
Children’s lung function in relation to changes in socioeconomic, nutritional, and household factors over 20 years in Lanzhou
Introduction
Lung function tests using standard spirometry have been useful in the care of children with chronic respiratory diseases, including asthma, cystic fibrosis, and interstitial lung diseases (1). Lung function assesses diagnosis, follow-up, monitoring, and objective determination of the children’s response to interventions. Lung function parameters are, however, affected by various factors, including anthropometric, nutritional status, ambient environment, and sociodemographic factors (2). People, particularly women and children, spend most of their time indoors, which makes these groups more susceptible to household air pollution (HAP). Lung function has been used to assess the health impact of HAP (3,4). Pre-clinical studies of lung and respiratory tract cell lines have established the adverse effects of particulate matter (PM) emissions from biomass combustion (5). The place of domicile and the socio-economic class that children belong to could be a surrogate for the level and duration of exposure to PM and noxious gases both outdoors and indoors. These factors may affect lung function of children (6-8). Associations between low socio-economic status (SES) and impaired lung function both in children and adults have been studied mainly in European countries and North America (9,10).
Urban environments can also adversely affect health. This effect can be ascribed to several factors in addition to air pollution, including dietary habits, socioeconomic status, infections, stress, allergic exposure, and parasitic infections (11). Inner-city children appear to have more episodes of acute respiratory infection than those living in rural areas (11) and lung function growth rate is higher in rural children than urban children (12), although this effect could be reversible (13).
Many of these factors potentially affecting lung function have changed substantially in the past decades in Chinese cities experiencing rapid economic growth and urban expansion. Between 1993–1996, a cohort of elementary school children in Lanzhou performed lung function tests and were and surveyed for their family’s socioeconomic status and household environmental conditions. To assess whether there are differences in children’s lung function between then and more recent years, we conducted a follow-up study in children of the same age range living in the same areas of the city. In this paper, we aim to compare children’s lung function between Period 1 (1996) and Period 2 (2017) and to explore the relative importance of an urban-rural setting and other potential risk factors for lung function. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-20-2232).
Methods
Site selection and subject recruitment
The two cross-sectional investigations took place in 1996 (Period 1) and 2017 (Period 2). Spirometry tests were conducted among children living in Lanzhou, a typical industrial city in northwestern China. The municipality of this provincial capital of Gansu is comprised of 5 districts including Chengguan, Qilihe, Xigu, Anning, and Honggu, covering an area of approximately 13,085 km2, with a population of about 3.7 million in 2017. Two primary schools were randomly selected from the urban district of Chengguan and the suburban district of Xigu, respectively. Children at these schools were enrolled into the study, upon their parents having signed a written consent form to voluntarily participate in a questionnaire survey and lung function tests. Children in grades 2 to 5 were randomly selected by gender and age for lung function tests. A total of 1,418 children underwent spirometry, 734 in Period 1 (53.1% urban; 50.9% male) and 684 in Period 2 (28.1% urban; 54.2% male). We excluded children from the study if they had lived at their current home for less than three years. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Biomedicine Research, Duke Kunshan University (No. FWA00021580).
Questionnaire survey
Questionnaires were filled out by children’s parents to collect information on parental smoking habits, whether children sleep in own beds, whether children sleep in their own rooms, the style of kitchen, fuel for cooking, types of kitchen ventilators, parental occupation, and parental education levels. In the same year as the questionnaire survey, children performed spirometry in their schools. The questionnaire used in Period 1 was adapted from the American Thoracic Society (ATS) questionnaire (14). The questionnaire used in Period 2 maintained the same questions as long as they were still relevant (e.g., what became irrelevant).
Variables associated with housing factors are defined as, parental smoking [or environmental tobacco smoke (ETS)], sleeping in own room, sleeping in own bed, kitchen style, coal fuel use, and ventilation use. District refers to urban and suburban. ETS was defined as ETS at home, including paternal and maternal smoking. Kitchen style refers to separated and open kitchen types. Coal fuel use was defined from the question “What was the main fuel type for cooking?” and “What was the main fuel type for heating?” Ventilation use was defined by the “Yes” answer to the question “Was there a ventilation device in the kitchen when cooking?”
