
Rational decision making in a wide scenario of different minimally invasive lumbar interbody fusion approaches and devices
Introduction
Once a patient has met the criteria to be a candidate for spinal fusion, there are many procedure options to employ (1), and include either open or minimally invasive exposures (i.e., mini-open, endoscopic, tubular, and percutaneous) for anterior [direct anterior (ALIF), lateral anterior lumbar interbody fusion (LLIF)] or posterior approaches [posterior (PLIF) or transforaminal (TLIF)]. Regardless of surgical approach or procedure chosen, the goals of spinal fusion surgery remain the same: decompression of the neural elements, maximization of final construct stiffness through the placement of a large intervertebral implant and/or rigid fixation in order to promote fusion over as large a fusion area as possible while preserving or restoring segmental alignment and overall spinal balance.
These different procedures vary in their inherent ability to fulfill each surgical goal. Patient and pathologic considerations largely guide which procedures are possible and surgeon preference drives which of the viable procedures is selected for use. With the proliferation of a variety of minimally invasive surgical (MIS) approaches, particularly ones that use direct visualization (mini-open), there is a need for updated criteria for patient and procedural selection for the modern surgeon (2,3). The purpose of this work is to critically evaluate and present decision-making criteria in selecting the appropriate MIS approach (PLF, PLIF, TLIF, ALIF and/or LLIF) based on patient pathology and surgeon/institution considerations.
Procedural considerations and decision-making
MIS transforaminal lumbar interbody fusion (TLIF)
Rationale/indications
MIS TLIF is applicable to common degenerative conditions leading to fusion indications including degenerative disc disease (DDD), stenosis, recurrent herniated discs and grade II or less spondylolisthesis. or any combination of the above (4). The most common levels for MIS TLIF are L4–5 and/or L5–S1, but it’s used at any lumbar level. The procedure is ideal at one or two levels and in stenotic patients with instability but with preserved disc height or locked facets or posterior elements, where indirect decompression may not be obtainable. A patient who is relatively low demand and without osteoporosis with low back pain and a unilateral radiculopathy requiring direct decompression, especially at L5–S1, is an ideal candidate for MIS TLIF. In general, TLIF allows for single-position surgery in patients with multiple baseline medical comorbidities or in those who may not tolerate a potentially larger 360° procedure. Further, MIS TLIF can be expeditiously performed with less paraspinal muscle dissection (no dissection of the insertions of the multifidus) and blood loss than other posterior approaches, including open TLIF.
Drawback/limitations
Disc space preparation can be challenging in MIS TLIF, though recent reports have shown that it is possible to obtain a broad fusion bed with MIS TLIF approaches. Attention is needed on performing as extensive a discectomy as possible with grafting in the ipsilateral, anterior, and posterolateral corner of the contralateral side (using curved instruments). The extent of discectomy possible may be limited by the patients’ surgical depth, epidural scarring from previous surgery, or an inability to distract the endplates in patients with poor bone quality. Since this is a unilateral approach, the cage to endplate contact surface area ratio can be less than desired which can lead to disc height and, more importantly, alignment loss (5,6). In general, posterior approaches have the potential to be kyphosing but can be mitigated and overcome through the placement of lordotic insert-and-rotate cages (to minimize the risk of endplate damage during insertion) or expandable cages. Additionally, the selective use of compression through posterior instrumentation or bilateral facet mobilization can maximize lordosis in patients that require more meaningful gains in lordosis.
