
Chinese expert consensus on the diagnosis, treatment, and management of asthma in women across life
Highlight box
Key recommendations
• The overall management of asthma in female is the same as that of asthma in general, but it has its own characteristics in different life cycles, and these characteristics should be paid attention to in order to carry out more precise treatment.
What was recommended and what is new?
• Asthma is a heterogeneous disease, and its incidence, prevalence, and severity vary by sex and age. Recommendations of management of female asthma in different age groups and special periods had been proposed in this expert consensus.
• This is the first expert consensus on the diagnosis, treatment, and management of asthma in women across life.
What is the implication, and what should change now?
• There is a lack of high-quality studies on the effects of sex hormones, physiological, and endocrine characteristics at different stages of the female life cycle on the pathogenesis and progression of asthma and the response to different treatments, which remains to be studied.
Introduction
Currently, more than 330 million people worldwide suffer from asthma, and the prevalence rate and the per capita medical expenditure of asthma continue to increase (1). A previous epidemiological survey conducted in China between June 22, 2012, and May 25, 2015, found that the overall prevalence of asthma was 4.2%, representing 45.7 million Chinese adults (2). The incidence of childhood asthma in China is also increasing year by year (3), which has a huge impact on the spirit and economy of the affected children’s families. A systematic review estimated that by 2020 the highest incidence of childhood asthma in China would be in 4-year-old urban boys (10.27%), while the lowest would be in 14-year-old rural girls (1.11%) (4).
Indoor and outdoor pollutants affect respiratory health, increasing the prevalence and triggering the symptoms of asthma, and this effect is more pronounced in all age groups in women. Among children with allergic predisposition, positive associations between air pollutants and respiratory symptoms and diseases have been detected in females (5). Among girls, but not boys, lifetime exposures to black carbon [a constituent of traffic-related particulate matter 2.5 (PM2.5)] and PM2.5 are associated with greater odds of early and mid-childhood asthma (6). Adult women living in households that use biomass and solid fuels have a significantly higher risk of asthma than those living in households that use cleaner fuels, even after controlling for a number of potentially confounding factors, but this effect has not been found among men (7). PM2.5 exposure increases the risk of developing asthma, and PM2.5 and nitrogen dioxide (NO2) increase the risk of developing wheezing, the cardinal symptom of asthma, in adult women (8). Mishra (9) examined the effect of cooking smoke on the reported prevalence of asthma among elderly men and women, and found that the adjusted effect of cooking smoke on asthma was greater among women than among men.
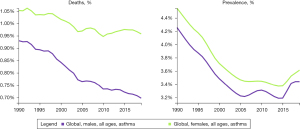
Asthma is a heterogeneous disease, and its incidence, prevalence, and severity vary by sex and age. In the pediatric population, boys have a higher prevalence of asthma than girls due to different sex hormone production and relatively delayed lung development, but the prevalence and severity of asthma are higher in women than in men in adulthood (Figure 1) (1,10). The incidence, prevalence, and severity of asthma in men and women are not only influenced by important factors such as sex hormones, genetics, society, and environment, but are also associated with patients’ response to asthma treatment. In female patients, the fluctuation in hormone levels in infancy, adolescence, menstrual cycle, pregnancy and lactation, climacterium, menopause, and postmenopausal period; and the transition of various social roles such as from marriage to child rearing, family burden, and responsibility lead to the onset of asthma, making the diagnosis, treatment, and management of asthma in women quite challenging. In addition, gene expression, epigenetic modification, and different patients’ responses to environmental stimuli such as SARS-CoV-2 infection are also associated with the differences in the incidence, prevalence, and symptoms of asthma in men and women.
Global Initiative for Asthma (GINA) and Chinese guidelines for asthma prevention and treatment provide important guidance for healthcare practitioners on the prevention, diagnosis, and treatment of asthma. However, there is hitherto no comprehensive and standardized asthma guidance document for female patients. This expert consensus on the management of female asthma throughout the life cycle has been formulated by female experts in China. These experts have extensive experience in the fields of female growth and development, endocrine and sex hormone changes and their effects on the female immune system, as well as the diagnosis, treatment, and management of female asthma. The experts have collected many basic research results and clinical evidence-based medical data and reviewed the effects of sex hormones, classical genetics, and epigenetics on the clinical presentation and treatment response of female patients with asthma under different environmental effects, in order to provide diagnosis, treatment, and research reference for clinical and basic medical workers through this consensus.
Methods
This consensus used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) to evaluate the strength of recommendation and quality of evidence. The strength of recommendation was divided into strong and weak (Table 1), and the quality of evidence was divided into high, moderate, low, and very low (Table 2). Due to the lack of multicenter, randomized, controlled trials, all recommendations in this consensus were conditional, and the level of evidence for guiding recommendations was generally low.
Table 1
| Strength of recommendation | Description |
|---|---|
| Strong [1] | Clearly show that benefits of an intervention outweigh its harms or harms of an intervention outweigh its benefits |
| Weak [2] | Benefits and harms are unclear or evidence of high or low quality of evidence shows that benefits and harms are comparable |
Table 2
| Level of evidence | Description |
|---|---|
| High (A) | The consensus panel is very confident that the true value is close to the effect estimate |
| Moderate (B) | The consensus panel is moderately confident in the effect estimate: the true value is probably to be close to the estimate, but there remains a possibility that the two are fundamentally different |
| Low (C) | The consensus panel has limited confidence in effect estimate: the true value may differ substantially from the estimate |
| Very low (D) | The consensus panel has little confidence in effect estimate: the true value is probably to be very different from the estimate |
Corresponding search strategies were determined based on clinical concerns regarding female asthma. Search terms included “sex hormones and lung development”, “sex hormone changes and asthma”, “hormones and asthma immune response”, “women, asthma”, “children, asthma”, “puberty, asthma”, “menstruation, asthma”, “pregnancy, asthma”, “lactation, asthma”, “menopause, asthma”, “obesity, asthma”, and “women, refractory, severe asthma”. Literature was retrieved from PubMed/Medline, Embase, Cochrane Library, China Biology Medicine disc, China National Knowledge Infrastructure, Wanfang Data with the search date of July 30, 2022 as the last day.
Changes of endocrine function and sex hormones in females at different ages and during special periods
Human lung development and maturation and airway responsiveness to external stimuli are influenced by sex. The differences in the structure and function of the lungs between males and females at different stages indicate that sex hormones play an important role.
Lung development and sex differences in lung development at different ages (11)
The lungs continue to develop after birth. The lungs of full-term infants are mature at birth and fully developed at the age of three years. Sex differences in lung development are significant (Table 3).
Table 3
| Developmental period | Time of organogenesis | Major developmental process | Sex differences |
|---|---|---|---|
| Embryonic period | About 26 days to 6 weeks of pregnancy | Appearance of lung buds, formation of tracheal and bronchial buds | No significant difference |
| Pseudoglandular period | About 6–16 weeks of pregnancy | Bronchial development and airway bifurcation | Growth and respiratory movements detected early in female fetuses |
| Canalicular period | About 17–28 weeks of pregnancy | Enlargement of bronchial lumen; differentiation of distal airways into tubules and vascularization of lung tissues; differentiation of type I and type II cells and production of alveolar surfactants | Estrogen promotes the secretion of surfactants and maturation of phospholipids, while androgens show inhibitory effects |
| Terminal sac period | About 29–36 weeks of pregnancy | Cell differentiation, maturation of type II cells and secretion of surfactants, establishment of blood-air interface; formation and expansion of terminal sac; Overall increase in lung volume | The production of alveolar surfactants and phospholipid in females is higher than that in males |
| Alveolar period | About 36 weeks of pregnancy to maturity | Formation and proliferation of alveoli, linear increase of lung with age, and increase of airway resistance | The lung volume is smaller, and the airway resistance is lower in females than that of males |
Sex hormones play a regulatory role in human lung development (12,13). Estrogen stimulates lung maturation, and estrogen receptors α and β (ERα and ERβ) are expressed in the lungs. Estrogen, ERα, and ERβ are important for alveolar surfactant production and alveolar development, and androgens partially regulate the morphology of pulmonary branches and inhibit the production of surfactant (14-16). During the fetal period, female individuals produce alveolar surfactant earlier than males, which promotes the opening of respiratory bronchioles and alveoli, thereby speeding up air flow and reducing airway resistance. Therefore, the morbidity and mortality of neonatal respiratory distress syndrome are higher in males (13).
Early postnatal lung development and maturation are mainly achieved by increasing the alveolar number and volume growth. Women have smaller lung volumes than men, and their airways grow proportionally to their lungs, resulting in higher forced expiratory flow. Airway growth lags behind the lung growth in men, and the total number and surface area of alveoli are greater in men throughout childhood. Therefore, boys have smaller airway diameters relative to their lung volume and present more pronounced lung dysplasia associated with airway flow velocity (17).
During adolescence, men produce greater respiratory pressure than women due to androgens and changes in the shape of the chest and respiratory muscles. Forced vital capacity increases more rapidly in women, however, it is higher in men, which results in a larger alveolar surface area and greater carbon monoxide diffusion capacity in men (13). When growth and development stop, men have greater vital capacity, total lung capacity, and peak flow compared with women of the same height. However, women have greater expiratory flow.
Secretion and regulation of sex hormones at different life stages and their relationship with asthma
Adolescence
In early childhood (before eight years of age), the function of the hypothalamus–pituitary-ovary axis is in an inhibitory state. In late childhood (after eight years of age), the inhibitory state of gonadotropin-releasing hormone (GnRH) is relieved, and follicles in the ovaries develop to some extent and secrete sex hormones, but they are still immature. In girls, adolescence begins at the age of 8–10 years. GnRH is released in pulses; follicles develop and secrete estrogen, and estrogen reaches adult levels by the age of 12 years. In boys, testosterone rises markedly at the age of 10–11 years. During this time estradiol begins to decline, adolescence begins, and androgen reaches adult levels after the age of 14 years. Since adolescence, the prevalence and severity of asthma in women are higher than those in men.
Menstrual period
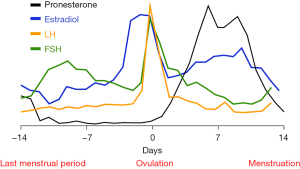
On the seventh day of menstruation, estrogen secreted by follicles increases rapidly and reaches the peak before ovulation. The release of estrogen in the follicular fluid after ovulation results in the decrease of circulating estrogen. One-to-two days after ovulation, the corpus luteum secretes estrogen, which leads to the increase of circulating estrogen, and secretes a small amount of progesterone at the same time. By the time the corpus luteum matures seven-to-eight days after ovulation, the secretion of estrogen and progesterone reaches the peak. Afterwards, the corpus luteum atrophies, and the levels of estrogen and progesterone fall to their lowest level during menstruation (Figure 2) (18). Some patients with asthma experience exacerbation of asthma symptoms or decreased lung function before or during menstruation, which gradually resolves after menstruation, and this is called perimenstrual asthma (PMA) (19).
