paradoxically high protein content of chylothoraces has been attributed to high rates of fluid and solute reabsorption from the pleural cavity into the intravascular space, leading to high protein concentration within the pleural space (26,27,62,67). By comparison, the low levels of LDH observed in chylothoraces may be explained by its large molecular size, limiting filtration from capillaries into the pleural space (62). Consequently, an elevated LDH in a chylous effusion indicates the presence of alternative pathology that should be further investigated. As outlined above, in around 14% of cases, a chylous effusion may be transudative in nature, particularly in the setting of decompensated liver disease (47) or heart failure (26).
In addition to measurement of pleural fluid triglyceride and cholesterol levels, it is important that pleural fluid cytology is performed, particularly given the possibility of underlying malignant aetiology. Notably, chylothorax is characterized by a lymphocyte predominant exudate (typically >70% of the differential cell count), reflecting the cellular composition of chyle (27); however, the normally mature lymphocytes may demonstrate atypical features in the context of haematological disease (69,70). While pseudochylothorax also demonstrates a lymphocyte-predominant cell differential, in 39% of cases there may be a polymorphonuclear cell predominance, which would not be typical of chylothorax (71).
Imaging
Chest radiography has limited value in the specific diagnosis of chylothorax, though can confirm the presence of a pleural effusion more broadly (49). It may serve as a screening tool for underlying aetiologies, such as the identification of a lung mass or hilar lymphadenopathy relating to malignancy, or chest wall injury in traumatic chylothorax.
Thoracic ultrasound has become a widely adopted tool in pleural medicine (72). However, much like chest radiography, this modality alone is unable to distinguish chylothorax from other causes of pleural effusion, and its primary role is to facilitate ultrasound-guided pleural intervention.
Computed tomography (CT) (with or without contrast) remains central to thoracic imaging and is useful in identifying non-traumatic causes of chylothorax (such as malignancy, ascites, or lymphadenopathy) as well as detecting traumatic injuries to the lymphatic system (2,49). In cases where no obvious cause for chylothorax is identified, imaging the lymphatics may be warranted to aid both diagnosis and management options. In particular, CT lymphangiography involves administration of water-soluble contrast directly into the cisterna chyli under CT-guidance, allowing demonstration of thoracic duct anatomy and identification of chyle leak (2). Thoracic images constructed from CT lymphangiography may also help detect underlying parenchymal disease, such as LAM (73).
Where CT imaging fails to identify the site of chyle leak, nuclear medicine imaging techniques—such as lymphoscintigraphy and single photon emission computed tomography (SPECT)—may be utilized (2). Through these methods, a water-soluble radiotracer is injected subcutaneously and subsequently absorbed by the lymphatics to be distributed throughout the lymphatic circulation (74), visible by CT/SPECT. This not only allows identification of the potential site of chyle leak, but also offers functional assessment of lymphatic flow, which may indicate presence of a primary lymphatic disorder (74-77).
Magnetic resonance lymphangiography can also help to identify chyle leaks related to primary lymphatic disorders such as LAM and lymphangiomas, non-Hodgkin’s lymphoma, and trauma. This can be performed either with or without contrast or using the more novel technique of dynamic contrast-enhancement (78-80). Whilst this modality has the advantages of high spatial resolution and no exposure to ionizing radiation, its availability may be more limited (79).
Conventional lymphangiography remains the gold standard investigation for evaluating the lymphatic circulation (81). This is performed by injection of a poppyseed-based oil into a lymphatic vessel of the foot or ankle and following the flow of contrast under fluoroscopic guidance (81). The technique can successfully identify lymphatic defects and anomalous anatomy and may additionally have a role in treating the chyle leak once established (82). Nonetheless, lymphangiography is not without risk, and has been associated with various complications including oil embolization, lipoid pneumonia, infection, pulmonary oedema, and urticaria at the site of contrast injection (83-85). These risks, alongside its invasive nature and limited availability, make conventional lymphangiography a less attractive initial diagnostic option (2,49). It is therefore largely reserved for a select group of patients who are likely to gain diagnostic and therapeutic benefit from the procedure.
Management
There are currently no established guidelines regarding the management of chylothorax. High quality evidence, including randomised controlled trials, is lacking, and so management approaches are based largely on clinical consensus, case series data and observational studies. A multi-disciplinary approach is crucial, involving respiratory physicians, thoracic surgeons, oncologists, dieticians, and pharmacists (15). It is also important to consider the underlying cause of the chylothorax (including the rate of chyle leak), as well as the patients’ performance status and nutritional state, to develop an individualised management plan. Figure 3 depicts a proposed algorithm for the management of chylothorax.
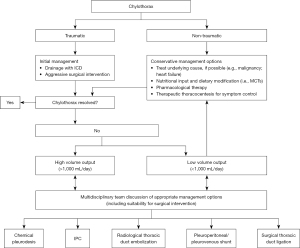
In general, a conservative approach, including drainage of the effusion, nutritional modifications, and pharmacological adjuncts, is adopted first line for a limited period of time, before more invasive interventional measures are considered (15). The primary aim of these conservative measures is to reduce the flow of chyle through the thoracic duct to allow the leak to heal itself (86,87).
Some authors have advocated for the stratification of cases—including subsequent management approaches—into high and low output chylothorax, where high output chylothorax represents an estimated or known volume loss of greater than 1,000 mL chyle per day, and low output chylothorax represents an estimated or known volume loss of less than 1,000 mL per day (88,89). Notably, low output chylothorax is more commonly observed in patients with non-traumatic causes, whereas high output chylothorax is most commonly encountered in post-surgical patients and those with liver cirrhosis. It is suggested that patients with high output chylothorax are more likely to fail with initial conservative management approaches, and early intervention should be considered in these cases (90).