A relative limitation to using a unilateral MIS TLIF includes patients with Grade III or higher spondylolistheses, which will result in a substantially limited ability to place a larger TLIF cage, which will compromise fusion bed and graft amount. In additional the increased sacral slope (those occur mostly at L5–S1) makes it difficult to safely access the disc space and decompress the L5 nerve root. In this instance, a Midline incision TLIF with bilateral facet resections and neural decompression would likely be more appropriate. Other limitations to the approach include some patients with extensive ipsilateral epidural scarring and a recurrent herniated disc following a prior laminectomy. In these cases, the ability to mobilize the dura may be difficult and hinder the surgeon from safely being able to deliver the interbody graft. In those cases where dural mobilization may be difficult MIS TLIF still offers an advantage over MIS PLIF, which requires more dural retraction. Also, patients with poor bone quality (osteoporosis or osteopenia) may have a limited ability to distract the endplates using the pedicle screws and can ultimately lead to subsidence and malalignment. Other procedures with a larger cage, that means bigger endplate surface area ratios, may be better suited in these patients. Finally, patients with multi-level (>2) disease indicated for interbody fusion may be challenging using this approach, as it may lead to elevated blood loss, operative time, and likelihood of non-union. In these cases of multi-level disease, procedures with single exposures that can treat multiple levels may be better suited. While TLIF can be used in multi-level deformity, it is typically deployed through the open exposure required for conventional deformity correction techniques.
Description of technique
There are varieties of MIS TLIF systems available for use. The resultant posterior elements and neural exposure is familiar to surgeons trained in conventional open techniques. The learning curve, therefore, is relatively small though there are still concerns about the ability to fully meet the surgical goals of fusion using an MIS approach compared to when using an open approach. In MIS TLIF, the primary concerns are protection of sensitive structures (i.e., nerve roots and the dura) during access to the disc space and then an adequate disc space preparation for fusion. What follows is a systematic description of a pedicle-based MIS TLIF technique integrated into a single procedural platform.
Following a paramedian incision, a split blade retractor is used along the standard TLIF approach trajectory with the blades of the retractor attached to percutaneous pedicle screws placed without the tulips engaged. The placement of pedicle screws provides visual orientation through the working window of anatomy and its orientation and their connection to the retractor blades allow for stabilization of the retractor on local anatomy for distraction to facilitate disc space preparation and interbody grafting. Three blades are used, two in cranial and caudal orientations, each affixed to the placed pedicle screws and one blade positioned medially for medial retraction. While the incision is smaller than for a traditional TLIF, all relevant surgical anatomy are visualized, including the ipsilateral facet, lamina, dura, exiting and traversing nerve roots, and pedicles. Once the disc space has been exposed, standard TLIF disc space preparation and grafting techniques are used. This unilateral approach also allows for contralateral direct decompression of the neural elements through the same surgical corridor. The contralateral facet can be optionally released and fused for complete segment mobilization for improved disc and foraminal height restoration and to provide more segmental lordosis (Figure 1). Contralateral pedicle screws can then be delivered through the same incision and provide contralateral disc space distraction (Figure 1).
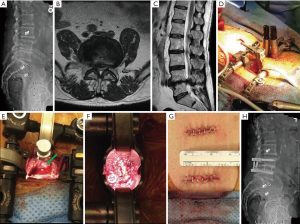
Case example
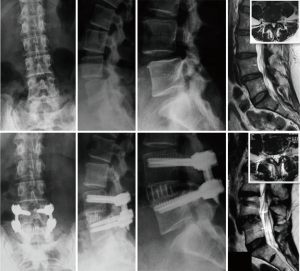
A 76-year-old male presented with back and bilateral leg pain (predominantly right) with associated weakness and sensory changes. Radiographically, the patient had preoperative pelvic incidence-lumbar lordosis mismatch of 21° with disc height loss at L4–5 and a moderately high iliac crest (Figure 1A). Magnetic resonance imaging (MRI) revealed partial L5 sacralization with an anteriorly positioned lumbar plexus and severe multifactorial stenosis (Figure 1B,C). Following failure of conservative treatment, he was counseled and consented for surgical treatment.
The patient underwent an MIS TLIF (Figure 1D) with direct decompression of the neural elements (Figure 1E) and contralateral facet exposure for release (Figure 1F) through two small incisions (Figure 1G). The patient was seen at 6 months postoperative with mild back stiffness and some residual weakness, but with full resolution of leg pain. Radiography showed improved lordosis, foraminal, and disc height with restoration of the pelvic incidence-lumbar lordosis relationship and (Figure 1H).