Distribution of indoor air pollution related variables (i.e., parental smoking, kitchen style, coal use for cooking and ventilation use) and socioeconomic factors (parental occupation and education) across the different studies were explored. We combined information on coal use for cooking and that for heating to create a binary variable: household coal use (yes vs. no). Similarly, the presence of all ventilation devices (chimney, hood, and exhaust fan) was collapsed to create a binary variable: ventilation use (yes vs. no). We combine parental information on education to make a binary variable: below senior high school vs. with or above high school. Parental information on occupation was classified by the manual laborer (e.g., farmers, factory workers and plasterer) versus nonmanual laborer (e.g., teachers, governmental office workers and doctor).
Anthropometric measurements
Children were measured for their body weight using a weighing scale (Jiangsu Suhong Medical Equipment Co. Ltd., China) to the nearest 0.5 kg and height using an RGZ-120 stadiometer (Jiangsu Suhong Medical Equipment Co. Ltd., China) to the closest 0.5 cm. Each study participant stood erect without shoes on the stadiometer. The children were required to put their feet together with their heels, buttocks, and occiput touching the wall while they look straight ahead to ensure accurate measurement of their height. Body mass index (BMI) in kg/m2 was calculated from body weight and height. The evaluation criteria for stunting in children used in the present study followed the WHO 2006 definition (15). The National Health Commission of the People’s Republic of China also issued the latest child development standards in 2018 (16). Height for age and sex measures were classified as no stunting (z-scores ≥−2SD) and stunting (z-scores <−2SD) (17). The evaluation criteria for overweight and obesity in teenagers (6–18 years) refer to the “Screening for overweight and obesity among school-age children and adolescents”, published by The National Health Commission of the People’s Republic of China (18). For example, for 6-year-old boys, they were overweight if having a BMI between 16.4 and 17.7 kg/m2 and obesity if having a BMI ≥17.7 kg/m2.
Measurement of lung function
The standardized spirometry protocol of the ATS was followed to measure lung function using computerized Warren E. Collins Survey II 8-liter water-seal volume Spirometers in Period 1 and Spiro-lab MIR, Italy in Period 2 by trained technicians (1). Children performed spirometry in the standing position in a dedicated room at each school. For this analysis, we assessed two lung function indices including forced vital capacity (FVC, mL) and the forced expiratory volume in one second (FEV1, mL). For each participant, the spirometry test was repeated (up to five times) until acceptable, reproducible flow-volume loops were generated (1). Between one test and the next, the device evaluates the repeatability of the following parameters: repeatable when the difference between the two highest FVC value is ≤150 mL and the FEV1 value is ≤150 mL. Acceptable spirograms were defined as a smooth flow-volume curve without artifacts and satisfactory exhalation of forced expiratory duration >6 s (3 s for children younger than 10 years). If the difference between the two largest FVC values were within 150 mL, the test was concluded. The highest acceptable values of FVC and FEV1 were selected for statistical analysis.
Statistical methods
Descriptive analysis was utilized to characterize the study sample and lung function indices. In each study group, the mean ± SD was calculated for continuous variables, and frequencies (percentage) were calculated for categorical variables. Differences in lung function (continuous variable) between categorical variables (e.g., gender) or between the two study periods were analyzed using Student’s t-test, whereas categorical variables between two study periods were analyzed using Pearson’s Chi-square test and Fisher’s exact test, as appropriate. Spearman’s rank correlation tests were carried out to evaluate the relationship between anthropometric measurements and lung function indices.
We primarily examined the overall associations between residential location (urban vs. suburban), as well as overweight or obesity (overweight vs. normal; obesity vs. normal), and lung function parameters during Periods 1 and 2, respectively. Generalized linear regression models (GLM) were used to assess changes of lung function and their 95% confidence intervals (95% CIs) associated with the residential location and overweight or obesity in Periods 1 and 2. Age, height, weight, gender, and other socioeconomic and household factors were controlled in the models. In addition, we performed a sensitivity analysis to examine the associations between residential location/overweight/obesity and lung function among girls and boys separately. The GLM was conducted using SPSS package (version 22, IBM Inc., Armonk, New York, NY, USA). We tested the significance of all the statistics at a significance level of 0.05, and calculated 95% CIs.
Results
Study participants
A total of 1,418 (734 Period 1 and 684 Period 2) school children took part in the study. The mean (SD) age of school children was 10.2 (1.7) years in Period 1 and 9.4 (1.3) years in Period 2. There was no significant difference in the gender of the children in Period 1 (51.0% male) and Period 2 (54.2% male). Likewise, no difference was found in the breastfeeding rate. We included more urban participants in Period 1 (53.1%) compared to those in Period 2 (28.1%).