Conservative approaches
Treatment should be directed towards the underlying cause of the chylothorax, if known. For instance, corticosteroids may be utilised in sarcoidosis; guideline-directed medical therapy (in particular, diuretic medication) can be used in both chylous ascites and chylothorax secondary to heart failure; and mTOR inhibitors (such as sirolimus) can be used in chylothorax associated with LAM (2,11,48,91). In malignant chylothorax, treatments targeted at the underlying malignancy, such as radiotherapy or chemotherapy, can be effective in managing the chylothorax (12,14,87,92).
Oral or enteral dietary modifications are often adopted first line in low output chylothorax (11,15,93). Patients should be assessed by dieticians and instructed to stay on a low or no-fat, high protein diet, ensuring that any fats ingested are MCTs (12,87,94). This serves to decrease fat absorption from the gut, thereby reducing the volume of chyle flow through the thoracic duct. In addition, MCTs, which may be taken as dietary supplements, are directly absorbed into the portal circulation, bypassing the lymphatic system altogether (in contrast to LCTs) (15,64,87,95). Additional nutritional support is often required, as deficiencies of fat-soluble vitamins and essential fatty acids may occur rapidly (e.g., within the first 5 days) of a low or no-fat dietary regimen. In addition, these regimens may not be suitable in malignant chylothorax since these patients are often malnourished or frail at presentation (15).
Total parenteral nutrition (TPN) may be adopted preferentially in high output chylothorax, as a major reduction in chyle flow can encourage healing of the leak and avert acute nutritional deficiencies (90). TPN is also considered when oral or enteral dietary modifications have failed to reduce chyle flow sufficiently (12,64,94). Although there is no specified duration of oral or enteral low-fat regimens, some authors have suggested that a lack of response at 2 weeks indicates the need to transition to TPN (96). TPN should, however, be used with caution: there are significant associated risks, such as line infection and cholestasis, and current evidence does not suggest a significant difference between oral or enteral dietary regimens and TPN in the rate of chylothorax resolution (97). Of note, no fat restrictions are required for TPN since this is administered intravenously and fat is therefore not absorbed as chyle in the gut.
Pharmacological therapies
Several case series have demonstrated the use of somatostatin and octretotide (a long-acting synthetic somatostatin analogue) in aiding resolution of chyle leaks from a variety of aetiologies, including congenital chylothorax, malignancy, post-operative chylothorax and yellow-nail syndrome (98-103). These are usually adopted in combination with dietary modifications or TPN, as well as drainage of the effusion if required. The exact mechanism of action of these agents in treating chylothorax remains unclear. Somatostatin and octreotide are known to reduce gastric, biliary, and pancreatic secretions, and to induce smooth muscle constriction in splanchnic and lymphatic vessels, thereby reducing lymph production and flow though the thoracic duct. They also increase faecal fat secretion (4,102,104-106).
The optimal timing, dosing, and route of administration of these agents is not known, and varying regimens have been proposed (101,106). In a case series of adult subjects, somatostatin was administered either by intravenous infusion 6 mg/day for 2 weeks or by subcutaneous injection of 50 micrograms every 8 hours. The authors report complete resolution of the chylothorax with adoption of both regimens (101). Reported side effects include flushing, diarrhoea, nausea, thrombocytopaenia, hepatotoxicity and cardiac arrythmias (99). It is advised that caution should be exercised in patients with underlying vascular disease, since the impact on splanchnic vasoconstriction may induce vascular compromise (98).
Isolated case reports have further described the use of alpha-adrenergic agonists, such as midodrine and etilefrine, in the treatment of refractory, idiopathic and post-operative chylothorax (107-109). The numbers reported are very low, with the largest case series to date including only ten patients (98). Alpha-adrenergic agonists are conventionally used to treat postural hypotension but, through smooth muscle contraction, can lead to splanchnic vasoconstriction and modulation of portal blood flow. This may result in reduced chyle production and flow, augmenting spontaneous closure of chyle leaks in chylothorax (107,108). The optimal dosing regimens and route of administration for alpha-adrenergic agonists is not clear. One case report described using midodrine 20 mg three times daily, alongside octreotide and TPN (110). Etilefrine is less readily available and administered via continuous intravenous infusion, carrying an added treatment burden (109).
Pleural interventions
Drainage of chylothoraces may be required for symptomatic benefit. This can be achieved through therapeutic thoracocentesis, placement of an intercostal chest drain (ICD), or use of an indwelling pleural catheter (IPC). The choice of pleural intervention is often dictated by the underlying aetiology and rate of accumulation of pleural fluid. In post-operative and traumatic cases, an ICD if often already in situ.
Thoracocentesis, IPC insertion or ICD placement may be offered in patients with non-traumatic chylothorax that present with large symptomatic effusions. For patients with slowly accumulating chylous effusions, repeated thoracocentesis is likely to be of some symptomatic benefit (11). In malignant chylothorax, periodic thoracocentesis may be used as a palliative measure to alleviate dyspnoea.
ICD insertion may represent a better option for chylous effusions that accumulate rapidly, since it allows quantification of the rate of chylous fluid production and subsequent stratification of cases into low and high output chylothorax. As indicated previously, this may inform further management strategies, including early surgical intervention where appropriate (90). It has been postulated that draining the effusion may assist in sealing the chyle leak by allowing time for other treatments to work, or for the site of leak to heal (86).
Drainage of chylothoraces is not without risk, however. Prolonged chest tube drainage can result in the loss of immunoglobulins, protein, and lymphocytes through chyle, thereby causing malnutrition and immunosuppression (4). Serial monitoring of electrolytes, lymphocyte counts, total protein and albumin is required, as well as monitoring of the patient’s nutritional state. As a general principle, pleural drainage via ICD is limited to less than 2 weeks and may be shortened further in more frail patients (111,112). The most appropriate size of ICD for chylothorax is not known, with case series reporting success using both small and large-bore tubes; there is no evidence to support preferential use of one size over the other (3,113-117).