Pregnancy and lactation period
Estrogen and progesterone are maintained at a high level during pregnancy. Chorionic gonadotropin is secreted by the placenta. It can be detected from maternal serum on the first day after the implantation of the fertilized egg, peaks at 8–10 weeks of pregnancy, rapidly decreases later, and disappears within two weeks after delivery. Estrogen is mainly produced by the corpus luteum of the ovary in the first trimester and is synthesized mainly by the placenta after 10 weeks of pregnancy. The level of estrogen in pregnancy gradually increases, reaching a peak in the third trimester, and then drops rapidly to a lower level in a short period after delivery. Progesterone mainly comes from the corpus luteum of the ovary within six weeks of pregnancy and is mainly secreted by the placenta in the second and third trimesters of pregnancy. The level of progesterone in pregnancy gradually increases, reaching the highest value before delivery, and then falls to normal level within a few days after delivery. Asthma is a common complication during pregnancy. It can worsen, improve, or remain stable during pregnancy, and sex hormones play an important role. However, the exact mechanism of how hormones affect asthma is unknown. Oxytocin and prolactin are often elevated in lactating women, which may cause the fluctuation of asthma, but the exacerbation of postpartum asthma is rare.
Menopause
In the early stage of menopause, the estrogen level fluctuates greatly and is occasionally higher than that in the normal follicular phase. With the gradual exhaustion of ovarian function, follicles completely stop developing, the estrogen level rapidly decreases, and progesterone decreases earlier than estrogen. It has been shown that women in the menopausal transition, early postmenopausal period, and late postmenopausal period are at an increased risk of new-onset asthma, and the incidence of nonallergic asthma may increase (20,21).
Postmenopausal period
Postmenopausal ovarian failure reduces the level of estrogen, and the main source of estrogen in the body is the peripheral transformation of androstenedione. Compared with premenopausal period, new-onset asthma in menopause and postmenopausal period often shows more obvious symptoms, relatively poor treatment response, fewer allergic symptoms, more chronic sinusitis, higher eosinophil levels, and more common airway hyperreactivity and acute exacerbation in clinical practice.
Effect of female endocrine function and sex hormones on immune responses in asthma
Effect of sex hormones on immune responses in asthma
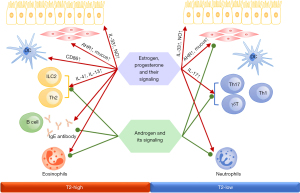
Sex hormones have a wide range of immunomodulatory effects and are involved in the regulation of immunological mechanisms in asthma by binding to hormone receptors on the surface or within the cytoplasm of immune cells. Clinical and animal studies have shown that sex hormones have both synergistic and antagonistic effects on inflammatory cells (22). Changes in sex hormone levels during different developmental stages influence the prevalence, (endotype) phenotype, severity, and treatment response of asthma. Changes in sex hormone levels caused by oral contraceptives and menopausal hormone therapy can also affect the risk of asthma. Figure 3 shows the effect of sex hormones on immune system response in asthma.
Estrogen and progesterone
Animal and cellular experiments have revealed that estrogen and progesterone and their signaling pathways positively regulate Th2-, Th17-, and type 2 innate lymphoid cell (ILC2)-mediated airway inflammation. Estrogen or progesterone stimulation not only upregulates the expression of Th2 inflammatory factors and transcription factors such as interleukin (IL)-4, IL-13, and GATA binding protein 3 (GATA-3) (23,24), but this stimulation also upregulates the expression of IL-17A protein and the differentiation of Th17 cells through the regulation of the IL-23/IL-23R signaling pathway and the production and migration of γδ T cells (25-27). It also promotes ILC2-mediated airway inflammation by upregulating IL-33 and C–C motif chemokine 2 (CCL2) production in airway epithelial cells through the ERα signaling pathway (28,29).
Moreover, estrogen treatment can promote the migration of mature dendritic cells (DC) and induce enhanced expression of CD86 in DCs, thereby increasing the activation and proliferation of Th2 cells and the expression of inflammatory factors such as IL-4, IL-5, IL-13, and monocyte chemotactic protein (MCP)-1, as shown in an asthmatic mouse model (30,31). In contrast, treatment with high-concentration progesterone negatively regulates the differentiation and activation of estrogen-mediated DCs, which in turn decreases the expression of proinflammatory cytokines IL-12, IL-1, and IL-6 (32).
In vitro cellular experiments have demonstrated that estrogen treatment promotes goblet cell differentiation and mucus production through ERβ signaling (33). Estrogen treatment also promotes nitric oxide (NO) production in airway epithelial cells by regulating ERα or ERβ signaling pathways, which in turn affects airway hyperreactivity in female patients with asthma (34). Progesterone affects airway mucus clearance by reducing ciliary beat frequency of airway epithelial cells (35). In addition, ERα and ERβ signaling are required for pulmonary alveolar development, and alveolar loss was observed in ERα- and ERβ-deficient mice (36). This could also explain the earlier lung maturation as well as lower incidence and severity of asthma in women than in men during infancy.
Androgen
Contrary to estrogen and progesterone, the androgen-androgen receptor (AR) signaling pathway attenuates Th2-, Th17-, and ILC2-mediated airway inflammation (27,37). Testosterone treatment results in significantly reduced eosinophil and neutrophil infiltration and decreased serum IL-4, IL-13, IL-17, and interferon (IFN)-γ levels in a house dust mite–induced allergic asthma mouse model. In vitro experiments have shown that testosterone and AR signaling attenuate Th2- and Th17-mediated airway inflammation through different mechanisms (27,38). Testosterone and its derivative 5α-dihydrotestosterone negatively regulate IL-2 receptor (IL-2R)-mediated ILC2 proliferation in the lungs through the AR signaling pathway and reduce the expression of cytokines such as IL-33, thymic stromal lymphopoietin (TSLP), IL-5, and IL-13, as well as ILC2-mediated allergic airway inflammation (37,39,40).
Effect of other endocrine functions on immune response in asthma
Psychological stress is considered a risk factor of asthma exacerbation. Women are more sensitive to psychological stress than men, which to some extent contributes to asthma exacerbation in women (41). Psychological stress enhances Th2 and Th17 inflammation in humans and rodents by activating the hypothalamus-pituitary-adrenal axis and the autonomic nervous system, followed by the secretion of neuroendocrine transmitters into blood, such as glucocorticoids, epinephrine, norepinephrine, and acetylcholine (41).
Obesity is another important contributor to the increased incidence of asthma in women. Previous studies have shown that obesity contributes to the development of asthma through immune mechanisms specific to the obese state (42-44). Furthermore, obesity can also induce inflammation by upregulation of oxidative stress level. Increased adipokines secretion by adipocytes in obese state leads to elevated IL-6, tumor necrosis factor (TNF)-α, and eotaxin levels, which trigger systemic inflammation. In addition, abnormal secretion of hormones of obesity contributes to asthma pathology; for instance, upregulation of a proinflammatory factor leptin and downregulation of an anti-inflammatory factor adiponectin lead to increased airway hyperresponsiveness (AHR) and inflammation (45,46), while insulin affects airway smooth muscle contraction and lung function by altering blood glucose concentration (47,48).
Incidence and management of asthma in different age groups and special periods
Asthma is a heterogeneous chronic airway inflammatory disease that in women is influenced by growth, development, and fluctuation in sex hormones at different ages. The diagnosis, treatment, and management of asthma can be quite challenging due to the variability in lung volume, airway resistance, immune response, and airway reactivity in female patients with asthma at different ages. Thus, treating patients requires special attention of medical staff in clinical practice.
Characteristics of allergy and wheezing in infancy and childhood, and therapeutic management
Recommendations for diagnosis and treatment
- If medical history and clinical symptoms and signs suggest asthma, it is recommended to examine the lung function of children over five years of age to find out whether there is significant reversible airflow restriction. In children under five years of age, experimental treatment may be beneficial, and the reversal of symptoms and signs within the expected time of diagnostic treatment suggests asthma. If the treatment is ineffective, or the medical history and symptoms and signs suggest other diagnoses, other causes of wheezing in children should be excluded (1C).
- After the diagnosis of asthma is confirmed, the severity of asthma should be assessed based on the clinical manifestations, lung function, and future risks of asthma exacerbation of the child so as to decide the appropriate therapeutic regimen. The most commonly used asthma control drugs for children are inhaled corticosteroid (ICS), inhaled long-acting beta agonist (LABA), or oral leukotriene receptor antagonist (LTRA) (1B). Short-acting beta agonist (SABA) is the preferred drug for the treatment of acute onset of asthma in children (1B). Systemic corticosteroids are indicated in children with moderate to severe acute asthma exacerbation and also in children with mild asthma exacerbation refractory to inhaled beta agonists alone (1C). For children aged six years and more with moderate to severe persistent allergic asthma whose symptoms cannot be effectively controlled with ICS and LABA, a biologic agent omalizumab may be used as appropriate if there are no contraindications (1B).
Overview
Asthma originates early in life. Genetic factors, maternal asthma, reduced vitamin D levels, respiratory tract infections, air pollution, and gastroesophageal reflux in the first year of life may affect the occurrence and development of asthma in children (49). Race, mode of delivery, breastfeeding, and cord blood vitamin D levels are associated with infant gut microbiome composition, with potential long-term implications for immune system modulation and asthma/allergic disease incidence (50). A Western lifestyle, which overly hygienically limits the general microbial exposure, alters the infant gut microbiome, subsequently disrupting the development of the immune system and ultimately leading to allergic disease (51). A study performed in Southern China also showed that rural children from an agricultural background exhibited a reduced risk of asthma owing to rural environmental exposure modulating the gut microbiota, which was essential in reducing allergy in children (52,53).
Characteristics and difficulties of allergy and wheezing in infancy and childhood
Cough and wheezing are the most common symptoms of asthma in children, and cough-variant asthma is one of the most common causes of chronic cough in children over three years old. About 80% of cases of children asthma occur before the age of five years, but asthma is easily misdiagnosed or missed due to a lack of objective indicators of lung function (54). Allergic diseases are associated with the onset, severity, and persistence of asthma. Food allergy and eczema are the most common atopic manifestations in infants and young children, while asthma and allergic rhinitis are more common in older children. For young children, there is a lack of objective measures of lung function, and some children with congenital and hereditary diseases may also have symptoms similar to wheezing, so appropriate tests should be selected according to the clinical manifestations of the child.