Difference in SES and household environmental factors between the two periods
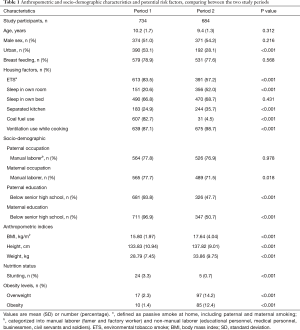
Details about household characteristics and socioeconomic level are shown in Table 1. The proportion of children with ETS exposure (paternal or maternal smoking) was significantly greater in Period 1 (83.5%) than in Period 2 (57.2%). More families used coal as cooking and/or heating fuel in Period 1 than Period 2 (82.7% versus 4.5%). Using a ventilation device during cooking was more common in Period 2 (98.7%) than in Period 1 (87.1%). A higher proportion of children lived in overcrowded homes in Period 1 than in Period 2, for example, 20.6% of children slept in their own rooms in Period 1 versus 52.0% in Period 2. Fewer homes had closed kitchens in Period 1 (24.9%) than in Period 2 (35.7%).
Full table
The proportion of manual laborers among children’s fathers was unchanged from Period 1 to Period 2, whereas the proportion among children’s mothers was decreased from 77.7% in Period 1 to 71.5% in Period 2 (P=0.018). The proportions of lower educational attainment (below high school) in both fathers and mothers were significantly smaller in Period 2 than in Period 1 (see Table 1).
Nutritional status, anthropometric indices and lung function
The Periods 1–2 disparities in anthropometric indices, nutritional factors, and lung function parameters are presented in Tables 1,2. The mean (SD) weight of the 734 schoolchildren of Period 1 was 28.79 (7.45) kg, ranging from 16.0 to 63.8 kg. Their height ranged from 107.00 to 167.00 cm with a mean (SD) height of 133.83 (10.94) cm. Children of Period 2 were heavier and taller than Period 1 participants. Likewise, their BMI was significantly higher than that of the children in Period 1 (Table 1). Considering nutritional status, more children in Period 1 were stunted (P<0.001) compared to Period 2. Greater proportions of children in Perion 2 were overweight and obese in comparison to the children of Period 1 (P<0.001).

Full table
In Period 1, the mean (SD) FVC of the school children was 2,516.4 (635.3) mL, ranging from 1,092 to 4,932 mL. The mean (SD) FEV1 was 2,098.9 (549.6) mL with arrange from 898 to 4,098 mL. In Period 2, the mean (SD) FVC of the participants was 1,933.4 (432.7) mL, ranging from 820 to 4,010 mL. The mean (SD) FEV1 was 1,740.5 (374.6) mL, which ranged from 820 to 3,080 mL. Both the mean FEV1 and mean FVC were significantly higher in Period 1 children than those of the Period 2 counterparts. The differences between the two periods remained statistically significant in gender stratified comparisons (Table 2).
Association of lung function with anthropometric indices, socio-demographic variables, household environmental factors, and nutritional status
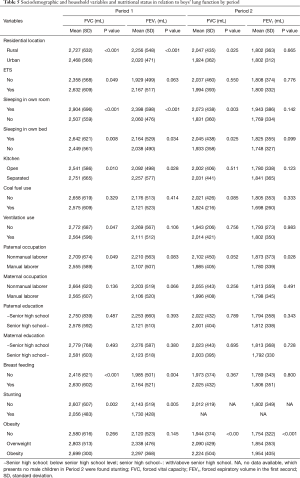
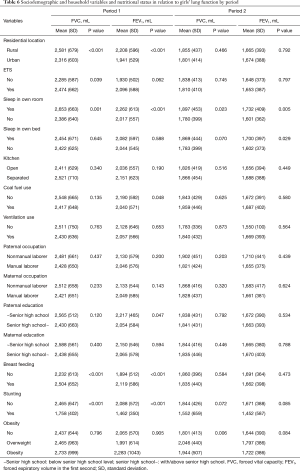
In addition to age, anthropometric parameters like weight, height, and BMI were positively correlated with FEV1 and FVC of the children (P<0.05) (Tables 3,4). Both FEV1 and FVC had stronger correlations with age, weight, height, and BMI among the Period 1 children than among the Period 2 children.