Chemical pleurodesis through ICD may be considered in patients that fail to improve with conservative management and are not candidates for surgery. Several case reports have successfully used agents such as talc, bleomycin, tetracycline, povidone, elemene, and hypertonic glucose for this purpose (12,14,118-120). Talc, which can be used thoracoscopically or via slurry, has been reported to have a 100% success rate in a small case series of 24 patients (121). However, the success rate of pleurodesis may be lower in chylothorax than malignant effusions particularly in high-output leaks where adequate pleural apposition is not achieved.
There is a growing body of evidence to support the use of IPCs in chylothorax, albeit largely in the form of case series. Notably, DePew et al. described the successful removal of IPCs in 9 of 14 chylothoraces following drainage, without significant nutritional or immunological compromise (a primary concern with ongoing drainage of chylous fluid) (122). Similarly, the use of IPCs has been successfully demonstrated in both malignant and recurrent chylothoraces, akin to their use in malignant or refractory pleural effusions (123,124). As with ICDs, IPCs permit monitoring of the rate of chyle production and quantification of chyle leak (86), which may influence future management decisions.
Surgical techniques
As previously indicated, surgical intervention may be considered when conservative management of chylothorax fails. This is largely the case for non-traumatic chylothoraces, where there is no strong indication for immediate surgical intervention. The typical criteria for surgery in patients undergoing initial conservative management include large daily chyle leak of more than 1.5 L in an adult, longer than 2 weeks of chest tube output, and rapidly declining nutritional status (125-127). By contrast, post-traumatic or post-surgical chylothorax requires aggressive early surgical intervention (14).
The most commonly described surgical technique is that of thoracic duct ligation. The chyle leak may be identified by giving the patient a drink of fat or cream immediately prior to surgery (128), or using a lipophilic dye such as Sudan red or black, methylene blue, or indocyanine green (27,129-131). Once identified, the leak is repaired via video-assisted thoracoscopy or robotic-assisted thoracic surgery (132). This procedure is well-described in the context of traumatic chylothorax, with a success rate of more than 90% in this cohort (86,127,132,133). The outcomes in non-traumatic cases are less well established (11). Complications associated with thoracic duct ligation include ongoing chyle leak and development of multiloculated chylothorax, in addition to general peri-operative risks of pneumonia, bleeding, and injury to surrounding structures (130). Mortality from the procedure is quoted in the region of 3% (134).
For refractory chylothoraces, pleuroperitoneal shunts (PPS) or pleurovenous shunts (PVS) may be considered (135,136). A PPS diverts the chylous effusion into the peritoneum where it is systemically absorbed but should clearly not be used in patients with concomitant chylous ascites. A PVS, by comparison, redirects chyle into the subclavian or jugular veins. Both PPS and PVS are placed subcutaneously or externally and can be activated manually (Denver shunt) or passively (LeVeen shunt) (86). Despite being less invasive than video-assisted thoracoscopic surgery (VATS) thoracic duct ligation, shunts carry a high risk of complications, including displacement, skin erosion, infection, blockage, and pneumoperitoneum (86).
Interventional radiology
An alternative to the surgical approaches outlined above is thoracic duct embolization (TDE), which aims to seal the chyle leak at its site of origin. This is typically performed percutaneously by interventional radiology colleagues. The technique involves first performing pedal or intra-nodal lymphangiography to opacify the cisterna chyli or other enlarged lymphatic vessels; canulating the thoracic duct with a catheter; performing a thoracic ductography to identify the leak site; and employing embolization coils to repair the defect (137). The overall success rate of TDE is estimated as 60%, with a higher rate of success (approximately 74%) in non-traumatic cases (137,138). The procedure is contraindicated in the presence of untreated coagulopathies, and patients with abdominal lesions such as abdominal aortic aneurysm, where percutaneously traversing the abdomen to the retroperitoneum would be hazardous (139). Reported complications include fat embolism, biliary leak, chronic leg or abdominal swelling, and chronic diarrhoea (138,140). The pooled complication rate of TDE is quoted as 2.4% in a systematic review and meta-analysis performed by Kim et al. (138), whilst long-term complication rates were estimated at 14.3% in a retrospective review of cases with successful TDE (140). As such, TDE offers a relatively safe, less-invasive alternative to surgery in the management of chylothorax, with a modest success rate.
In recent years, novel interventional radiology techniques including radiofrequency and microwave ablation of thoracic duct, and needle disruption of retroperitoneal lymph nodes, have been identified as potential therapeutic options in the management of chylothorax (141-143). However, to date these techniques have only been reported in small case series and animal models, and thus require further evaluation before consideration as suitable alternatives to TDE.
Outcomes in chylothorax
The outcome for patients with chylothorax is heavily influenced by the underlying aetiology, and whether this can be successfully reversed. Notably, mortality in post-traumatic chylothorax has greatly improved due to aggressive early therapeutic strategies which counter the ill-effects of chyle loss and, consequently, lead to improved outcomes (134). However, the long-term outcomes for patients with non-traumatic chylothorax remain relatively poor. In a retrospective study of 74 patients, the rate of resolution of chylothorax in non-traumatic cases was worse than traumatic cases (27% versus 50%, P=0.048), even if surgical intervention was utilized as a management strategy for the former (11). Malignancy, chronic chylous effusion, and bilateral chylothorax are all indicative of poor prognosis (144), emphasising the vital role of the multidisciplinary team in formulating appropriate management strategies for these patients.
Conclusions
Chylothorax is an uncommon but important cause of pleural effusion. The clinical presentation can vary between individuals, with symptoms typically related to the rate of chyle accumulation and the underlying cause. Diagnosis depends on appropriate pleural fluid analysis, supported by various imaging studies to help determine the underlying pathology and locate the site of chyle leak. The identification of chylomicrons within pleural fluid is the most definitive diagnostic criterion, although this is not frequently employed in practice, where demonstration of elevated triglyceride and reduced cholesterol levels within the pleural fluid is key (see Table 3). Crucially, management of chylothorax must be individualised, considering the anatomical and aetiological factors associated with its development. Conservative approaches, including management of the underlying cause, dietary modification, and pharmacological intervention, are often the first line of treatment. Drainage of the effusion may be required for symptom relief. When conservative methods fail, definitive interventions such as thoracic duct ligation or embolization may be considered. Determining the optimal management of chylothorax represents a nascent field, with a lack of current guidelines. Ongoing research and collaboration among healthcare professionals is essential to improve our understanding and management of this challenging condition.