MIS posterior lumbar interbody fusion (PLIF)
Rationale/indications
The MIS PLIF approach allows for single exposure direct bilateral decompression, interbody fusion, and posterior fixation. This exposure provides for a larger disc space preparation and potential fusion mass compared to MIS TLIF and mini-open direct anterior approaches (7). In general, MIS PLIF can be applied to any lumbar spinal level for standard degenerative and deformity applications indicated for interbody fusion and is best suited for treating one to two level disease (similar to MIS TLIF, where multi-level surgery may not experience the same benefits as smaller constructs due to the additional exposures required. Compared to Unilateral MIS TLIF, MIS PLIF may potentially be better able to treat higher-grade spondylolisthesis through the placement of bilateral intervertebral spacers, though it may still be a challenging access. In those patients, especially with a need for bilateral direct decompression, MIS TLIF with bilateral cage insertion is a viable option. Especially below longer fusion constructs, such as in deformity or in conjunction with superior LIF, MIS PLIF at L5–S1 provides a more stable base/foundation than MIS TLIF for maintaining the construct above. This is of particular concern in high demand patients.
Drawback/limitations
As previously mentioned, the screw trajectory used in MIS PLIF may require passing through a learning curve and can be technically difficult in patients with spondylolysis or multi-level deformity. Patients with prior posterior spine surgery with scarring or in patients with prior instrumented spine surgery have additional technical concerns for all posterior approaches, including MIS PLIF. However, in patients with adjacent segment disease and prior adjacent conventional outside-in pedicle screws, the use of cortical screws and MIS PLIF at the adjacent level may be able to be used without revision of the prior posterior construct. In lordosis correction, the cortical pedicle screws may be more challenging to compress and provide lordosis correction in addition to any correction gained by intervertebral spacer placement. In patients with high-grade stenosis with bone-on-bone endplate contact, exposure to the disc space may be difficult. In addition, in patients with small diameter pedicles, the cortical pedicle screw trajectory may be challenging. Finally, a drawback of the approach is that standard posterolateral fusion (PLF) in the lateral gutters cannot be performed in this procedure due to the medialized exposure.
Description of technique
PLIF was first performed by Ralph Cloward in 1943, described in the literature in 1953, and refined further in 1985 (8) as a technique for simultaneous direct neural decompression and interbody fusion. What originally was a technically demanding procedure grew in popularity with the advent of more advanced instrumentation. Roy-Camille et al. and later Steffe et al. developed and popularized pedicle-screw-based fixation and its incorporation into interbody fusion procedures, namely PLIF (9,10). The PLIF approach with pedicle screw and rod fixation are both regularly taught as part of spinal orthopedic training and remains a commonly performed interbody fusion procedure. This traditional procedure, however, typically requires exposure out to the transverse processes for both broad direct decompression (laminectomy with or without facetectomy), which is associated with elevated procedural morbidity, namely neural injuries and postoperative infections (11).
Wherein MIS TLIF uses the same general instrumentation and techniques as in open TLIF, just with a smaller incision and more standardized surgical steps, MIS PLIF requires a change in instrumentation and procedural steps for effective use. The MIS PLIF surgical technique begins with a midline incision, and medialized mini-open exposure out to approximately the medial border of the facet capsule. In this exposure, the multifidus and longissimus muscles are largely spared and there is no dissection of the tissues around the superior facet complex. A direct decompression is performed and endplate preparation is performed using standard surgical techniques. Specialized bilateral PLIF implants are placed near each lateral border of the apophyseal ring to resist subsidence and maximize rigidity and lordosis and are narrow enough to be placed with minimal or no cauda equina retraction. The biggest difference between MIS and open PLIF is in the style of pedicle screw placed. In conventional PLIF with exposure to the transverse processes, traditional outside-in pedicle screw trajectories are used. In MIS PLIF, with only a medial exposure of the posterior complex, cortical pedicle screws are utilized, with an inside-out trajectory (12). The placement of medial, cortical pedicle screws represents the largely potential learning curve in adopting this MIS procedure. Otherwise, this approach has the same overall benefits of PLIF with less procedural morbidity.