Full table

Full table
Associations of lung function with sociodemographic variables, household factors, nutritional status in two periods are presented for boys in Table 5 and for girls in Table 6. In Period 1, boys and girls from the urban area had significantly lower FVC and FEV1 than those from the suburban area. For example, boys had mean (SD) FVC of 2,468 (566) in the urban vs. 2,727 (632) in the suburban area (P<0.001) and mean (SD) FEV1 of 2,020 (471) vs. 2,256 (548) (P<0.001). Children from overcrowded homes (e.g., shared room and shared bed) had lower FVC and FEV1 (P<0.05) than those who slept in their own bed/room. Boys from open kitchen families had significantly lower FVC and FEV1 than those from separated kitchen families.
Full table
Full table
Boys with paternal non-manual laborer occupation had significantly higher FVC and FEV1 than those with paternal manual laborer occupation. Stunting was significantly associated with lower FVC and FEV1 among boys from Period 1 [mean (SD) FVC 2,056 (483) vs. 2,607 (607) mL; P=0.002) and mean (SD) FEV1 1,730 (428) vs. 2,143 (519) mL; P=0.005)]. A similar effect of stunting was also observed in Period 1 girls. Boys and girls who were breastfed had significantly higher FVC and FEV1 than those never breastfed. In Period 2, overweight and obese boys (but not girls) had a significantly higher FVC and FEV1 than those with normal BMI from unadjusted analysis.
Effects of residential location and overweight/obesity on lung function in Period 1 and Period 2
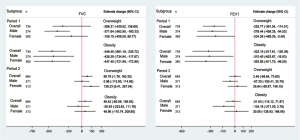
Further multivariate regression analysis of residential location and overweight/obesity effect on lung function was conducted. Significant effects of living in the urban area on FVC and FEV1 were observed among male and female participants in Period 2 but not in Period 1 (Figure 1). After adjusting for potential confounding variables, we estimated that living in the urban area was associated with a −223.8 mL (95% CI: −294.0, −153.6 mL) reduction in FVC and an −89.7 mL (95% CI: −150.1, −29.4 mL) reduction in FEV1 among the overall children during Period 2 (Figure 1). The largest differences were observed for boys’ FVC (−245.0 mL; 95% CI: −339.2, −150.8 mL) and for girls’ FEV1 (−109.8 mL; 95% CI: −203.4, −16.2 mL).

Due to the low prevalence of reported stunting during Period 2, no further analysis was conducted for the effects of stunting. The declines of lung function, after adjusting for height, weight and variables in Table 1, in overweight and obese children were significant in Period 1 but not in Period 2 (Figure 2). We estimated an FVC reduction of −371.64 mL (95% CI: −562.95, −180.33 mL) in overweight boys compared with normal weight boys in Period 1. The FVC reduction was observed in girls as well, although statistical significance was not reached. For FEV1, a significant reduction was seen for both boys and girls in Period 1. Significant effects of obesity on lung function were observed among children in Period 1. Multivariable linear regression analysis confirmed an estimate change of −448.45 (95% CI, −661.19, −235.72) and −323.10 (95% CI: −517.81, −128.39) in Period 1 for FVC and FEV1, respectively. Negative associations between obesity and lung function were more noticeable in girls during Period 1, with an estimated reduction of −447.45 (95% CI: −721.99, −172.90) for FVC and −353.85 (95% CI: −611.70, −96.00) for FEV1.
Discussion
In the current analysis, we investigated the differences in lung function and associations with socio-economic, nutritional, and housing (household) factors between school children measured in 2017 (Period 2) and school children measured in 1996 (Period 1). Both cohorts of children had the same age range and lived in the same areas of Lanzhou. Socioeconomic level, household, and individual characteristics changed across the two study periods, as evidenced by substantial prevalence changes in ETS, room crowdedness, coal fuel use, cooking ventilation conditions, parental education attainment, overweight, and obesity. FVC and FEV1 of boys and girls were all lower in the 2017 cohort than in the 1996 cohort. We observed an adverse effect of living in the urban area on FVC and FEV1 during Period 2, after adjusting for potential confounders and in gender stratified analyses. Overweight and obesity were each significantly associated with lower FEV1 in both boys and girls and with FVC in boys in Period 1.
Lung function depends on a variety of individual, behavioral and environmental factors (19-22). It is evident that these characteristics changed in China across two decades. Therefore, it is expected that lung function values would have changed with changing influencing factors (23). Some previous studies reported the changes of FVC and FEV1 in children in Chinese cities, which present confusing trends in different cities in recent decades (24-26). Although differences in equipment or methodology may explain some of these variations, much of it appears to be real and must be a result of other causes such as socioeconomic changes. We found decreased lung function in Lanzhou across two cross-sectional study periods separated by more than twenty years (Table 2).