Table 3
| Chylothorax is a rare cause of pleural effusion which carries a high risk of mortality |
| Clinicians should consider the diagnosis in cases of pleural effusion with ongoing output of uncertain cause |
| The aetiology is wide ranging, but may be categorised broadly as either ‘traumatic’ or ‘non-traumatic’ in nature |
| In practice, diagnosis is based on demonstrating elevated pleural fluid triglyceride levels and reduced cholesterol levels in the presence of a characteristic milky fluid appearance |
| Occasionally, direct demonstration of chylomicrons within pleural fluid using lipoprotein electrophoresis is necessary to confirm the diagnosis |
| Various imaging modalities (including CT and MR lymphangiography) may be utilised to support the diagnosis and identify the site of chyle leak |
| Management is often challenging, involving a combination of conservative methods (e.g., dietary modification, medication, intermittent thoracocentesis) and more invasive radiological or surgical intervention (e.g., thoracic duct embolization or ligation) |
| In cases of persistent chyle leak, treatment decisions should be guided by adopting a multidisciplinary approach to optimise individual patient care |
CT, computed tomography; MR, magnetic resonance.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Avinash Aujayeb) for the series “Malignant and Benign Pleural Effusions” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1636/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1636/coif). The series “Malignant and Benign Pleural Effusions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sassoon CS, Light RW. Chylothorax and pseudochylothorax. Clin Chest Med 1985;6:163-71.
- Cholet C, Delalandre C, Monnier-Cholley L, et al. Nontraumatic Chylothorax: Nonenhanced MR Lymphography. Radiographics 2020;40:1554-73. [Crossref] [PubMed]
- Shah RD, Luketich JD, Schuchert MJ, et al. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg 2012;93:897-903; discussion 903-4. [Crossref] [PubMed]
- Agrawal A, Chaddha U, Kaul V, et al. Multidisciplinary Management of Chylothorax. Chest 2022;162:1402-12. [Crossref] [PubMed]
- Macfarlane JR, Holman CW. Chylothorax. Am Rev Respir Dis 1972;105:287-91. [Crossref] [PubMed]
- Toliyat M, Singh K, Sibley RC, et al. Interventional radiology in the management of thoracic duct injuries: Anatomy, techniques and results. Clin Imaging 2017;42:183-92. [Crossref] [PubMed]
- Liu ME, Branstetter BF 4th, Whetstone J, et al. Normal CT appearance of the distal thoracic duct. AJR Am J Roentgenol 2006;187:1615-20. [Crossref] [PubMed]
- Mauro MA, Murphy KPJ, Thomson KR, et al. Image-guided interventions e-book: expert radiology series. 2nd edition. Elsevier Health Sciences; 2013.
- Shields TW, Locicero J 3rd, Reed CE, et al. General Thoracic surgery. 7th edition. Philadelphia: LWW; 2009.
- Phang K, Bowman M, Phillips A, et al. Review of thoracic duct anatomical variations and clinical implications. Clin Anat 2014;27:637-44. [Crossref] [PubMed]
- Maldonado F, Cartin-Ceba R, Hawkins FJ, et al. Medical and surgical management of chylothorax and associated outcomes. Am J Med Sci 2010;339:314-8. [Crossref] [PubMed]
- McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med 2010;104:1-8. [Crossref] [PubMed]
- Bessone LN, Ferguson TB, Burford TH. Chylothorax. Ann Thorac Surg 1971;12:527-50. [Crossref] [PubMed]
- Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg 2007;32:362-9. [Crossref] [PubMed]
- Ur Rehman K, Sivakumar P. Non-traumatic chylothorax: diagnostic and therapeutic strategies. Breathe (Sheff) 2022;18:210163. [Crossref] [PubMed]
- Zilversmit DB. The composition and structure of lymph chylomicrons in dog, rat, and man. J Clin Invest 1965;44:1610-22. [Crossref] [PubMed]
- Doerr CH, Allen MS, Nichols FC 3rd, et al. Etiology of chylothorax in 203 patients. Mayo Clin Proc 2005;80:867-70. [Crossref] [PubMed]
- Chen C, Wang Z, Hao J, et al. Chylothorax after Lung Cancer Surgery: A Key Factor Influencing Prognosis and Quality of Life. Ann Thorac Cardiovasc Surg 2020;26:303-10. [Crossref] [PubMed]
- El-Farra MH, Pham N, Smith J, et al. Treatment of Chylothorax After Coronary Artery Bypass Grafting. Ann Thorac Surg 2021;112:e349-52. [Crossref] [PubMed]
- Wu D, Chesnokova AE, Akvan S, et al. Postoperative Chylothorax After Thoracoabdominal Aortic Aneurysm Repair. Semin Thorac Cardiovasc Surg 2018;30:215-9. [Crossref] [PubMed]
- Ziedalski TM, Raffin TA, Sze DY, et al. Chylothorax after heart/lung transplantation. J Heart Lung Transplant 2004;23:627-31. [Crossref] [PubMed]
- McWilliams A, Gabbay E. Chylothorax occurring 23 years post-irradiation: literature review and management strategies. Respirology 2000;5:301-3. [Crossref] [PubMed]