Treatment, monitoring, and management of allergy and wheezing in infancy and childhood
The treatment of childhood asthma should be based on current clinical presentation, impairment of lung function, and future risk assessment. ICS and oral LTRA are the most commonly used medications; inhaled LABA can be used for concomitant medication in older children. Inhaled SABA can rapidly relax airway smooth muscle and relieve acute asthma symptoms, so it is the preferred drug for the treatment of acute onset of asthma in children. Common SABAs include albuterol and terbutaline. Ipratropium bromide is an anticholinergic drug that can be combined with SABA by atomizing inhalation for the treatment of moderate to severe acute asthma exacerbation in children. Glucocorticoids have anti-inflammatory effects and can effectively reduce respiratory edema and secretion during acute asthma exacerbation. Systemic corticosteroids have a slow onset of action, with oral formulations taking effect after at least two hours; they are indicated in children with moderate to severe acute asthma exacerbation and also in children with mild asthma exacerbation refractory to inhaled beta agonists alone. Montelukast, a LTRA, can effectively reduce or prevent asthma symptoms in children with intermittent asthma and viral infection-related wheezing. For children over the age of six years with moderate to severe persistent allergic asthma whose symptoms cannot be effectively controlled with ICS and LABA, a biologic agent omalizumab may be used as appropriate if there are no contraindications (55). After the initiation of controlled therapy, the patients should be regularly assessed for the therapeutic effect at intervals of two-to-four weeks for moderate to severe persistent asthma and four-to-six weeks for mild persistent asthma to determine the response to the specific interventions. Children with well-controlled asthma are usually followed up every 3 months to assess the efficacy, but the frequency of follow-up may also need to be decided based on the severity of asthma. Patients whose asthma is not adequately controlled or is poorly controlled should be followed at intervals of 2-to-6 weeks or 2 weeks, respectively, to assess their response to escalation therapy.
Characteristics of allergy and asthma in adolescence and therapeutic management
Recommendations for diagnosis and treatment
- Adolescent patients should be asked about their childhood manifestations. If they have had long-term cough, nocturnal cough without respiratory infection, or have been diagnosed with “recurrent bronchitis” or “asthmatic bronchitis”, the possibility of asthma should be considered (1C).
- Effective management of asthma patients requires preventive measures; regular assessment of symptoms, lung function, exposure to triggers, and coexisting diseases; adjustment of medication; and continuous patient education (1C).
Overview
In adolescence, the prevalence of asthma in women is higher than that in men, which may be related to factors such as sex-specific lung development and sex hormone levels (56). Obesity, chronic rhinosinusitis, airway obstruction in early life, severe asthma symptoms in childhood, personal or family history of allergic diseases, perinatal stress, premature delivery (23–27 weeks), and early menarche are risk factors for asthma in adolescent females (57).
Characteristics and difficulties of adolescent asthma
Asthma symptoms are often alleviated before and after adolescence, but may also recur several years later. Childhood history is useful for diagnosing asthma in adolescents, particularly if there was a prolonged or nocturnal cough without respiratory infection, or a diagnosis of ‘recurrent bronchitis’ or ‘asthmatic bronchitis’. Other clues that are highly suggestive of asthma include recurrent episodic symptoms and the presence of typical triggers (e.g., exercise, cold air, allergen exposure). Adolescent asthma patients may also have atopic dermatitis, which manifests as typical lichenoid plaques distributed in intertriginous sites, especially the antecubital fossa, popliteal fossa, palmar surface of the wrist, ankles, and neck. Asthma and its treatment, which may bring some stress on adolescent patients, combined with the high incidence of social anxiety disorder, may affect the treatment compliance (58).
Treatment, monitoring, and management of asthma in adolescence
Effective management of asthma patients requires preventive measures; regular assessment of symptoms, lung function, exposure to triggers, and coexisting diseases; adjustment of medication; and continuous patient education. For all patients, avoidance of triggers and patient education are essential in addition to drug therapy. Avoidance of triggers helps to avoid recurrent exacerbations. Patient education should be continuous and individualized and should specify how to self-administer at baseline and during exacerbation.
For patients with intermittent asthma, it is recommended to use SABA to relieve symptoms as needed, or use low-dose ICS. For patients with mild persistent asthma, low-dose ICS or ICS + LABA compound inhaler should be used regularly; daily oral LTRA can also be used, and SABA can be used as needed to relieve symptoms. Patients with moderate persistent asthma are treated with daily control regimen to prevent exacerbation, combined with rescue medication to control symptoms. Low-dose ICS is recommended as the main control drug, and low-dose ICS + LABA compound inhaler can be used. Severe persistent asthma refers to asthma requiring high-dose ICS, or requiring continuous oral corticosteroids (OCS) for control, or never achieving control despite the treatment. Initial control drugs for such patients include OCS or ICS and LABA, and LTRA can be selected for patients who cannot be adequately controlled with high-dose ICS combined with LABA. Patients whose symptoms are not controlled by high-dose ICS and one or more non-GC control drugs should have their diagnosis reviewed. Biologics may be considered appropriate in children who meet age and other conditions. Potential options and investigational therapies include immunomodulatory therapy and macrolide antibiotics (59). However, a thorough evaluation by an experienced specialist is required.
Patients with active asthma should undergo routine follow-up every 1 to 6 months based on the severity of asthma. Asthma control, lung function, episodes, inhaler usage, compliance, adverse drug reactions, quality of life, and patient satisfaction with treatment should be assessed at the follow-up. Each follow-up visit requires an assessment of symptoms past 4 weeks, including daytime symptoms, nighttime awakening due to asthma symptoms, frequency of symptom relief with SABA, and difficulty with normal activities and movements.
Characteristics of PMA and therapeutic management
Recommendations for diagnosis and treatment
- Standardized recording of respiratory symptoms and peak flow levels during the menstrual cycle is an effective tool for diagnosing the disease. For example, the monitoring of peak expiratory flow rate (PEFR) can reveal a decrease in PEFR during the menstrual cycle (1C).
- The treatment and management principles for PMA are the same as those for typical asthma. If premenstrual asthma is identified, prophylactic drugs, such as montelukast or ketotifen, may be administered orally several days before the day of menstruation (1C); progesterone should be injected promptly before menstruation to prevent a sudden decrease in progesterone levels; and danazol should be used as appropriate for patients with premenstrual anxiety (2C).
Overview
Some female patients with asthma experience exacerbation of asthma symptoms or decreased lung function before or during menstruation, which gradually improves after menstruation. This phenomenon is called PMA (19,60). The incidence of PMA is approximately 19–40% (61). Fluctuations in estrogen levels during ovulation and premenstrual period as well as changes in progesterone secreted during the luteal phase and its subsequent rapid decline may be involved in the pathogenesis of PMA (62,63).
Characteristics and difficulties of PMA
PMA is divided into premenstrual asthma and menstrual asthma. The former refers to the exacerbation of asthma symptoms during the luteal phase, which are relieved spontaneously after menstruation; the latter refers to the exacerbation of asthma symptoms on the first day of menstruation. The clinical manifestations are periodic exacerbations of cough, wheezing, chest tightness, and dyspnea during the perimenstrual period (64,65), which require the addition of bronchodilators and are accompanied with a decline in PEFR. The severity of PMA increases with age, and its duration prolongs until around menopause (66). PMA is a specific phenotype of asthma. Older patients and those with a higher body mass index are more likely to experience more severe and longer asthma with PMA and develop aspirin-sensitive asthma. Asthma medications are used more frequently in PMA patients, and such patients often experience dysmenorrhea, premenstrual syndrome, short menstrual cycle, and long bleeding time.
Treatment, monitoring, and management of PMA
PMA patients need more active antiasthma treatment during menstrual cycle. The 2022 GINA suggests that PMA patients may potentially benefit from adding oral contraceptives and/or LTRAs to their conventional asthma therapy (67). Several studies (68-72) have suggested that hormone replacement therapy (HRT) (including different combinations of estrogen and/or progesterone) has a therapeutic effect on PMA, which can improve lung function and reduce the number of acute exacerbations. For example, exogenous estradiol may relieve asthma symptoms (68). It has been reported that intramuscular injection of high-dose progesterone in PMA patients’ refractory to high-dose glucocorticoid therapy helps to relieve symptoms (73). LTRAs can improve symptoms and PEFR variation rate by inhibiting inflammation (74). However, the sample size of the above-mentioned studies is too small, and further verifications are required. In addition, the degree of PMA varies from person to person and is generally mild to moderate; however, some patients have very severe disease, even near-fatal asthma (NFA). Caution should be taken to prevent NFA in PMA patients who have experienced aggravated menstrual symptoms. The symptoms of asthma and the lung function during menstrual period should be recorded in the self-management plan of such patients (75). Danazol may be used in PMA patients with premenstrual anxiety (76). Table 4 shows the drugs for the treatment of PMA.
Table 4
| Treatment | Representative medications or surgeries |
|---|---|
| Conventional asthma medication | ICS + LABA, leukotriene modifiers |
| Sex hormone therapy | Progesterone, testosterone, selective estrogen receptor antagonists |
| Intervention with gonadotropin-releasing hormone analogue | Goserelin |
| Blocking of menstrual cycle | Hysterosalpingo-oophorectomy |
PMA, perimenstrual asthma; ICS, inhaled corticosteroid; LABA, long-acting beta agonist.
Characteristics and management of asthma during pregnancy and lactation
Recommendations for diagnosis and treatment
- It is recommended to strengthen the education and assessment of patients with asthma during pregnancy, including but not limited to smoking cessation, avoiding exposure to allergens, avoiding infection, compliance to medication and follow-up, controlling gestational weight gain, monitoring lung function and fractional exhaled nitric oxide (FENO), and formulating an asthma action plan (1C). It is recommended that pregnant women with asthma are managed by respiratory specialists, obstetricians, and gynecologists in a multidisciplinary and collaborative manner (1C).
- Drug therapy is recommended to achieve good asthma control and prevent acute exacerbation, and step-down therapy should be avoided during pregnancy (1B).
- The drug therapy for acute exacerbation of asthma in pregnant women is almost the same as that in nonpregnant women. Acute asthma exacerbation during pregnancy requires immediate high-flow oxygen inhalation to maintain oxygen saturation level above 95%, along with continuous fetal monitoring, close contact between respiratory specialists and obstetricians, and early referral to intensive care unit (ICU) doctors if necessary (1C).
- Acute exacerbation of asthma is rare at delivery and can be treated with commonly used asthma medications. Women receiving more than 7.5 mg prednisolone per day, for more than 2 weeks prior to delivery, should receive hydrocortisone 50 mg every 6–8 hours during delivery. Intraspinal anesthesia is superior to general anesthesia if a parturient with asthma requires anesthesia during delivery. Hyperventilation during delivery may lead to bronchoconstriction, and SABAs should be used. If high doses of SABAs are administered during delivery, blood glucose should be tested in the first 24 hours after birth (1C).