Compared with a survey conducted in 24 centers in Northeast, North, Northwest, Southwest, South, East, and Central China, the percentage differences of previous Chinese studies ranged from −17.8% to 11.4%, which were found to significantly overestimate or underestimate lung function (27). This discrepancy in the temporal trend of lung function might be partly due to the sample size, age ranges, small local regions, as well as different study protocols and quality control. A previous study in Hong Kong reported spirometric values of this population have increased compared to Chinese populations of similar sex, age, and height two decades ago (28). All measured lung function parameters were strongly correlated with age. Among study subjects of the Hong Kong study (28), the average age was greater than our study.
This study demonstrated significantly smaller lung volumes and capacities (FVC and FEV1) among children from Period 2 than age and sex-matched in Period 1. Furthermore, the study also highlighted that children from urban, overcrowded home, parental manual laborer, undernourished children and those exposed to noxious particulate and gaseous substances from the use of coal fuel and open kitchen have significantly lower lung function than their counterparts. Hegewald and Crapo (29) in a review of published work on the effects of socioeconomic class on the lung function of children and adults proposed a number of associated factors. Moreover, suboptimal housing environment like overcrowded homes commonly associated with low socioeconomic status has been associated with significantly lower lung function volumes and capacities (30). However, these characteristics were much improved in Period 2 compared to Period 1. In northwestern China where Lanzhou is located, the coal-burning source was generally associated with extensive burning for domestic heating (31). The priorities for an air pollution control area for Chinese administrators were also focused on that region, such as the replacement of coal with natural gas and electricity in North China (32,33). This regulated the use of coal, which then contributed to a decline in household coal use.
Undernourishment was more prevalent among the children in Period 1 during which 3.3% of the children were stunted compared to 0.7% in Period 2 children. The prevalence of overweight and the prevalence of obesity in Period 2 children were significantly higher than in Period 1 children, with 14.2% vs. 2.3% and 12.4% vs. 1.4% for overweight and obesity, respectively. The high prevalence of overweight or obesity among children in recent years in Lanzhou was also pointed out by Zhang et al. (34) and Xue et al. (35). Changes in the physical growth of children in urban areas of Lanzhou from 2001 to 2010 were obvious, with increases in body height and body weight. However, problems such as overweight and obesity emerged in recent years (34). A latest study which covered four districts in Lanzhou reported the prevalence rate of obesity among 3,283 children was 5.76% (36). Currently, children are socially advantaged with access to basic necessities of life, including food and quality health care. This may be related to the fact that children in Period 2 were taller and more well-nourished than their Period 1 counterparts as shown in this study.
Our finding of adverse effects of living in urban areas on lung function among elementary school children in Lanzhou city in Period 2 was in conformity with those from earlier studies (37-39), including some conducted among developing countries (37,40). An international population-based study (38) investigated the influence of childhood living environment and biodiversity indicators on lung function, reporting fewer atopic sensitization and higher FEV1 among those with early-life farm exposure. Further, accessibility and actual exposures related to farming environment obviously differ between urban and rural (or suburban in this study). Relevant studies have observed the “farm-effect” in children relative to the cumulative incidence of asthma, always resulted in poor lung function (41-43). Another two cross-sectional cohort study, conducted among school-age children (5–15 years of age) from the capital and the rural district in Mongolia (40), reported normal FEV1 was actually 40% higher in rural Mongolian children than in urban children. The differences observed in lung function between the rural and urban areas were also pointed by other studies (37,44) and attributed to outdoor air pollution (12), exposure to farm animals (45), farming activities (46), and allergen exposure (11). While we initially intended to consider ambient air pollution as a risk factor, it was difficult to do this in our two cross-sectional design, due to difficulties in measuring personal exposure to outdoor and indoor air pollution. However, there are inconsistent conclusions about the urban-suburban disparity in lung function from previous studies (6,47). Children from rural areas are raised in homes with unclean fuel, homes which use biomass for cooking, lighting, and heating, and generally fall within the low socioeconomic class, which may contribute to lower lung function (47). The inconsistent results might be caused by differences in the study period and stage of city development. In our study, over a span of 20 years, the impact of urbanization was much more pronounced in Period 2. The expansion of urban and the more prominent problem of urbanization could partly explain the conflicting results.