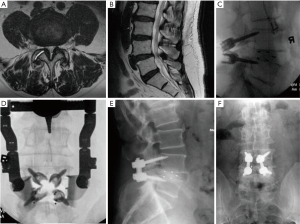
Case example
A 75-year-old male presented to the clinical with a long history of mechanical back pain with the more recent development of neurogenic claudication. The patient had failed medical management and several rounds of epidural steroid injections. Radiography and MRI revealed a low-grade L4–5 spondylolisthesis with severe bilateral recess and central stenosis with grade IV facet degeneration (Figure 2A,B) (13). The patient was treated with MIS PLIF with direct midline decompression, bilateral cage and cortical pedicle screw placement (Figure 2). The estimated blood loss, operative time, and length of stay were 100 minutes, 150 mL, and 2 days, respectively. At 6 months postoperative follow up, the patient had significant improvement in claudication and back pain symptoms with preserved lumbar lordosis and alignment.
MIS PLF
Rationale/indications
The rationale for the use of MIS over conventional PLF is the lowered risk of procedural morbidity with the medialized exposure with the same goals of surgery achieved in select patients using specialized instrumentation for medialized fixation and fusion. A low demand patient with central stenosis with or without low-grade or “stable” spondylolisthesis and who has generally good bone stock is the ideal patient for MIS PLF, especially if they have baseline medical conditions that indicate that the patient may not tolerate a more extensive, interbody, surgery as well without the unnecessary elevated risk of complication. Since the procedure does not pass through to the disc space, the risk to neural structures is low. This is generally a one- to three-level procedure, able to be used at lumbar spinal levels. Additionally, this procedure has been used adjacent to superior levels in multi-level constructs to possibly reduce adjacent segment disease or junctional breakdown.
Drawback/limitations
Drawbacks to the use of the procedure include the somewhat limited patient pool, with higher demand or more advanced disease, including deformity, not particularly well treated with this type of procedure. As with other posterior approaches, MIS PLF has the potential for kyphosis if instrumentation and interspinous spacers are not placed, with special attention to segmental and regional alignment. While the ideal candidate for this procedure is a low demand patient with stenosis with or without low-grade spondylolisthesis, it is not suited for patients with poor bone quality (osteopenia or osteoporosis), who might otherwise fit the description for treatment. In patients with poor bone quality, more points of fixation and interbody fusion with a large cage: endplate interface ratio are likely needed.
Description of technique
PLF without interbody fusion is one of the oldest forms of spinal fusion techniques, introduced by Hibbs in the early 1900s (14), and remains a common form of spinal fusion. The same general exposure for conventional PLIF is required for PLF, with exposure from midline out to the transverse processes for direct posterior decompression with intertransverse and posterolateral gutter fusion, typically with bilateral pedicle screw and rod fixation. In modern, MIS approaches for PLF the same general principles are applied of direct posterior decompression and posterior-only fusion, though with a medialized exposure that does not violate the multifidus lateral to the lateral margin of the facet joint and does not involve exposure of the transverse process. This is identical to the exposure of the current MIS PLIF. The basic principle of MIS PLF is performing a PLF without the morbidity of the conventional open posterolateral intertransverse fusion. This method utilizes a lamina preservation technique of a distractive laminoplasty for decompression with placement of an interlaminar Hibbs-like graft with the addition of posterior instrumentation, either cortical pedicle screws or interspinous plating. The grafting principle is very similar to the Brooks Gallie fusion of the cervical spine.