- Asthma patients during lactation have similar treatment and management goals to other asthma patients, but it is necessary to evaluate the advantages and disadvantages in order to prescribe drugs. Breastfeeding is recommended as much as possible after delivery (1C).
Overview
The incidence of asthma during pregnancy ranges from 3% to 8% (77). Pregnant women with asthma have a higher risk of pregnancy complications than those without asthma; complications include preeclampsia, premature delivery, low birth weight or intrauterine growth restriction, congenital infant malformations, and perinatal death (78,79). The occurrence of these pregnancy complications is significantly associated with uncontrolled asthma, suggesting that proper asthma control is beneficial for improving pregnancy outcomes (80). The secretion of estrogen is greatly increased in lactating women, which may also have an influence on the control of asthma, but postpartum asthma exacerbations are less common (81). Numerous studies have demonstrated that breastfeeding can reduce the risk of allergic diseases such as eczema, atopic dermatitis, asthma, food allergy, and infectious diseases in young children (82). Therefore, breastfeeding is recommended as much as possible after delivery.
Characteristics and difficulties of asthma during pregnancy and lactation
Patients with asthma may experience changes in asthma control due to the changes in respiratory physiology, hormone levels, immunology, and psychology during pregnancy (83). Previous studies have shown that exacerbations occur in one-third of patients (84). Asthma exacerbation during pregnancy mostly occurs in the second trimester (85). A recent meta-analysis has shown that old gestational age, obesity, smoking, multipara, anxiety and depression, and moderate to severe asthma increase the risk of asthma exacerbation during pregnancy (86). Other risk factors for asthma exacerbation during pregnancy include poor compliance to medication, respiratory viral infection, and rapid weight gain during pregnancy (85,87). There is a lack of safety data on different drugs for treating asthma in pregnant women. To be used during pregnancy, an antiasthma medication should be evaluated to fully weigh the benefits and risks to the pregnant woman and the fetus, and the patient’s opinion should be considered. Patients with asthma also require good control of their disease postpartum, but postpartum asthma exacerbations are less common.
Treatment and management of asthma during pregnancy and lactation
Diagnosis and management of asthma during pregnancy
(I) Diagnosis of asthma during pregnancy
The diagnosis is relatively straightforward in patients who have been diagnosed with asthma before pregnancy. For patients with atypical clinical symptoms, poor response to treatment, or no previous history of asthma, it is necessary to further clarify the etiology of respiratory symptoms, differentiating between asthma, gestational dyspnea, gastroesophageal reflux, rhinitis during pregnancy, hyperventilation, vocal cord dysfunction, pulmonary embolism, and other causes. The diagnosis of asthma during pregnancy is based on the diagnostic criteria for common asthma; it is based on the combination of symptoms and their characteristics, signs, objective examination of variable airflow limitation, and exclusion of other diseases. Bronchial provocation test is not recommended as there is a lack of evidence on its safety during pregnancy. Serum specific IgE can be detected to understand the presence or absence of allergic factors and guide allergen avoidance during pregnancy, but allergen skin prick test is not recommended because it may induce serious adverse reactions.
(II) Education on asthma during pregnancy
The content of education for patients with asthma during pregnancy and the reasons are shown in Table 5.
Table 5
| Content of education | Reasons |
|---|---|
| Stop smoking | Smoking increases perinatal complications, risks and severity of asthma exacerbation |
| Avoid exposure to allergens | Fewer allergic asthma exacerbation leads to fewer medication |
| Prevent respiratory infections and vaccinate | Pregnant women are susceptible to respiratory infectious diseases which may lead to asthma exacerbations |
| Control the speed and range of weight gain during pregnancy | Excessive weight gain during pregnancy is an independent risk factor for asthma exacerbations during pregnancy |
| Improve medication compliance and emphasize the benefits of good control of asthma during pregnancy | Patient worries about medication risks and medication compliance decreases |
| Use drugs correctly | The correct use of inhaled drugs is low |
| Monitor peak flow rate | It is helpful for differential diagnosis of dyspnea during pregnancy and prediction of asthma exacerbation |
| Develop an asthma action plan | It is used to guide the treatment of asthma exacerbation |
| Multidisciplinary management | Pregnancies in patients with asthma should be considered as high-risk pregnancies and require close cooperation and coordination among obstetrics and gynecology, respiratory and pediatrics |
(III) Treatment of asthma during pregnancy
The treatment goal for asthma during pregnancy is to achieve control of asthma with the best regimens to ensure the health and quality of life of the mother and the normal development of the fetus. The initial and maintenance treatments for asthma during pregnancy are similar to those for nonpregnant women with asthma, which can be obtained from GINA and Chinese guidelines for the prevention and treatment of bronchial asthma (76). Many drugs for treating asthma still lack safety data for pregnant women. The currently available safety data of drugs for asthma treatment during pregnancy are shown in Table 6 (88). Inhaled drugs are recommended to reduce systemic absorption and potential effects on the fetus. It is recommended to continuously evaluate, adjust, and review the responses to treatment for asthma during pregnancy. GINA recommends that step-down treatment should be considered only after delivery, and ICS should not be stopped during pregnancy preparation or pregnancy. The safety profile of omalizumab before and during pregnancy has been reported (89). Allergen-specific immunotherapy and omalizumab cannot be used as new treatments during pregnancy.
Table 6
| Drug classification | Drugs | Adverse fetal/neonatal outcomes |
|---|---|---|
| Oral antihistamines | ||
| First generation antihistamines | Chlorphenamine | Animal experiments have shown no increased risk of malformations, and human studies have reported association with a variety of birth defects |
| Diphenhydramine | Based on animal studies and available human data, diphenhydramine is not expected to increase the risk of congenital anomalies | |
| Second generation antihistamines | Cetirizine | Based on animal and human data, medication is not expected to increase the risk of adverse pregnancy outcomes |
| Desloratadine | Based on animal data, medication during pregnancy is not expected to increase the risk of congenital anomalies No human data available | |
| Fexofenadine | Based on animal data and human reports for the parent compound fexofenadine, exposure during pregnancy is not expected to increase the risk of adverse consequences | |
| Levocetirizine | Based on animal and human data reported, medication is not expected to increase the risk of adverse pregnancy outcomes | |
| Loratadine | Based on animal data and human reports, medication is not expected to increase the risk of adverse pregnancy outcomes | |
| Rupatadine | Based on animal data, medication is not expected to increase the risk of congenital anomalies | |
| Inhaled glucocorticoids | Budesonide | Budesonide, beclomethasone and fluticasone are preferred because these ICSs have more safety information. However, if the patient’s asthma has been well controlled by other ICSs (e.g., ciclesonide, mometasone) before becoming pregnant, no change in treatment is necessary |
| Fluticasone | ||
| Beclomethasone | ||
| Mometasone furoate | ||
| Ciclesonide | ||
| Inhaled bronchodilators | ||
| Short-acting bronchodilators | Salbutamol | Salbutamol is the preferred and most studied β-agonist for the treatment of asthma. Currently available human data for salbutamol are reassuring and show it is safe during pregnancy, and the reported malformations may be associated with asthma severity or incidental findings |
| Long-acting bronchodilators | Formoterol | Based on animal studies, inhaled formoterol for asthma is not expected to increase the risk of congenital anomalies Human data are limited, but results show formoterol is safe |
| Salmeterol | Based on experimental animal studies and human experience, use of salmeterol during pregnancy is not expected to increase the risk of congenital anomalies | |
| Systemic glucocorticoids | ||
| Difficult to pass through the placenta | Prednisolone | Glucocorticoids are not major teratogens in humans, but there is a mild increase in the risk of cleft lip and palate, which is not a concern with the use of glucocorticoids after the 12th week of life since palate formation is completed at 12 weeks of fetal life. The risk of disease left untreated, progression, and maternal and infant mortality should be weighed against the potential for increased risk of uncontrolled disease in the mother and fetus |
| Methylprednisolone | ||
| Easy to pass through the placenta | Cortisone | |
| Hydrocortisone | ||
| Prednisone | ||
| Triamcinolone acetonide | ||
| Leukotriene receptor antagonist | Montelukast | According to available human data, leukotriene receptor antagonist can be regarded as the second-line treatment during pregnancy |
| Zafirlukast | ||
| Biological targeted drug | Benralizumab | Monkey experiments show no side effects, and no human study data are published |
| Dupilumab | Monkey experiments show no side effects, and no human study data are published | |
| Mepolizumab | Monkey experiments show no side effects, and no human study data are published | |
| Omalizumab | Monkey experiments show no side effects, and human studies show that omalizumab does not increase the risk of adverse pregnancy outcomes | |
| Reslizumab | Rat and rabbit experiments show no side effects, and no human study data are published | |
ICS, inhaled corticosteroids.
Approximately 18% of pregnant women with asthma have at least one emergency department visit during pregnancy, and 62% of pregnant women with asthma require hospitalization during asthma exacerbation. The treatment and management of asthma exacerbation are approximately the same as those for the ordinary patients and should be started as early as possible (67,90). The arterial blood gas analysis of pregnant women indicates the presence of compensatory respiratory alkalosis. Acute exacerbation of asthma can lead to increased alkalosis, which can cause fetal hypoxia (20,21). In contrast, acute respiratory acidosis may occur when PaCO2 exceeds the normal level of 28–32 mmHg. Maternal acidosis can cause a loss of CO2 pressure difference between maternal venous blood and fetal umbilical artery blood, resulting in decreased fetal excretion of CO2. During physical examination of patients with asthma in the second and third trimesters of pregnancy, the hemodynamic effects of an enlarged uterus in the supine position should be evaluated. Patients with moderate to severe asthma exacerbation should be monitored for vital signs, and obstetricians should also participate in the monitoring of fetal vital signs. Patients who frequently visit the emergency room, have been hospitalized, and that have required intubation or been admitted to ICU for severe asthma exacerbations should be considered the patients with high-risk or fatal asthma exacerbation. Prevention of maternal and fetal hypoxia and relaxation of the airway are most important in the management of severe asthma exacerbation during pregnancy. Pregnant women can be treated with oxygen inhalation, and the blood oxygen saturation level must be maintained above 95%. Nebulized salbutamol is recommended every 20 minutes during the first hour, and 500 µg ipratropium bromide can be added to each nebulization. If this treatment fails to relieve the symptoms or the patients have moderate to severe acute exacerbation at the beginning, intravenous or OCS should be given. Intravenous theophylline does not further induce bronchodilation. If the patient has already taken oral theophylline, plasma theophylline concentration should be measured before intravenous administration of theophylline. Patients must be monitored and evaluated every hour in the emergency room for their response to treatment, and the decision on hospitalization or discharge home should be based on their response to treatment for the first four hours. In general, patients requiring further hospitalization are those who have little or no response to initial treatment in the emergency department, or those with life-threatening asthma exacerbations. Patients who require urgent intubation, whose condition worsens after therapy, and whose PaCO2 continues to increase are suggested to be admitted to the ICU for further management.