- Kurekci E, Kaye R, Koehler M. Chylothorax and chylopericardium: a complication of a central venous catheter. J Pediatr 1998;132:1064-6. [Crossref] [PubMed]
- Yekeler E, Ulutas H. Bilateral chylothorax after severe vomiting in a child. Ann Thorac Surg 2012;94:e21-3. [Crossref] [PubMed]
- Cammarata SK, Brush RE Jr, Hyzy RC. Chylothorax after childbirth. Chest 1991;99:1539-40. [Crossref] [PubMed]
- Maldonado F, Hawkins FJ, Daniels CE, et al. Pleural fluid characteristics of chylothorax. Mayo Clin Proc 2009;84:129-33. [Crossref] [PubMed]
- Huggins JT. Chylothorax and cholesterol pleural effusion. Semin Respir Crit Care Med 2010;31:743-50. [Crossref] [PubMed]
- Davis SN, Clark F. Multiple myeloma as a cause of chylothorax. J R Soc Med 1986;79:49. [Crossref] [PubMed]
- Teng CL, Li KW, Yu JT, et al. Malignancy-associated chylothorax: a 20-year study of 18 patients from a single institution. Eur J Cancer Care (Engl) 2012;21:599-605. [Crossref] [PubMed]
- O'Callaghan AM, Mead GM. Chylothorax in lymphoma: mechanisms and management. Ann Oncol 1995;6:603-7. [Crossref] [PubMed]
- Nadolski G. Nontraumatic Chylothorax: Diagnostic Algorithm and Treatment Options. Tech Vasc Interv Radiol 2016;19:286-90. [Crossref] [PubMed]
- Taveira-DaSilva AM, Moss J. Epidemiology, pathogenesis and diagnosis of lymphangioleiomyomatosis. Expert Opin Orphan Drugs 2016;4:369-78. [Crossref] [PubMed]
- Faul JL, Berry GJ, Colby TV, et al. Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med 2000;161:1037-46. [Crossref] [PubMed]
- Johnson SR, Cordier JF, Lazor R, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010;35:14-26. [Crossref] [PubMed]
- Ryu JH, Doerr CH, Fisher SD, et al. Chylothorax in lymphangioleiomyomatosis. Chest 2003;123:623-7. [Crossref] [PubMed]
- Almoosa KF, McCormack FX, Sahn SA. Pleural disease in lymphangioleiomyomatosis. Clin Chest Med 2006;27:355-68. [Crossref] [PubMed]
- Maldonado F, Tazelaar HD, Wang CW, et al. Yellow nail syndrome: analysis of 41 consecutive patients. Chest 2008;134:375-81. [Crossref] [PubMed]
- Valdés L, Huggins JT, Gude F, et al. Characteristics of patients with yellow nail syndrome and pleural effusion. Respirology 2014;19:985-92. [Crossref] [PubMed]
- Hoque SR, Mansour S, Mortimer PS. Yellow nail syndrome: not a genetic disorder? Eleven new cases and a review of the literature. Br J Dermatol 2007;156:1230-4. [Crossref] [PubMed]
- Rajagopala S, Kancherla R, Ramanathan RP. Tuberculosis-Associated Chylothorax: Case Report and Systematic Review of the Literature. Respiration 2018;95:260-8. [Crossref] [PubMed]
- Tanaka T, Saito N, Takaki M, et al. Refractory chylothorax in HIV/AIDS-related disseminated mycobacterial infection. Thorax 2016;71:960-1. [Crossref] [PubMed]
- Alexander R, Rizer M, Burke W, et al. Chylothorax in a patient with metastatic Kaposi sarcoma: differential diagnostic considerations. Radiol Case Rep 2015;10:1098.
- Promisloff RA, Hogue DJ. Chylothorax: the result of previous radiation therapy? J Am Osteopath Assoc 1997;97:164-6. [Crossref] [PubMed]
- Thomson AH, Sivalingham S, Rajesh PB, et al. Chylothorax after radiotherapy in oesophageal carcinoma. Lancet Oncol 2003;4:703-4. [Crossref] [PubMed]
- Resch B, Sever Yildiz G, Reiterer F. Congenital Chylothorax of the Newborn: A Systematic Analysis of Published Cases between 1990 and 2018. Respiration 2022;101:84-96. [Crossref] [PubMed]
- Attar MA, Donn SM. Congenital chylothorax. Semin Fetal Neonatal Med 2017;22:234-9. [Crossref] [PubMed]
- Romero S, Martín C, Hernandez L, et al. Chylothorax in cirrhosis of the liver: analysis of its frequency and clinical characteristics. Chest 1998;114:154-9. [Crossref] [PubMed]
- Villena V, de Pablo A, Martín-Escribano P. Chylothorax and chylous ascites due to heart failure. Eur Respir J 1995;8:1235-6. [Crossref] [PubMed]
- Expert Panel on Vascular Imaging and Interventional Radiology. ACR Appropriateness Criteria(®) Chylothorax Treatment Planning. J Am Coll Radiol 2017;14:S118-26. [Crossref] [PubMed]
- Servelle M, Noguès C, Soulié J, et al. Spontaneous, post-operative and traumatic chylothorax. J Cardiovasc Surg (Torino) 1980;21:475-86.
- Ammori JB, Pickens A, Chang AC, et al. Tension chylothorax. Ann Thorac Surg 2006;82:729-30. [Crossref] [PubMed]
- Yadav H, Nolan ME, Nichols FC 3rd, et al. Tension chylothorax following pneumonectomy. Respir Med Case Rep 2015;14:16-8. [Crossref] [PubMed]
- Lim KG, Rosenow EC 3rd, Staats B, et al. Chyloptysis in adults: presentation, recognition, and differential diagnosis. Chest 2004;125:336-40. [Crossref] [PubMed]
- Light RW. Pleural diseases. In: Evidence-based Respiratory Medicine. Wiley; 1995:521-36.