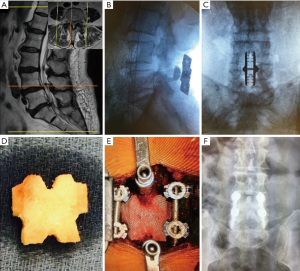
Case example
A 79-year-old female presented with right leg and back pain. The patient had failed conservative care. The exams (Figure 3A) revealed L4–5 spondylolisthesis with a tall disc and L5–S1 herniated disc. The surgical procedure consisted of L5S1 discectomy and a L4–5 MIS PLF with interspinous plating (Figure 3B,C). Instrumentation option with cortical pedicle screws is shown in Case 2 (Figure 3D,E) and 1 year post operative anterior posterior (AP) X-ray (Figure 3F).
Anterior lumbar interbody fusion (ALIF)
Rationale/indications
In comparison to posterior accesses, the ALIF procedure can be associated with a shorter operative time, with less bleeding and postoperative pain, reducing the length of hospitalization and withdrawal from work (15). There is a wide range of indication for the application of this technique. Among them are several degenerative pathologies that affect intervertebral discs, leading to disc collapse, discogenic pain, low-grade spondylolisthesis, disc herniation and pseudoarthrosis (16-19). A great advantage of ALIF is the opportunity to restore disc height and gain lordosis (20,21). In revision surgeries, it avoids scars from previous surgery. The relative contraindications are those related to the patient clinical status and comorbidities, such as vascular diseases, abdominal aortic aneurysm, psychiatric disorders, single kidney (risk of ureter injury), active infection, and young men wishing to have children (risk of hypogastric plexus injury). High-grade spondylolistheses and severe osteoporosis increase the risk of cage subsidence, leading to loss of sagittal and coronal correction, as well as loss of indirect decompression acquired with ligamentotaxis (22).
Drawback/limitations
The advantages of the technique include the possibility to perform a wide discectomy, avoiding the paraspinal musculature violation and epidural adhesion and achieving indirect decompression of the intervertebral foramen by ligamentotaxis, better sagittal correction and less risk of nerve root damage (23). Among the disadvantages, the need for an access surgeon, the retraction of the large vessels, and the incidence of deep vein thrombosis and vascular intercurrences (24) are highlighted. Retrograde ejaculation is a possible complication frequently reported in the literature, related to hypogastric plexus damage during anterior access (25). Incisional hernias and abdominal muscle atony had reduced rates after minimizing anterior access (26).
The potential complications of ALIF are those related to anterior approach to the lumbar spine, as abdominal viscera lesions, great vessels, thromboembolic events, ureter injury and retrograde ejaculation in men (27-30). The male patient must be aware of this complication, to take the necessary precautions in case he still has the desire to have children. The proper choice of the device is crucial, because its size can influence surgical outcomes. Smaller cages can lead to device migration, or fusion in kyphosis. Pedicle screws can be used in order to supplement in case of pseudoarthrosis.
Description of technique
Capener (31) first described the anterior access to the lumbar spine to implant an intersomatic spacer in 1932, for the treatment of spondylolisthesis. Since then, the procedure evolved and minimized the damage to adjacent tissues (26). Surgical planning is crucial to the procedure’s success. Lateral X-ray of the lumbar spine helps in determining the depth of the intervertebral disc, sagittal balance, spinal-pelvic measurements, anterior and posterior disc height, thus providing the correct indication of the intersomatic device for each patient. If the line drawn over the upper and lower plateau of the level to be accessed crosses the pubis, the ALIF should not be indicated. In patients with very high sacral inclination, the L5–S1 level is commonly very angulated, difficultly accessed by surgery (23).
The patient is positioned in supine position in a radiopaque surgical table, and the maintenance of the lumbar lordosis is essential. The fluoroscopy is fundamental for the correct positioning and surgical navigation. The access can be performed even transperitoneal or retroperitoneal, being the incision made longitudinal or transverse according to the preference and skills of each surgeon.