Treatment and management of asthma during delivery
Asthma exacerbation during delivery is uncommon, but it can pose serious threats to the parturient and fetus. It is currently considered that all asthma control drugs must be continued as usual during delivery. The vital signs of the parturient and fetus should be well monitored during delivery. Patients who take long-term oral glucocorticoids (e.g., prednisolone ≥7.5 mg/day for more than two weeks) should consider continuing glucocorticoids at delivery. It is recommended to use at least 50 mg hydrocortisone (intravenously or intramuscularly) every six hours from the start of the first stage of labor until six hours after delivery. Intraspinal anesthesia allows patients to maintain spontaneous breathing without endotracheal intubation and significantly reduces the risk of bronchospasm in patients with asthma. Hyperventilation during delivery may lead to bronchoconstriction, and SABAs should be used. If high doses of SABAs are administered during delivery, blood glucose of the fetus should be tested in the first 24 hours after birth. At present, there is no absolute indication for a termination of pregnancy in pregnant women with asthma. Whether or not to terminate pregnancy in emergency situations should be jointly decided by doctors of respiratory department, obstetrics and gynecology, pediatrics, and even intensive care medicine after multidisciplinary consultation. If the fetus has matured, early termination of pregnancy can be considered.
Treatment and management of asthma during lactation
The treatment and management of asthma during lactation should not only control asthma well, but should also reduce the entry of drugs into breast milk to reduce the impact of the drugs on infants. According to both domestic and international guidelines for asthma, there is no significant difference in therapeutic medication between asthma during lactation and other asthma patients, but it is necessary to weigh the advantages and disadvantages of using certain drugs because of lactation. Inhaled asthma drugs are primarily absorbed by the airway mucosa, and the dose of the drugs entering breast milk through the blood is small. In principle, inhaled asthma drugs can be safely used in lactating women with asthma. The safety profile of the commonly used asthma medications during lactation is presented in Table 7.
Table 7
| Asthma drugs | Safety during lactation |
|---|---|
| Short-acting β receptor agonists (represented by albuterol) | No published data. Because of low bioavailability and low plasma concentration, it is expected that the drug concentration of such drugs in breast milk will be very low |
| ICS such as beclomethasone, budesonide, fluticasone, triamcinolone acetonide | |
| Tiotropium bromide | |
| LTRA such as montelukast | The drug content in breast milk is very low |
| Systemic hormones | Prednisone: very low level in breast milk; no adverse effects have been identified in breastfed infants. High doses of hormones may cause temporary suppression of lactation |
| Tiotropium bromide | No published data. Because of low bioavailability and low plasma concentration, it is expected that the drug concentration of such drug in breast milk will be very low |
| Biological targeted drugs: omalizumab (anti-IgE), benralizumab (anti-IL-5Rα), dupilumab (anti-IL-4Rα) | No published data. The large molecular weight proteins of the monoclonal antibody may be destroyed in the gastrointestinal tract of infants |
ICS, inhaled corticosteroid; LTRA, leukotriene receptor antagonist; IL, interleukin.
Characteristics of menopausal asthma and therapeutic management
Recommendations for diagnosis and treatment
Due to unstable hormone levels and emotional factors, menopausal women with asthma may subjectively feel that their asthma symptoms are aggravated, which needs to be carefully screened. Patients with asthma should be treated in accordance with the prevention and control guidelines for bronchial asthma. HRT is optional depending on the actual situation of menopause (2C).
Overview
Most women enter menopause after the age of 45 years and have different menstrual cycle lengths once menopause begins as a result of the changes in ovarian function and depletion of the follicular pool. Previous studies have shown that women in the menopausal transition, early postmenopausal period, and late postmenopausal period are at an increased risk of new-onset asthma. However, studies in this area are inconsistent. Some studies have shown that natural menopause does not lead to an increased risk of new-onset asthma in the overall population and in women with normal body mass index; natural menopause has an increased risk of new-onset asthma in overweight and obese women; and surgical menopause has an increased risk of new-onset asthma compared with premenopausal and natural menopause women (91).
Characteristics and difficulties of menopausal asthma
The unstable hormone levels of menopausal women lead to more obvious asthma symptoms. Restrictive pulmonary dysfunction caused by decreased lung function and osteoporosis increase the perception of asthma symptoms in menopausal patients (92). The incidence of nonallergic asthma may increase in menopausal women. Studies have shown that, compared with women who have asthma before menopause and healthy menopausal women, in women who have menopausal asthma, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels are decreased, the proportion of neutrophils in sputum is increased, exhaled IL-6 levels are increased, and urinary leukotriene E-4 (LTE-4) levels are decreased (93).
Treatment, monitoring and management of menopausal asthma
There is some controversy over the use of HRT for menopause (94). Early studies have shown that HRT increases the risk of asthma, with the highest risk in women who use conjugated estrogens (95). Such women also have a lower body mass index and do not smoke (96,97). Subsequently, HRT after menopause has been linked to an increased probability of new-onset asthma (94). Estrogen therapy alone and combined estrogen and progestin therapy increase the risk of new-onset asthma, while progestin therapy alone decreases the risk of new-onset asthma. In patients who stop HRT, the possibility of asthma disappearance is increased (98). Another recent retrospective study on perimenopausal and postmenopausal women aged 45 to 70 years who have had no asthma for five years or more has shown a decreased risk of new-onset asthma in women who have received either form of HRT compared with women who have not received HRT, with a further reduction in the risk of new-onset asthma in those who have used HRT for a longer period of time (99). The inconsistency of the above results is also related to the different efficacy of the compound medroxyprogesterone acetate used in the United States and other preparations used in Europe (100). Thus, it is clear that estrogen, progesterone, and androgen interact in a complex manner.
Characteristics of postmenopausal asthma and therapeutic management
Recommendations for diagnosis and treatment
In clinical practice, for menopausal women with new-onset asthma or aggravated symptoms of preexisting asthma after menopause, it is important to note whether HRT has been performed, and discontinuation of HRT may be considered if necessary (2C). For elderly patients with asthma, final treatment recommendations should be carefully assessed and weighed against the risks and benefits when formulating treatment regimens, taking into account comorbidities that may exacerbate asthma or affect treatment outcome, as well as drug interactions, or potential adverse events (1C).
Overview
Epidemiological studies on menopause and asthma are scarce, and existing studies have yielded conflicting results. Most studies suggest that women are at an increased risk of developing asthma after menopause, which may be associated with the decrease of estrogen levels in the body (21,95,101). A survey that included nationwide databases in Korea indicated the highest prevalence of asthma among elderly women (102). Airway inflammation in patients with postmenopausal asthma appears to differ from that in patients with early-onset asthma and is characterized by the poor response to anti-inflammatory therapy and more frequent acute asthma exacerbations (103). After menopause, women gradually enter the old age. Due to organ degeneration, physiological function and immune function decline, and many other diseases, asthma management is more complicated in old age.
Characteristics and difficulties of postmenopausal asthma
Compared with premenopausal asthma, new-onset asthma occurring during menopause and postmenopausal period has more severe clinical symptoms, relatively poor treatment response, fewer allergic symptoms, higher coincidence of chronic sinusitis, higher eosinophil levels, and more common acute exacerbations (103). Acute exacerbations tend to cause respiratory failure (104). Compared with asthma patients in other age groups, elderly patients with postmenopausal asthma have more comorbidities, often including cardiovascular diseases, endocrine diseases, neuropsychiatric diseases, and chronic obstructive pulmonary diseases. Comorbidities may change the pharmacokinetics and pharmacodynamics of drugs for asthma, and increase the risk of adverse drug reactions.
Treatment, monitoring, and management of postmenopausal asthma
At present, the role of HRT in postmenopausal asthma remains controversial, with mixed results (95,98,99). Therefore, in clinical practice, discontinuation of HRT should be considered if respiratory symptoms aggravate when HRT is prescribed.
The prescription of asthma medication and management of elderly patients with postmenopausal asthma must also be considered (104). In addition to complying with the management principles of adult asthma, special attention should be paid to the following characteristics: (I) due to many complications in the elderly patients, inhaled drugs are the optimal choice of prescription, and the potential risks of long-term use of inhaled high-dose ICS and β2 receptor agonists on blood glucose and cardiovascular diseases should be paid attention to. (II) Most elderly people have problems with inspiratory flow rate and movement coordination, therefore, they may require individualized and careful guidance on the selection of inhalation devices and drug inhalation techniques.
The treatment principles of acute exacerbation of asthma in the elderly are consistent with those in adults, but elderly patients have more complications and rapid changes in their conditions, requiring careful and timely monitoring as well as assessment of response after treatment. In the treatment of acute exacerbation, special consideration should be given to the treatment of comorbidities and the impact of comorbidities on asthma.
The goals of senile asthma management are consistent with those of adult asthma, and special attention should be paid to the following points. Since elderly patients can have lower cognitive level and poorer learning ability, education needs to be strengthened in particular, and caregivers/family members should be educated together when necessary. It is also necessary to repeatedly educate and carefully guide patients to use inhaled drugs correctly and regularly. The use of multiple inhalers should be avoided to reduce inhalation errors. Patients should be educated as to use emergency relief medications in a timely manner during acute exacerbations and to enhance self-management.
The management model for postmenopausal asthma in the elderly requires comprehensive consideration of the complex comorbidities, impaired cognitive and behavioral abilities of elderly patients, and age-related psychosocial factors and drug interactions, as well as multidimensional evaluation and multidisciplinary intervention.
Characteristics of obese-asthma and therapeutic management
Recommendations for diagnosis and treatment
Obese-asthma patients have poor treatment outcomes, and weight loss has many beneficial effects. Weight loss and weight control are priorities for asthma management in obese patients. Nonsurgical weight loss methods can effectively improve the outcomes of asthma. Weight loss through caloric restriction combined with exercises is the main intervention to improve the outcomes of asthma (1C).