- Romero S. Nontraumatic chylothorax. Curr Opin Pulm Med 2000;6:287-91. [Crossref] [PubMed]
- Wasmuth-Pietzuch A, Hansmann M, Bartmann P, et al. Congenital chylothorax: lymphopenia and high risk of neonatal infections. Acta Paediatr 2004;93:220-4. [Crossref] [PubMed]
- Orange JS, Geha RS, Bonilla FA. Acute chylothorax in children: selective retention of memory T cells and natural killer cells. J Pediatr 2003;143:243-9. [Crossref] [PubMed]
- Dumont AE, Mayer DJ, Mulholland JH. The suppression of immunologic activity by diversion of thoracic duct lymph. Ann Surg 1964;160:373-83. [Crossref] [PubMed]
- Strange C, Nicolau DP, Dryzer SR. Chylous transport of amiodarone. Chest 1992;101:573-4. [Crossref] [PubMed]
- Taylor MD, Kim SS, Vaias LJ. Therapeutic digoxin level in chylous drainage with no detectable plasma digoxin level. Chest 1998;114:1482-4. [Crossref] [PubMed]
- Repp R, Scheld HH, Bauer J, et al. Cyclosporine losses by a chylothorax. J Heart Lung Transplant 1992;11:397-8.
- Agrawal V, Doelken P, Sahn SA. Pleural fluid analysis in chylous pleural effusion. Chest 2008;133:1436-41. [Crossref] [PubMed]
- Rahman NM, Chapman SJ, Davies RJ. Pleural effusion: a structured approach to care. Br Med Bull 2004;72:31-47. [Crossref] [PubMed]
- de Beer HG, Mol MJ, Janssen JP. Chylothorax. Neth J Med 2000;56:25-31. [Crossref] [PubMed]
- Staats BA, Ellefson RD, Budahn LL, et al. The lipoprotein profile of chylous and nonchylous pleural effusions. Mayo Clin Proc 1980;55:700-4.
- Chan KP, Yip WH, Chan TYC, et al. Changing pleural fluid triglyceride levels in cirrhotic chylothorax. Respirol Case Rep 2022;10:e0907. [Crossref] [PubMed]
- Agrawal V, Sahn SA. Lipid pleural effusions. Am J Med Sci 2008;335:16-20. [Crossref] [PubMed]
- Merrigan BA, Winter DC, O'Sullivan GC. Chylothorax. Br J Surg 1997;84:15-20.
- Sammartino D, Khanijo S, Koenig S, et al. Chylothorax in Patients with Chronic Lymphocytic Leukemia: A Case Series. J Hematol 2018;7:14-8. [Crossref] [PubMed]
- Cetin N, Pandey S, Lorsbach RB. Immature lymphoid cells are a consistent feature of chylothorax in neonates and infants: cytologic features and flow cytometry aid in its distinction from lymphoblastic leukemia/lymphoma. Diagn Cytopathol 2015;43:335-8. [Crossref] [PubMed]
- Lama A, Ferreiro L, Toubes ME, et al. Characteristics of patients with pseudochylothorax-a systematic review. J Thorac Dis 2016;8:2093-101. [Crossref] [PubMed]
- Banka R, Skaarup S, Mercer R, et al. Thoracic ultrasound: A key tool beyond procedure guidance. In: Maskell NA, Laursen CB, Lee YCG, et al. editors. Pleural Disease (ERS Monograph). European Respiratory Society; 2020:73-89.
- Zhang C, Chen X, Wen T, et al. Computed tomography lymphangiography findings in 27 cases of lymphangioleiomyomatosis. Acta Radiol 2017;58:1342-8. [Crossref] [PubMed]
- Yoshida RY, Kariya S, Ha-Kawa S, et al. Lymphoscintigraphy for Imaging of the Lymphatic Flow Disorders. Tech Vasc Interv Radiol 2016;19:273-6. [Crossref] [PubMed]
- Stavngaard T, Mortensen J, Brenoe J, et al. Lymphoscintigraphy using technetium-99m human serum albumin in chylothorax. Thorac Cardiovasc Surg 2002;50:250-2. [Crossref] [PubMed]
- Suga K, Kume N, Hara A, et al. Abnormal lymphatic flow demonstrated by lymphoscintigraphy in chylothorax correlation with lymphography. Clin Nucl Med 1999;24:716-7. [Crossref] [PubMed]
- Restrepo JM, Caride VJ. Lymphoscintigraphy and radionuclide venography in chylothorax. Clin Nucl Med 2004;29:440-1. [Crossref] [PubMed]
- Yu DX, Ma XX, Wang Q, et al. Morphological changes of the thoracic duct and accessory lymphatic channels in patients with chylothorax: detection with unenhanced magnetic resonance imaging. Eur Radiol 2013;23:702-11. [Crossref] [PubMed]
- Kim EY, Hwang HS, Lee HY, et al. Anatomic and Functional Evaluation of Central Lymphatics With Noninvasive Magnetic Resonance Lymphangiography. Medicine (Baltimore) 2016;95:e3109. [Crossref] [PubMed]
- Chavhan GB, Lam CZ, Greer MC, et al. Magnetic Resonance Lymphangiography. Radiol Clin North Am 2020;58:693-706. [Crossref] [PubMed]
- Guermazi A, Brice P, Hennequin C, et al. Lymphography: an old technique retains its usefulness. Radiographics 2003;23:1541-58; discussion 1559-60. [Crossref] [PubMed]
- Alejandre-Lafont E, Krompiec C, Rau WS, et al. Effectiveness of therapeutic lymphography on lymphatic leakage. Acta Radiol 2011;52:305-11. [Crossref] [PubMed]
- Sokol GH, Clouse ME, Kotner LM, et al. Complications of lymphangiography in patients of advanced age. AJR Am J Roentgenol 1977;128:43-4. [Crossref] [PubMed]
- Knochel JQ, Koehler PR, Miller FJ. Need for chest radiographs during and after lymphography. AJR Am J Roentgenol 1979;132:981-2. [Crossref] [PubMed]
- Richardson P, Crosby EH, Bean HA, et al. Pulmonary oil deposition in patients subjected to lymphography: Detection by thoracic photoscan and sputum examination. Can Med Assoc J 1966;94:1086-91.