We found that obesity was negatively associated with FVC and FEV1. This supports earlier work in children aged 6–11 years that found that FVC, FEV1, forced expiratory flow 25–27%, and FEV1/FVC were markedly reduced in overweight or obese children compared with those of normal weight (48). The reductions in FVC and FEV1 reports in our study supports the notion that obesity induces symptoms similar to restrictive lung disease by increasing the external load on the chest wall, resistance in the respiratory system, respiratory muscle function, and airway structure or function (49). Obesity may alter airway function by increasing bronchial hyperreactivity (50). In addition, lung inflammation may be increased in obese children because adipose tissue is a source of pro-inflammatory cytokines and chemokines (50). A meta-analysis study report subjects who were obese (as compared with overweight) had an even further decreased FEV1 and FVC (51). Physicians should be aware of adverse effects of obesity on lung function, and weight control should be considered in the management of airway disease among obese individuals.
There are several limitations to our study. First, we used indoor air pollution related household factors instead of actual personal exposure measurements. Second, the main aim of the lung function test performed in this study was to compare healthy Lanzhou children across the two periods. The study was limited in providing any causal evidence in observed relationships between lung function and exposure. Third, data on some potential risk factors were unavailable, including birth weight, household pets, or the number of children in the household. Finally, the questionnaire was self-reported and we were not in a position to confirm the accuracy of response.
Conclusions
Children with the same age range and living in the same areas of Lanzhou had significantly lower lung function (FVC and FEV1) in 2017 than in 1996. Children’s lung function measured in 1996 was adversely affected by overcrowded home conditions, parents being manual laborers, being undernourished, household use of coal for heating and cooking, being obese or overweight, and having an open kitchen. Living in the urban area had a negative effect on lung function in 2017. As overweight and obesity prevalence has increased markedly in recent years in Lanzhou and elsewhere, we commend the provision of weight control, in addition to air pollution exposure control, as a strategy to improve children’s lung function.
Acknowledgments
We are particularly indebted to the children, their parents and the schools for their time and enthusiastic participation. We are grateful to the leaders and teachers of the two primary schools for their kind assistance with recruitment and sample collection. We also appreciate all those who helped us during the implementation of the project.
Funding: This work was supported by the National Science Foundation (41977374), the National Key Research and Development Program of China (2016YFC1302501) and Beijing Natural Science Foundation (7202106).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Junfeng Zhang, Howard Kipen and Haidong Kan) for the series “Children’s Respiratory Health and Air Quality” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-20-2232
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-20-2232
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-20-2232). The series “Children’s Respiratory Health and Air Quality” was commissioned by the editorial office without any funding or sponsorship. JZ served as the unpaid Guest Editor of the series. JG and JZ serves as editorial board members of Journal of Thoracic Disease. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Committee on Ethics of Biomedicine Research, Duke Kunshan University, Jiangsu (No. FWA00021580). All patients enrolled completed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stocks J, Lum S. Pulmonary Function Tests in Infants and Preschool Children. In: Wilmott R, Bush A, Boat T, et al. Kendig & Chernicks Disorders of the Respiratory Tract in Children. Saunders, 2012:169-210.
- Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005;26:153-61. [Crossref] [PubMed]
- Bruce N, Dan P, Rehfuess E, et al. WHO indoor air quality guidelines on household fuel combustion: Strategy implications of new evidence on interventions and exposure–risk functions. Atmos Environ 2015;106:451-7. [Crossref]
- Arora P, Jain S, Sachdeva K. Physical characterization of particulate matter emitted from wood combustion in improved and traditional cookstoves. Energy Sustain Dev 2013;17:497-503. [Crossref]
- Hawley B, Volckens J. Proinflammatory effects of cookstove emissions on human bronchial epithelial cells. Indoor Air 2013;23:4-13. [Crossref] [PubMed]
- Kuti BP, Oladimeji OI, Kuti DK, et al. Rural-urban disparity in lung function parameters of Nigerian children: effects of socio-economic, nutritional and housing factors. Pan Afr Med J 2017;28:230. [Crossref] [PubMed]
- Luis R, Gochicoa-Rangel L G, González G C, et al. Comparative study of respiratory function tests in children from a rural and an urban community in Mexico. Paediatr Respir Rev 2013;14:S81. [Crossref]
- Sonnappa S, Lum S, Kirkby J, et al. Disparities in pulmonary function in healthy children across the Indian urban-rural continuum. Am J Respir Crit Care Med 2015;191:79-86. [Crossref] [PubMed]
- Lawlor DA, Ebrahim S, Davey Smith G. Association between self-reported childhood socioeconomic position and adult lung function: findings from the British Women's Heart and Health Study. Thorax 2004;59:199-203. [Crossref] [PubMed]
- Slachtova H, Gehring U, Hoek G, et al. Parental education and lung function of children in the PATY study. Eur J Epidemiol 2011;26:45-54. [Crossref] [PubMed]
- Priftis KN, Mantzouranis EC, Anthracopoulos MB. Asthma symptoms and airway narrowing in children growing up in an urban versus rural environment. J Asthma 2009;46:244-51. [Crossref] [PubMed]
- He QQ, Wong TW, Du L, et al. Effects of ambient air pollution on lung function growth in Chinese schoolchildren. Respir Med 2010;104:1512-20. [Crossref] [PubMed]
- Renzetti G, Silvestre G, D'Amario C, et al. Less air pollution leads to rapid reduction of airway inflammation and improved airway function in asthmatic children. Pediatrics 2009;123:1051-8. [Crossref] [PubMed]
- Zhang JJ, Hu W, Wei F, et al. Children's respiratory morbidity prevalence in relation to air pollution in four Chinese cities. Environ Health Perspect 2002;110:961-7. [Crossref] [PubMed]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 2006;450:76-85. [PubMed]
- National Health Commission of China. Standard for height level classification among children and adolescents aged 7–18 years. Accessed December 2, 2019. Available online: http://www.nhc.gov.cn/ewebeditor/uploadfile/2018/07/20180705095132166.pdf
- World Health Organization (WHO), United Nations International Children's Emergency Fund (UNICEF). WHO child growth standards and the identification of severe acute malnutrition in infants and children: A Joint Statement by the World Health Organization and the United Nations Children's Fund. Geneva: World Health Organization, 2009.
- National Health Commission of China. Screening for overweight and obesity among school-age children and adolescents. Accessed February 23, 2018. Available online: http://www.nhc.gov.cn/ewebeditor/uploadfile/2018/03/20180330094031236.pdf
- Ostrowski S, Barud W. Factors influencing lung function: are the predicted values for spirometry reliable enough? J Physiol Pharmacol 2006;57:263-71. [PubMed]
- Quanjer PH, Hall GL, Stanojevic S, et al. Age- and height-based prediction bias in spirometry reference equations. Eur Respir J 2012;40:190-7. [Crossref] [PubMed]
- Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med 2008;177:253-60. [Crossref] [PubMed]
- Al-Riyami BM, Al-Rawas OA, Hassan MO. Normal spirometric reference values for Omani children and adolescents. Respirology 2004;9:387-91. [Crossref] [PubMed]
- Du J. Economic reforms and health insurance in China. Soc Sci Med 2009;69:387-95. [Crossref] [PubMed]
- Chia SE, Wang YT, Chan OY, et al. Pulmonary function in healthy Chinese, Malay and Indian adults in Singapore. Ann Acad Med Singap 1993;22:878-84. [PubMed]
- Neukirch F, Chansin R, Liard R, et al. Spirometry and maximal expiratory flow-volume curve reference standards for Polynesian, European, and Chinese teenagers. Chest 1988;94:792-8. [Crossref] [PubMed]
- Gao C, Zhang X, Wang D, et al. Reference values for lung function screening in 10- to 81-year-old, healthy, never-smoking residents of Southeast China. Medicine (Baltimore) 2018;97:e11904 [Crossref] [PubMed]