Overview
Obesity is a complex chronic disease, and studies have shown that it can increase the severity of asthma (104-106). Data from the National Center for Health Statistics (NCHS) evaluating the incidence of asthma in obese women ≥20 years of age in the United States have shown that the incidence of asthma is 7.9% in women with normal weight, 9.1% in overweight women, and 14.6% in obese women. The incidence of asthma is 6.1% in men with normal weight, 6.7% in overweight men, and 7.1% in obese men (107). A study of 4,619 subjects who were followed up for 25 years showed that the correlation between body mass index and female asthma events was statistically significant, and complicated metabolic syndrome could predict the occurrence of asthma events in women; however, it could not predict the occurrence of asthma events in men. Compared with complicated metabolic syndrome, body mass index can better predict the occurrence of asthma events in women (108). Dietary intake plays an important role in obesity and asthma. Tarazona-Meza found that better diet quality was associated with lower odds of asthma (109). According to Kim, the higher consumption of fish and seaweed and the high ratio of n-3 to n-6 polyunsaturated fatty acids (PUFA) may be associated with a lower prevalence of asthma in adult women (110). A cross-sectional study has suggested that dietary arachidonic acid is a promoter of allergic disease in women (111). In elderly women, a healthy diet is associated with fewer asthma symptoms, and, among women with asthma, healthy diet is linked to reduced uncontrolled asthma and metabolic-related multimorbidity (112). There is a complex association between asthma and obesity, especially in females. Vieira investigated the prevalence of atopy among healthy obese and nonobese women and found that the frequency of specific IgE in the obese group was almost three times higher than that in the nonobese group, confirming a direct relation between obesity and a T helper 2 cell immune response in women (113). Body fat distribution affects the obesity-asthma relationship, and when stratifying by sex, the association between trunk-predominant adiposity (higher trunk/total fat and trunk/legs fat, or lower legs/total fat) and asthma has only been found in adult women (114). The root cause of sex differences in asthma risk may be related to different hormone levels.
Characteristics and difficulties of obese-asthma
In general, patients with obese-asthma have significant respiratory symptoms, are more severely ill, are not easily controlled, and respond poorly to ICS (115-117). Residual volume (RV) and total lung capacity (TLC) are reduced in patients with obese-asthma, manifesting as normal or restrictive pulmonary dysfunction (118). Serum total IgE is normal or decreased, with no significant airway eosinophilic inflammation and low FeNO level (119). Most patients suffer from obesity-related comorbidities, such as obstructive sleep apnea and gastroesophageal disease (120). Some patients have history of nasal polyps. The response to glucocorticoid therapy is slow. Due to the limited effectiveness of inhaled glucocorticoids, traditional treatment methods do not work well for patients with obese-asthma, and the prognosis is worse than that of asthma patients with normal weight (121,122).
Treatment, monitoring, and management of obese-asthma
Current asthma treatments are less effective in obese patients, and weight loss has many beneficial effects. Weight loss and weight control are priorities for asthma management in obese patients (123). Weight loss strategy: weight loss surgery can improve the control of obese asthma patients (124). However, some studies have shown that weight loss surgery may not be effective for some patients (125). Nonsurgical weight loss can effectively improve the outcomes of asthma. Weight loss through caloric restriction combined with exercises is the main intervention to improve the outcomes of asthma. Metformin, a drug for diabetes with a weight-loss effect (126,127), may facilitate asthma control because of its potential anti-inflammatory or immunomodulatory effects, or because of its improvement of insulin resistance (128). Obese patients with refractory asthma are hormone-insensitive and have systemic inflammation. Biological targeted drug such as dupilumab can significantly reduce the OCS dose in severe asthma patients with OCS dependence, significantly reduce acute exacerbations, improve lung function, and achieve good tolerance (129).
Management of acute asthma exacerbation and severe asthma in women
Recommendations for diagnosis and treatment
- The management of acute exacerbations of bronchial asthma in women lacks multicenter studies and still follows the strategy of asthma control guidelines for management: identification of risk factors for mortality in acute asthma exacerbations, assessment of acute exacerbation severity, and selection of therapeutic measures depending on the disease severity and the response to treatment (1C).
- The incidence of severe asthma in women is high. So far, the principle of treatment continues to be individualized treatment based on the guidelines (1C).
Overview
Acute exacerbation of bronchial asthma refers to cough, asthma, chest tightness, and progressive decline of pulmonary function that occurs or rapidly worsens in a short period of time and requires additional reliever drugs for treatment. Common causes of bronchial asthma are exposure to allergens, various physicochemical irritants, or upper respiratory tract infections (67). The incidence, severity, exacerbation, hospitalization, and mortality of asthma are generally higher in women than in men (130). In women, asthma symptoms fluctuate due to fluctuation in sex hormone levels at different stages. About 30% to 40% of women experience worsening symptoms during menstruation, and PMA is associated with emergency department visits, hospitalization, ICU admission for endotracheal intubation, and even fatal events (19). Currently, there is still lack of multicenter studies on the treatment of acute asthma exacerbation in women, and treatment still follows the guidelines and strategies for asthma prevention and treatment. For PMA and acute exacerbation of asthma during pregnancy, please refer to this expert consensus; for asthma during other periods, please refer to the Chinese Guidelines for Prevention and Treatment of Bronchial Asthma (76).
Severe asthma refers to asthma that is uncontrolled despite adherence with maximal optimized high-dose ICS-LABA treatment and management of contributory factors, or that worsens when high-dose treatment is decreased. A previous study reported that almost two-thirds of patients with severe asthma were female (131). A global registry study of severe asthma showed that 59.3% of women had severe asthma (132). Italian network data of severe asthma showed that the disease was characterized by late onset, more complications, and poor control (133). Therefore, the incidence of severe asthma in women is high. The pathogenesis of severe asthma in women is not sufficiently clear, and asthma that occurs after menopause is usually a non-type 2 inflammatory asthma (134). There are not many studies on its treatment. A study of a biological agent in the treatment of patients with severe asthma has shown that sex is not a determinant of response to the biological agent therapy. However, elderly women treated with biological agents have significantly higher improvement in pulmonary function than men of the same age group. Presently, the principle of treatment for severe asthma in women continues to be individualized treatment according to the guidelines.
Conclusions
The prevalence, incidence, and severity of asthma vary by sex and may be related to genetic/epigenetic factors, sex hormones, socio-environmental factors, and response to treatment. In addition, the complex heterogeneity of asthma makes it difficult for medical staff to fully understand the specific impact of sex differences on the diagnosis and management of asthma. The increased incidence, severity, control level, and comorbidities of asthma are all related to the hormone levels and endocrine balance in human body. There have been many large cohorts and basic studies at the animal and cell level that have explored the pathogenesis of female asthma, and the diagnosis, treatment, and management specifications at different physiological stages. However, there is still a lack of high-quality studies on the effects of sex hormones, physiological, and endocrine characteristics at different stages of the female life cycle on the pathogenesis and progression of asthma and the response to different treatments. It remains to be studied whether there are sex differences in the efficacy of type 2 and non-type 2 inflammatory asthma according to the current inflammatory pathway, and whether there are sex differences in the susceptibility of asthma patients to SARS-CoV-2. This unmet need suggests further clinical and epidemiological research is required to clarify the pathogenesis of asthma caused by the changes in sex hormones or the use of exogenous hormone therapy, as well as the impact on asthma treatment response. In addition, as more biologics are approved for the treatment of asthma, we must further clarify their differences in the efficacy by sex and age. These studies will strengthen our understanding of the pathogenesis of asthma and provide individualized treatment and management plans for different populations at all stages.
Acknowledgments
We thank LetPub (www.letpub.com) for linguistic assistance and pre-submission expert review.
Funding: This work was supported by
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1069/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1069/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211-59. [Crossref] [PubMed]
- Xiao D, Chen Z, Wu S, et al. Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. Lancet Respir Med 2020;8:1081-93. [Crossref] [PubMed]
- Shu W, Li ML, Li ZA, et al. Meta-analysis of asthma prevalence of children aged 0-14 in surveillance cities of China. Zhonghua Yu Fang Yi Xue Za Zhi 2020;54:875-83. [Crossref] [PubMed]
- Li X, Song P, Zhu Y, et al. The disease burden of childhood asthma in China: a systematic review and meta-analysis. J Glob Health 2020;10:010801. [Crossref] [PubMed]
- Dong GH, Chen T, Liu MM, et al. Gender differences and effect of air pollution on asthma in children with and without allergic predisposition: northeast Chinese children health study. PLoS One 2011;6:e22470. [Crossref] [PubMed]
- Rice MB, Rifas-Shiman SL, Litonjua AA, et al. Lifetime air pollution exposure and asthma in a pediatric birth cohort. J Allergy Clin Immunol 2018;141:1932-1934.e7. [Crossref] [PubMed]
- Agrawal S. Effect of indoor air pollution from biomass and solid fuel combustion on prevalence of self-reported asthma among adult men and women in India: findings from a nationwide large-scale cross-sectional survey. J Asthma 2012;49:355-65. [Crossref] [PubMed]
- Young MT, Sandler DP, DeRoo LA, et al. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am J Respir Crit Care Med 2014;190:914-21. [Crossref] [PubMed]
- Mishra V. Effect of indoor air pollution from biomass combustion on prevalence of asthma in the elderly. Environ Health Perspect 2003;111:71-8. [Crossref] [PubMed]
- Schatz M, Camargo CA Jr. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol 2003;91:553-8. [Crossref] [PubMed]
- DiFiore JW, Wilson JM. Lung development. Semin Pediatr Surg 1994;3:221-32.
- Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 2012;33:1-47. [Crossref] [PubMed]
- Silveyra P, Fuentes N, Rodriguez Bauza DE. Sex and Gender Differences in Lung Disease. Adv Exp Med Biol 2021;1304:227-58. [Crossref] [PubMed]
- Chu AJ, Rooney SA. Estrogen stimulation of surfactant synthesis. Pediatr Pulmonol 1985;1:S110-4.