- Bender B, Murthy V, Chamberlain RS. The changing management of chylothorax in the modern era. Eur J Cardiothorac Surg 2016;49:18-24. [Crossref] [PubMed]
- Duletzke NT, Kiraly LN, Martindale RG. Chylothorax and chylous ascites: Overview, management, and nutrition. Nutr Clin Pract 2023;38:557-63. [Crossref] [PubMed]
- Bryant AS, Minnich DJ, Wei B, et al. The incidence and management of postoperative chylothorax after pulmonary resection and thoracic mediastinal lymph node dissection. Ann Thorac Surg 2014;98:232-5; discussion 235-7. [Crossref] [PubMed]
- Power R, Smyth P, Donlon NE, et al. Management of chyle leaks following esophageal resection: a systematic review. Dis Esophagus 2021;34:doab012. [Crossref] [PubMed]
- Martucci N, Tracey M, Rocco G. Postoperative Chylothorax. Thorac Surg Clin 2015;25:523-8. [Crossref] [PubMed]
- Jarman PR, Whyte MK, Sabroe I, et al. Sarcoidosis presenting with chylothorax. Thorax 1995;50:1324-5. [Crossref] [PubMed]
- Iqbal MH, Smith PR, Bande S. Chylothorax due to angioimmunoblastic T-cell lymphoma. Intern Med J 2009;39:67-8. [Crossref] [PubMed]
- Sriram K, Meguid RA, Meguid MM. Nutritional support in adults with chyle leaks. Nutrition 2016;32:281-6. [Crossref] [PubMed]
- Fernández Alvarez JR, Kalache KD, Graŭel EL. Management of spontaneous congenital chylothorax: oral medium-chain triglycerides versus total parenteral nutrition. Am J Perinatol 1999;16:415-20. [Crossref] [PubMed]
- Takuwa T, Yoshida J, Ono S, et al. Low-fat diet management strategy for chylothorax after pulmonary resection and lymph node dissection for primary lung cancer. J Thorac Cardiovasc Surg 2013;146:571-4. [Crossref] [PubMed]
- Smoke A, Delegge MH. Chyle leaks: consensus on management? Nutr Clin Pract 2008;23:529-32. [Crossref] [PubMed]
- Steven BR, Carey S. Nutritional management in patients with chyle leakage: a systematic review. Eur J Clin Nutr 2015;69:776-80. [Crossref] [PubMed]
- Roehr CC, Jung A, Proquitté H, et al. Somatostatin or octreotide as treatment options for chylothorax in young children: a systematic review. Intensive Care Med 2006;32:650-7. [Crossref] [PubMed]
- Evans J, Clark MF, Mincher L, et al. Chylous effusions complicating lymphoma: a serious event with octreotide as a treatment option. Hematol Oncol 2003;21:77-81. [Crossref] [PubMed]
- Hillerdal G. Yellow nail syndrome: treatment with octreotide. Clin Respir J 2007;1:120-1. [Crossref] [PubMed]
- Mincher L, Evans J, Jenner MW, et al. The successful treatment of chylous effusions in malignant disease with octreotide. Clin Oncol (R Coll Radiol) 2005;17:118-21. [Crossref] [PubMed]
- Al-Zubairy SA, Al-Jazairi AS. Octreotide as a therapeutic option for management of chylothorax. Ann Pharmacother 2003;37:679-82. [Crossref] [PubMed]
- Rosti L, Bini RM, Chessa M, et al. The effectiveness of octreotide in the treatment of post-operative chylothorax. Eur J Pediatr 2002;161:149-50. [Crossref] [PubMed]
- Berzigotti A, Magalotti D, Cocci C, et al. Octreotide in the outpatient therapy of cirrhotic chylous ascites: a case report. Dig Liver Dis 2006;38:138-42. [Crossref] [PubMed]
- Cheung Y, Leung MP, Yip M. Octreotide for treatment of postoperative chylothorax. J Pediatr 2001;139:157-9. [Crossref] [PubMed]
- Kalomenidis I. Octreotide and chylothorax. Curr Opin Pulm Med 2006;12:264-7. [Crossref] [PubMed]
- Rehman KU, Ahmed L, Sivakumar P. Refractory chylothorax: Midodrine as a novel therapeutic option. 2021;58:PA3142.
- Sivakumar P, Ahmed L. Use of an Alpha-1 Adrenoreceptor Agonist in the Management of Recurrent Refractory Idiopathic Chylothorax. Chest 2018;154:e1-4. [Crossref] [PubMed]
- Guillem P, Papachristos I, Peillon C, et al. Etilefrine use in the management of post-operative chyle leaks in thoracic surgery. Interact Cardiovasc Thorac Surg 2004;3:156-60. [Crossref] [PubMed]
- Patel D, Rahmanian M, Iqbal J, Lee J, Seethamraju H. Midodrine: Breaking new ground in the Treatment of Chylothorax. Chest 2019;156:A190-1.