- Jian W, Gao Y, Hao C, et al. Reference values for spirometry in Chinese aged 4-80 years. J Thorac Dis 2017;9:4538-49. [Crossref] [PubMed]
- Ip MS, Ko FW, Lau AC, et al. Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilization. Chest 2006;129:384-92. [Crossref] [PubMed]
- Hegewald MJ, Crapo RO. Socioeconomic status and lung function. Chest 2007;132:1608-14. [Crossref] [PubMed]
- Choudhury S, Alam MS, Begum QN. Lung function parameters of Bangladeshi male subjects in different living conditions. Bangladesh Med Res Counc Bull 1997;23:30-3. [PubMed]
- Qiu X, Duan L, Gao J, et al. Chemical composition and source apportionment of PM10 and PM2.5 in different functional areas of Lanzhou, China. J Environ Sci (China) 2016;40:75-83. [Crossref] [PubMed]
- Xie JJ, Yuan CG, Xie J, et al. Comparison of arsenic fractions and health risks in PM2.5 before and after coal-gas replacement. Environ Pollut 2020;259:113881 [Crossref] [PubMed]
- Qin Y, Wagner F, Scovronick N, et al. Air quality, health, and climate implications of China's synthetic natural gas development. Proc Natl Acad Sci U S A 2017;114:4887-92. [Crossref] [PubMed]
- Zhang GX, Yu XL, Ma JH, et al. Changes in the physical growth of children aged 3 to 6 years in urban areas of Lanzhou from 2001 to 2010. Zhongguo Dang Dai Er Ke Za Zhi 2012;14:539-42. [PubMed]
- Xue H, Li Z, Ma G, et al. Surveys on the status and associated factors of overweight and obesity of rural children under 7 year-old in Gansu Province. Wei Sheng Yan Jiu 2011;40:68-70. [PubMed]
- Ye XH, Chen H, Kang XG, et al. Association between obesity and sleep disorders among children in Lanzhou, China. Zhongguo Dang Dai Er Ke Za Zhi 2019;21:987-91. [PubMed]
- Al-Qerem W, Ling J. Pulmonary function tests in Egyptian schoolchildren in rural and urban areas. East Mediterr Health J 2018;24:325-32. [Crossref] [PubMed]
- Campbell B, Raherison C, Lodge CJ, et al. The effects of growing up on a farm on adult lung function and allergic phenotypes: an international population-based study. Thorax 2017;72:236-44. [Crossref] [PubMed]
- Morgan BW, Siddharthan T, Grigsby MR, et al. Asthma and Allergic Disorders in Uganda: A Population-Based Study Across Urban and Rural Settings. J Allergy Clin Immunol Pract 2018;6:1580-1587.e2. [Crossref] [PubMed]
- Dashdendev B, Fukushima LK, Woo MS, et al. Carbon monoxide pollution and lung function in urban compared with rural Mongolian children. Respirology 2011;16:653-8. [Crossref] [PubMed]
- Genuneit J. Sex-specific development of asthma differs between farm and nonfarm children: a cohort study. Am J Respir Crit Care Med 2014;190:588-90. [Crossref] [PubMed]
- Rennie DC, Karunanayake CP, Chen Y, et al. Early farm residency and prevalence of asthma and hay fever in adults. J Asthma 2016;53:2-10. [Crossref] [PubMed]
- Rennie DC, Lawson JA, Karunanayake CP, et al. Farm Exposure and Atopy in Men and Women: The Saskatchewan Rural Health Study. J Agromedicine 2015;20:302-9. [Crossref] [PubMed]
- Asgari MM, DuBois A, Asgari M, et al. Association of ambient air quality with children's lung function in urban and rural Iran. Arch Environ Health 1998;53:222-30. [Crossref] [PubMed]
- Morcos MM, Morcos WM, Ibrahim MA, et al. Environmental exposure to endotoxin in rural and urban Egyptian school children and its relation to asthma and atopy. Minerva Pediatr 2011;63:19-26. [PubMed]
- Ege MJ, Frei R, Bieli C, et al. Not all farming environments protect against the development of asthma and wheeze in children. J Allergy Clin Immunol 2007;119:1140-7. [Crossref] [PubMed]
- Sengupta P, Mal S, Mahata H, et al. Rural-Urban variations in pulmonary functions as related to age, sex and anthropometric variables among Bengelee population. Ergonomics and Rural Development, 2014;5:52-62. Available online: http://inet.vidyasagar.ac.in:8080/jspui/handle/123456789/245
- Spathopoulos D, Paraskakis E, Trypsianis G, et al. The effect of obesity on pulmonary lung function of school aged children in Greece. Pediatr Pulmonol 2009;44:273-80. [Crossref] [PubMed]
- Rabec C, de Lucas Ramos P, Veale D. Respiratory complications of obesity. Arch Bronconeumol 2011;47:252-61. [PubMed]
- Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol (1985) 2010;108:735-43. [Crossref] [PubMed]
- Forno E, Han YY, Mullen J, et al. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. J Allergy Clin Immunol Pract 2018;6:570-581.e10. [Crossref] [PubMed]