- Patrone C, Cassel TN, Pettersson K, et al. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol 2003;23:8542-52. [Crossref] [PubMed]
- Gortner L, Shen J, Tutdibi E. Sexual dimorphism of neonatal lung development. Klin Padiatr 2013;225:64-9. [Crossref] [PubMed]
- Hernandez L, Laucyte-Cibulskiene A, Ward LJ, et al. Gender dimension in cardio-pulmonary continuum. Front Cardiovasc Med 2022;9:916194. [Crossref] [PubMed]
- Chabbert Buffet N, Djakoure C, Maitre SC, et al. Regulation of the human menstrual cycle. Front Neuroendocrinol 1998;19:151-86. [Crossref] [PubMed]
- Rao CK, Moore CG, Bleecker E, et al. Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the Severe Asthma Research Program. Chest 2013;143:984-92. [Crossref] [PubMed]
- van den Berge M, Heijink HI, van Oosterhout AJ, et al. The role of female sex hormones in the development and severity of allergic and non-allergic asthma. Clin Exp Allergy 2009;39:1477-81. [Crossref] [PubMed]
- Triebner K, Johannessen A, Puggini L, et al. Menopause as a predictor of new-onset asthma: A longitudinal Northern European population study. J Allergy Clin Immunol 2016;137:50-57.e6. [Crossref] [PubMed]
- Koper I, Hufnagl K, Ehmann R. Gender aspects and influence of hormones on bronchial asthma - Secondary publication and update. World Allergy Organ J 2017;10:46. [Crossref] [PubMed]
- Ito C, Okuyama-Dobashi K, Miyasaka T, et al. CD8+ T Cells Mediate Female-Dominant IL-4 Production and Airway Inflammation in Allergic Asthma. PLoS One 2015;10:e0140808. [Crossref] [PubMed]
- Nejatbakhsh Samimi L, Fallahpour M, Khoshmirsafa M, et al. The impact of 17β-estradiol and progesterone therapy on peripheral blood mononuclear cells of asthmatic patients. Mol Biol Rep 2021;48:297-306. [Crossref] [PubMed]
- Andersson A, Grahnemo L, Engdahl C, et al. IL-17-producing γδT cells are regulated by estrogen during development of experimental arthritis. Clin Immunol 2015;161:324-32. [Crossref] [PubMed]
- Newcomb DC, Cephus JY, Boswell MG, et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol 2015;136:1025-34.e11. [Crossref] [PubMed]
- Fuseini H, Yung JA, Cephus JY, et al. Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation. J Immunol 2018;201:1843-54. [Crossref] [PubMed]
- Cephus JY, Gandhi VD, Shah R, et al. Estrogen receptor-α signaling increases allergen-induced IL-33 release and airway inflammation. Allergy 2021;76:255-68. [Crossref] [PubMed]
- Miyasaka T, Dobashi-Okuyama K, Kawakami K, et al. Sex Plays a Multifaceted Role in Asthma Pathogenesis. Biomolecules 2022;12:650. [Crossref] [PubMed]
- Bengtsson AK, Ryan EJ, Giordano D, et al. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood 2004;104:1404-10. [Crossref] [PubMed]
- Masuda C, Miyasaka T, Kawakami K, et al. Sex-based differences in CD103(+) dendritic cells promote female-predominant Th2 cytokine production during allergic asthma. Clin Exp Allergy 2018;48:379-93. [Crossref] [PubMed]
- Xiu F, Anipindi VC, Nguyen PV, et al. High Physiological Concentrations of Progesterone Reverse Estradiol-Mediated Changes in Differentiation and Functions of Bone Marrow Derived Dendritic Cells. PLoS One 2016;11:e0153304. [Crossref] [PubMed]
- Tam A, Wadsworth S, Dorscheid D, et al. Estradiol increases mucus synthesis in bronchial epithelial cells. PLoS One 2014;9:e100633. [Crossref] [PubMed]
- Townsend EA, Meuchel LW, Thompson MA, et al. Estrogen increases nitric-oxide production in human bronchial epithelium. J Pharmacol Exp Ther 2011;339:815-24. [Crossref] [PubMed]
- Jain R, Ray JM, Pan JH, et al. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol 2012;46:446-53. [Crossref] [PubMed]
- Massaro D, Massaro GD. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L866-70. [Crossref] [PubMed]
- Cephus JY, Stier MT, Fuseini H, et al. Testosterone Attenuates Group 2 Innate Lymphoid Cell-Mediated Airway Inflammation. Cell Rep 2017;21:2487-99. [Crossref] [PubMed]
- Yu CK, Liu YH, Chen CL. Dehydroepiandrosterone attenuates allergic airway inflammation in Dermatophagoides farinae-sensitized mice. J Microbiol Immunol Infect 2002;35:199-202.
- Laffont S, Blanquart E, Savignac M, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med 2017;214:1581-92. [Crossref] [PubMed]
- Blanquart E, Mandonnet A, Mars M, et al. Targeting androgen signaling in ILC2s protects from IL-33-driven lung inflammation, independently of KLRG1. J Allergy Clin Immunol 2022;149:237-251.e12. [Crossref] [PubMed]
- Miyasaka T, Dobashi-Okuyama K, Takahashi T, et al. The interplay between neuroendocrine activity and psychological stress-induced exacerbation of allergic asthma. Allergol Int 2018;67:32-42. [Crossref] [PubMed]
- Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol (1985) 2010;108:729-34. [Crossref] [PubMed]
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 2018;141:1169-79. [Crossref] [PubMed]
- Miethe S, Karsonova A, Karaulov A, et al. Obesity and asthma. J Allergy Clin Immunol 2020;146:685-93. [Crossref] [PubMed]
- Lu FL, Johnston RA, Flynt L, et al. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L856-65. [Crossref] [PubMed]
- Sood A, Shore SA. Adiponectin, Leptin, and Resistin in Asthma: Basic Mechanisms through Population Studies. J Allergy (Cairo) 2013;2013:785835. [Crossref] [PubMed]
- Walter RE, Beiser A, Givelber RJ, et al. Association between glycemic state and lung function: the Framingham Heart Study. Am J Respir Crit Care Med 2003;167:911-6. [Crossref] [PubMed]
- Wood DM, Brennan AL, Philips BJ, et al. Effect of hyperglycaemia on glucose concentration of human nasal secretions. Clin Sci (Lond) 2004;106:527-33. [Crossref] [PubMed]
- Russo D, Lizzi M, Di Filippo P, et al. Time-Specific Factors Influencing the Development of Asthma in Children. Biomedicines 2022;10:758. [Crossref] [PubMed]
- Sordillo JE, Zhou Y, McGeachie MJ, et al. Factors influencing the infant gut microbiome at age 3-6 months: Findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol 2017;139:482-491.e14. [Crossref] [PubMed]
- Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol 2013;9:15. [Crossref] [PubMed]
- Feng M, Yang Z, Pan L, et al. Associations of Early Life Exposures and Environmental Factors With Asthma Among Children in Rural and Urban Areas of Guangdong, China. Chest 2016;149:1030-41. [Crossref] [PubMed]
- Yang Z, Chen Z, Lin X, et al. Rural environment reduces allergic inflammation by modulating the gut microbiota. Gut Microbes 2022;14:2125733. [Crossref] [PubMed]
- Luo G, Nkoy FL, Stone BL, et al. A systematic review of predictive models for asthma development in children. BMC Med Inform Decis Mak 2015;15:99. [Crossref] [PubMed]
- Chinese Thoracic Society Asthma Group. Chinese expert consensus on the use of Omalizumab in allergic asthma (2021 version). Zhonghua Jie He He Hu Xi Za Zhi 2022;45:341-54. [Crossref] [PubMed]
- Calcaterra V, Nappi RE, Farolfi A, et al. Perimenstrual Asthma in Adolescents: A Shared Condition in Pediatric and Gynecological Endocrinology. Children (Basel) 2022;9:233. [Crossref] [PubMed]
- McCleary N, Nwaru BI, Nurmatov UB, et al. Endogenous and exogenous sex steroid hormones in asthma and allergy in females: A systematic review and meta-analysis. J Allergy Clin Immunol 2018;141:1510-1513.e8. [Crossref] [PubMed]
- Withers AL, Green R. Transition for Adolescents and Young Adults With Asthma. Front Pediatr 2019;7:301. [Crossref] [PubMed]
- Tong X, Guo T, Liu S, et al. Macrolide antibiotics for treatment of asthma in adults: a meta-analysis of 18 randomized controlled clinical studies. Pulm Pharmacol Ther 2015;31:99-108. [Crossref] [PubMed]
- Vrieze A, Postma DS, Kerstjens HA. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol 2003;112:271-82. [Crossref] [PubMed]
- Eliasson O, Scherzer HH, DeGraff AC Jr. Morbidity in asthma in relation to the menstrual cycle. J Allergy Clin Immunol 1986;77:87-94. [Crossref] [PubMed]
- Hamano N, Terada N, Maesako K, et al. Effect of sex hormones on eosinophilic inflammation in nasal mucosa. Allergy Asthma Proc 1998;19:263-9. [Crossref] [PubMed]
- Matteis M, Polverino F, Spaziano G, et al. Effects of sex hormones on bronchial reactivity during the menstrual cycle. BMC Pulm Med 2014;14:108. [Crossref] [PubMed]
- Agarwal AK, Shah A. Menstrual-linked asthma. J Asthma 1997;34:539-45. [Crossref] [PubMed]
- Pereira Vega A, Sánchez Ramos JL, Maldonado Pérez JA, et al. Variability in the prevalence of premenstrual asthma. Eur Respir J 2010;35:980-6. [Crossref] [PubMed]
- Shames RS, Heilbron DC, Janson SL, et al. Clinical differences among women with and without self-reported perimenstrual asthma. Ann Allergy Asthma Immunol 1998;81:65-72. [Crossref] [PubMed]
- Gina global strategy for asthma management and prevention (2022updated). Available online: https://ginasthma.org/
- Chandler MH, Schuldheisz S, Phillips BA, et al. Premenstrual asthma: the effect of estrogen on symptoms, pulmonary function, and beta 2-receptors. Pharmacotherapy 1997;17:224-34.
- Ensom MH, Chong E, Carter D. Premenstrual symptoms in women with premenstrual asthma. Pharmacotherapy 1999;19:374-82. [Crossref] [PubMed]
- Murray RD, New JP, Barber PV, et al. Gonadotrophin-releasing hormone analogues: a novel treatment for premenstrual asthma. Eur Respir J 1999;14:966-7. [Crossref] [PubMed]
- Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ 2001;323:776-80. [Crossref] [PubMed]
- Ensom MH, Chong G, Zhou D, et al. Estradiol in premenstrual asthma: a double-blind, randomized, placebo-controlled, crossover study. Pharmacotherapy 2003;23:561-71. [Crossref] [PubMed]
- Beynon HL, Garbett ND, Barnes PJ. Severe premenstrual exacerbations of asthma: effect of intramuscular progesterone. Lancet 1988;2:370-2. [Crossref] [PubMed]
- Pasaoglu G, Mungan D, Abadoglu O, et al. Leukotriene receptor antagonists: a good choice in the treatment of premenstrual asthma? J Asthma 2008;45:95-9. [Crossref] [PubMed]
- Martinez-Moragón E, Plaza V, Serrano J, et al. Near-fatal asthma related to menstruation. J Allergy Clin Immunol 2004;113:242-4. [Crossref] [PubMed]
- Asthma group of Chinese Throacic Society. Guidelines for bronchial asthma prevent and management (2020 edition) Asthma group of Chinese Throacic Society. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:1023-48. [Crossref] [PubMed]
- Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol 2003;13:317-24. [Crossref] [PubMed]
- Murphy VE, Schatz M. Asthma in pregnancy: a hit for two. Eur Respir Rev 2014;23:64-8. [Crossref] [PubMed]
- Hodyl NA, Stark MJ, Scheil W, et al. Perinatal outcomes following maternal asthma and cigarette smoking during pregnancy. Eur Respir J 2014;43:704-16. [Crossref] [PubMed]
- Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011;118:1314-23. [Crossref] [PubMed]
- Stenius-Aarniala BS, Hedman J, Teramo KA. Acute asthma during pregnancy. Thorax 1996;51:411-4. [Crossref] [PubMed]
- Huo X, Chu S, Hua L, et al. The effect of breastfeeding on the risk of asthma in high-risk children: a case-control study in Shanghai, China. BMC Pregnancy Childbirth 2018;18:341. [Crossref] [PubMed]
- Wang H, Li N, Huang H. Asthma in Pregnancy: Pathophysiology, Diagnosis, Whole-Course Management, and Medication Safety. Can Respir J 2020;2020:9046842. [Crossref] [PubMed]
- Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol 1988;81:509-17.
- Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax 2006;61:169-76. [Crossref] [PubMed]
- Robijn AL, Bokern MP, Jensen ME, et al. Risk factors for asthma exacerbations during pregnancy: a systematic review and meta-analysis. Eur Respir Rev 2022;31:220039. [Crossref] [PubMed]
- Murphy VE, Powell H, Wark PAB, et al. A prospective study of respiratory viral infection in pregnant women with and without asthma. Chest 2013;144:420-7. [Crossref] [PubMed]
- Pfaller B, Bendien S, Ditisheim A, et al. Management of allergic diseases in pregnancy. Allergy 2022;77:798-811. [Crossref] [PubMed]
- Majou D, Moreira B, Martin C, et al. Safety of Omalizumab During Pregnancy and Breast-Feeding With Assessment of Placental Transfer: A Case Report. Allergy Asthma Immunol Res 2021;13:515-6. [Crossref] [PubMed]
- National Heart, Lung, and Blood Institute. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol 2005;115:34-46. [Crossref] [PubMed]
- Matulonga-Diakiese B, Courbon D, Fournier A, et al. Risk of asthma onset after natural and surgical menopause: Results from the French E3N cohort. Maturitas 2018;118:44-50. [Crossref] [PubMed]
- Triebner K, Matulonga B, Johannessen A, et al. Menopause Is Associated with Accelerated Lung Function Decline. Am J Respir Crit Care Med 2017;195:1058-65. [Crossref] [PubMed]
- Foschino Barbaro MP, Costa VR, Resta O, et al. Menopausal asthma: a new biological phenotype? Allergy 2010;65:1306-12. [Crossref] [PubMed]
- Barr RG, Wentowski CC, Grodstein F, et al. Prospective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary disease. Arch Intern Med 2004;164:379-86. [Crossref] [PubMed]
- Troisi RJ, Speizer FE, Willett WC, et al. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med 1995;152:1183-8. [Crossref] [PubMed]
- Jarvis D, Leynaert B. The association of asthma, atopy and lung function with hormone replacement therapy and surgical cessation of menstruation in a population-based sample of English women. Allergy 2008;63:95-102. [Crossref] [PubMed]
- Romieu I, Fabre A, Fournier A, et al. Postmenopausal hormone therapy and asthma onset in the E3N cohort. Thorax 2010;65:292-7. [Crossref] [PubMed]
- Hansen ESH, Aasbjerg K, Moeller AL, et al. Hormone Replacement Therapy and Development of New Asthma. Chest 2021;160:45-52. [Crossref] [PubMed]
- Shah SA, Tibble H, Pillinger R, et al. Hormone replacement therapy and asthma onset in menopausal women: National cohort study. J Allergy Clin Immunol 2021;147:1662-70. [Crossref] [PubMed]
- Nwaru BI, Simpson CR, Soyiri IN, et al. Exogenous sex steroid hormones and asthma in females: protocol for a population-based retrospective cohort study using a UK primary care database. BMJ Open 2018;8:e020075. [Crossref] [PubMed]
- Real FG, Svanes C, Omenaas ER, et al. Lung function, respiratory symptoms, and the menopausal transition. J Allergy Clin Immunol 2008;121:72-80.e3. [Crossref] [PubMed]
- Park SY, Kim JH, Kim HJ, et al. High Prevalence of Asthma in Elderly Women: Findings From a Korean National Health Database and Adult Asthma Cohort. Allergy Asthma Immunol Res 2018;10:387-96. [Crossref] [PubMed]
- Balzano G, Fuschillo S, De Angelis E, et al. Persistent airway inflammation and high exacerbation rate in asthma that starts at menopause. Monaldi Arch Chest Dis 2007;67:135-41. [Crossref] [PubMed]
- Nong Y, Lin JT. Strengthen the management of asthma in the elderly and strive to improve the prognosis of the disease. Zhonghua Yi Xue Za Zhi 2021;101:1047-9. [Crossref] [PubMed]
- Freitas PD, Ferreira PG, Silva AG, et al. The Role of Exercise in a Weight-Loss Program on Clinical Control in Obese Adults with Asthma. A Randomized Controlled Trial. Am J Respir Crit Care Med 2017;195:32-42. [Crossref] [PubMed]
- Scott HA, Wood LG, Gibson PG. Role of Obesity in Asthma: Mechanisms and Management Strategies. Curr Allergy Asthma Rep 2017;17:53. [Crossref] [PubMed]
- Obesity tied to higher asthma prevalence in women. Medscape.Mar 17, 2016 Available online: https://www.medscape.com/viewarticle/860517?form=fpf
- Assad N, Qualls C, Smith LJ, et al. Body mass index is a stronger predictor than the metabolic syndrome for future asthma in women. The longitudinal CARDIA study. Am J Respir Crit Care Med 2013;188:319-26. [Crossref] [PubMed]
- Tarazona-Meza CE, Hanson C, Pollard SL, et al. Dietary patterns and asthma among Peruvian children and adolescents. BMC Pulm Med 2020;20:63. [Crossref] [PubMed]
- Kim EK, Ju SY. Asthma and Dietary Intake of Fish, Seaweeds, and Fatty Acids in Korean Adults. Nutrients 2019;11:2187. [Crossref] [PubMed]
- Tagliaferro AR, Windt MR, Ronan AM, et al. Obesity, dietary arachadonic acid and risk of allergic disease in women. The FASEB Journal 2006;20:A603.
- Ait-Hadad W, Bédard A, Chanoine S, et al. Healthy diet associated with better asthma outcomes in elderly women of the French Asthma-E3N study. Eur J Nutr 2022;61:2555-69. [Crossref] [PubMed]
- Vieira VJ, Ronan AM, Windt MR, et al. Elevated atopy in healthy obese women. Am J Clin Nutr 2005;82:504-9. [Crossref] [PubMed]
- Forno E, Han YY, Libman IM, et al. Adiposity and Asthma in a Nationwide Study of Children and Adults in the United States. Ann Am Thorac Soc 2018;15:322-30. [Crossref] [PubMed]
- Luthe SK, Hirayama A, Goto T, et al. Association Between Obesity and Acute Severity Among Patients Hospitalized for Asthma Exacerbation. J Allergy Clin Immunol Pract 2018;6:1936-1941.e4. [Crossref] [PubMed]
- Pate CA, Zahran HS, Bailey CM. Impaired health-related quality of life and related risk factors among US adults with asthma. J Asthma 2019;56:431-9. [Crossref] [PubMed]
- Stanescu S, Kirby SE, Thomas M, et al. A systematic review of psychological, physical health factors, and quality of life in adult asthma. NPJ Prim Care Respir Med 2019;29:37. [Crossref] [PubMed]
- Klepaker G, Svendsen MV, Hertel JK, et al. Influence of Obesity on Work Ability, Respiratory Symptoms, and Lung Function in Adults with Asthma. Respiration 2019;98:473-81. [Crossref] [PubMed]
- Fenger RV, Gonzalez-Quintela A, Vidal C, et al. Exploring the obesity-asthma link: do all types of adiposity increase the risk of asthma? Clin Exp Allergy 2012;42:1237-45. [Crossref] [PubMed]
- Oka S, Goto T, Hirayama A, et al. Association of obstructive sleep apnea with severity of patients hospitalized for acute asthma. Ann Allergy Asthma Immunol 2020;124:165-170.e4. [Crossref] [PubMed]
- Peters-Golden M, Swern A, Bird SS, et al. Influence of body mass index on the response to asthma controller agents. Eur Respir J 2006;27:495-503. [Crossref] [PubMed]
- Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med 2007;101:2240-7. [Crossref] [PubMed]
- Ilmarinen P, Pardo A, Tuomisto LE, et al. Long-term prognosis of new adult-onset asthma in obese patients. Eur Respir J 2021;57:2001209. [Crossref] [PubMed]
- Guerron AD, Ortega CB, Lee HJ, et al. Asthma medication usage is significantly reduced following bariatric surgery. Surg Endosc 2019;33:1967-75. [Crossref] [PubMed]
- Forno E, Zhang P, Nouraie M, et al. The impact of bariatric surgery on asthma control differs among obese individuals with reported prior or current asthma, with or without metabolic syndrome. PLoS One 2019;14:e0214730.
- Yerevanian A, Soukas AA. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr Obes Rep 2019;8:156-64. [Crossref] [PubMed]
- Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021;384:989-1002. [Crossref] [PubMed]
- Rastogi D. Evidence Builds for a Role of Metformin in Asthma Management. Ann Am Thorac Soc 2019;16:1497-9. [Crossref] [PubMed]
- Rabe KF, Nair P, Brusselle G, et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N Engl J Med 2018;378:2475-85. [Crossref] [PubMed]
- Zein JG, Erzurum SC. Asthma is Different in Women. Curr Allergy Asthma Rep 2015;15:28. [Crossref] [PubMed]
- Senna G, Latorre M, Bugiani M, et al. Sex Differences in Severe Asthma: Results From Severe Asthma Network in Italy-SANI. Allergy Asthma Immunol Res 2021;13:219-28. [Crossref] [PubMed]
- Wang E, Wechsler ME, Tran TN, et al. Characterization of Severe Asthma Worldwide: Data From the International Severe Asthma Registry. Chest 2020;157:790-804. [Crossref] [PubMed]
- Benoni R, Panunzi S, Batani V, et al. Clinical response to biologicals for severe asthma: any relevance for sex in different age ranges? ERJ Open Res 2022;8:00670-2021. [Crossref] [PubMed]
- Tidemandsen C, Halvard Hansen ES, Rasmussen SM, et al. Unique Aspects of Asthma in Women. Clin Chest Med 2021;42:497-506. [Crossref] [PubMed]