- Kim KW, Song JH. Emerging Roles of Lymphatic Vasculature in Immunity. Immune Netw 2017;17:68-76. [Crossref] [PubMed]
- Liao S, von der Weid PY. Lymphatic system: an active pathway for immune protection. Semin Cell Dev Biol 2015;38:83-9. [Crossref] [PubMed]
- Bacon BT, Mashas W. Chylothorax caused by blunt trauma: Case review and management proposal. Trauma Case Rep 2020;28:100308. [Crossref] [PubMed]
- Waikar HD, Kamalaneson P, Mohamad Zamri MS, et al. Chylothorax after off-pump coronary artery bypass graft surgery: Management strategy. Ann Card Anaesth 2018;21:300-3. [Crossref] [PubMed]
- Stager V, Le L, Wood RE. Postoperative chylothorax successfully treated using conservative strategies. Proc (Bayl Univ Med Cent) 2010;23:134-8. [Crossref] [PubMed]
- Cherian S, Umerah OM, Tufail M, et al. Chylothorax in a patient with HIV-related Kaposi's sarcoma. BMJ Case Rep 2019;12:e227641. [Crossref] [PubMed]
- Lee J, Cho JS. Delayed right chylothorax after left blunt chest trauma: a case report. J Med Case Rep 2017;11:98. [Crossref] [PubMed]
- Rizzardi G, Loy M, Marulli G, et al. Persistent chylothorax in lymphangioleiomyomatosis treated by intrapleural instillation of povidone. Eur J Cardiothorac Surg 2008;34:214-5. [Crossref] [PubMed]
- Jianjun Q, Song Z, Yin L, et al. Treatment of chylothorax with elemene. Thorac Cardiovasc Surg 2008;56:103-5. [Crossref] [PubMed]
- Zhang K, Li C, Zhang M, et al. Treatment of Chylothorax complicating pulmonary resection with hypertonic glucose Pleurodesis. J Cardiothorac Surg 2021;16:149. [Crossref] [PubMed]
- Mares DC, Mathur PN. Medical thoracoscopic talc pleurodesis for chylothorax due to lymphoma: a case series. Chest 1998;114:731-5. [Crossref] [PubMed]
- DePew ZS, Iqbal S, Mullon JJ, et al. The role for tunneled indwelling pleural catheters in patients with persistent benign chylothorax. Am J Med Sci 2013;346:349-52. [Crossref] [PubMed]
- Mohamed M, Tan J, Kalpurath KK. Non-Hodgkin lymphoma manifesting as massive malignant chylothorax: successful management with chemotherapy and ambulatory drainages using indwelling pleural catheter. Intern Med J 2015;45:980-3. [Crossref] [PubMed]
- Jimenez CA, Mhatre AD, Martinez CH, et al. Use of an indwelling pleural catheter for the management of recurrent chylothorax in patients with cancer. Chest 2007;132:1584-90. [Crossref] [PubMed]
- Selle JG, Snyder WH 3rd, Schreiber JT. Chylothorax: indications for surgery. Ann Surg 1973;177:245-9. [Crossref] [PubMed]
- Dugue L, Sauvanet A, Farges O, et al. Output of chyle as an indicator of treatment for chylothorax complicating oesophagectomy. Br J Surg 1998;85:1147-9. [Crossref] [PubMed]
- Marts BC, Naunheim KS, Fiore AC, et al. Conservative versus surgical management of chylothorax. Am J Surg 1992;164:532-4; discussion 534-5. [Crossref] [PubMed]
- Misthos P, Kanakis MA, Lioulias AG. Chylothorax complicating thoracic surgery: conservative or early surgical management? Updates Surg 2012;64:5-11.
- Varshney VK, Suman S, Garg PK, et al. Management options for post-esophagectomy chylothorax. Surg Today 2021;51:678-85. [Crossref] [PubMed]
- Platis IE, Nwogu CE. Chylothorax. Thorac Surg Clin 2006;16:209-14. [Crossref] [PubMed]
- Divisi D, Di Tommaso S, Crisci R. Preoperative red sudan administration to locate thoracic duct lesion in videothoracoscopy. Eur J Cardiothorac Surg 2007;31:1148-9; author reply 1149. [Crossref] [PubMed]
- Schild HH, Strassburg CP, Welz A, et al. Treatment options in patients with chylothorax. Dtsch Arztebl Int 2013;110:819-26. [Crossref] [PubMed]
- Reisenauer JS, Puig CA, Reisenauer CJ, et al. Treatment of Postsurgical Chylothorax. Ann Thorac Surg 2018;105:254-62. [Crossref] [PubMed]
- Cerfolio RJ, Allen MS, Deschamps C, et al. Postoperative chylothorax. J Thorac Cardiovasc Surg 1996;112:1361-5; discussion 1365-6. [Crossref] [PubMed]
- Tanaka E, Matsumoto K, Shindo T, et al. Implantation of a pleurovenous shunt for massive chylothorax in a patient with yellow nail syndrome. Thorax 2005;60:254-5. [Crossref] [PubMed]
- Murphy MC, Newman BM, Rodgers BM. Pleuroperitoneal shunts in the management of persistent chylothorax. Ann Thorac Surg 1989;48:195-200. [Crossref] [PubMed]
- Nadolski GJ, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol 2012;23:613-6. [Crossref] [PubMed]
- Kim PH, Tsauo J, Shin JH. Lymphatic Interventions for Chylothorax: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol 2018;29:194-202.e4. [Crossref] [PubMed]
- Stecker MS, Fan CM. Lymphangiography for Thoracic Duct Interventions. Tech Vasc Interv Radiol 2016;19:277-85. [Crossref] [PubMed]
- Laslett D, Trerotola SO, Itkin M. Delayed complications following technically successful thoracic duct embolization. J Vasc Interv Radiol 2012;23:76-9. [Crossref] [PubMed]
- McGregor H, Weise L, Brunson C, et al. Percutaneous Radiofrequency Ablation to Occlude the Thoracic Duct: Preclinical Studies in Swine for a Potential Alternative to Embolization. J Vasc Interv Radiol 2022;33:1192-8. [Crossref] [PubMed]
- Farahnak K, De Filippis Falcon A, Shepherd HM, et al. Utilization of microwave ablation as a novel approach for refractory chylothorax following esophagectomy: A case report. JTCVS Tech 2023;18:151-3. [Crossref] [PubMed]
- Kagawa H, Stringham J, Selzman C, et al. Case Report of Needle Disruption of the Retroperitoneal Lymph Nodes for Refractory Chylothorax After Double Lung Transplantation. Transplant Proc 2023;55:1981-3. [Crossref] [PubMed]
- Milsom JW, Kron IL, Rheuban KS, et al. Chylothorax: an assessment of current surgical management. J Thorac Cardiovasc Surg 1985;89:221-7.