After the skin incision, the rectus abdominis muscle is dissected and the peritoneum is incised or rebound to cranial. The identification of the great vessels is pivotal, as the discs are localized below them. Caution is needed in order to avoid vascular injury or thromboembolic events. The excess use of electrocautery increases the incidence of retrograde ejaculation in men (25). Once the correct disc space is confirmed by fluoroscopy, the anterior longitudinal ligament is incised in midline and bilaterally rebound for discectomy. The intersomatic spacer must enhance primary fusion and promote fusion through the graft. For supplementation, anterior plates or pedicle/facet screws are used, as shown in the case example. The closure is made in a standard fashion.
The patient is encouraged to walk as early as possible, usually in the first postoperative day in order to minimize the risk of thrombotic events. The patient is discharged 2 to 3 days after surgery.
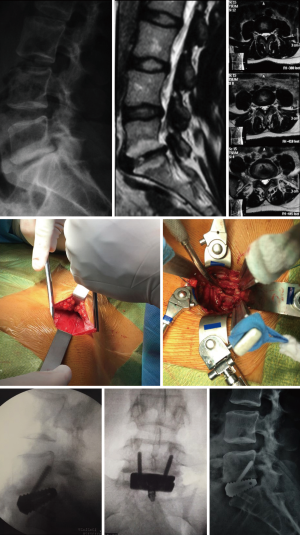
Case example
A 34-year-old female presented to the physician with low back pain and irradiating symptoms to the right lower limb. Conservative care consisted of analgesics and non-steroidal anti-inflammatory drugs (NSAIDs), along with physiotherapy followed be exercises for core strengthening. Patient returned after 8 months with increased pain symptoms. Preoperative imaging revealed L5–S1 DDD, with lateral recess stenosis and effusion of the facets. The surgical procedure was an L5–S1 stand-alone ALIF with a self-locking cage. Total surgical duration was 60 minutes with no intraoperative intercurrence. The patient was discharged on the first postoperative day without complication. Case images can be found in Figure 4.
Lateral lumbar interbody fusion (LLIF)
Rationale/indications
The LLIF technique allows access to the anterior spine and is an alternative to direct anterior ALIF without the requirement for an access or general surgeon for the approach. The direct advantages over ALIF include less muscular dissection, hastened postoperative patient mobilization, and drastic reduction of ALIF-specific complications, namely vascular and reproductive.
With similar benefits to ALIF in respect of preservation of the posterior elements, LLIF constructions brings some additional advantages related to the large intervertebral spacer and maintenance of ALL. Those included are: a wide endplate preparation area and fusion bed, the ability to align the spine and to correct unilateral disc collapse and stenosis (parallel disc distraction), superior segmental stability and biomechanical characteristics, subsidence resistance compared to posteriorly placed cages and the ability to choose anatomy/pathology specific implants with a variety of different cage morphologies available and able to be placed (e.g., implants with tabs/keels, different sizes and angles, coronal tapered).
LLIF preserves primary segmental stabilizing structures (ALL, PLL, ligamentum flavum, and facets), while in ALIF it is necessary resecting ALL and anterior annulus, and in PLIF/TLIF it is necessary resecting LF, PLL, posterior annulus and typically the facet joint(s). Along with the preservation of ligamentous structures, the lateral approach allows for preservation of posterior musculature and bony architecture. With the ALL and PLL intact it allows ligamentotaxis to align and indirectly decompress the segment. In many cases that normally would require a direct decompression afterwards, often it is capable of being treated with an indirect decompression following lateral based discectomy, disc height restoration and interbody fusion. Although a complete anatomic restitution of the spinal canal, lateral recess, and foraminal area would appear to be required, often less than total indirect decompression is able to adequately provide symptom relief or resolution (32,33).
Without direct decompression, a variety of fixation types or decompressions can be used. In posterior approaches, you are committed to a direct decompression and bilateral fixation, for example, in PLIF. Single position interbody fusion and fixation options with a variety of fixation are able to be placed with the patient in lateral position, including lateral plating, transpedicular facet screws, unilateral pedicle screws, bilateral pedicle screws (with a special technique), spinous process plating, and cortical bilateral pedicle screws.
General indications for the use of the LLIF procedure follow: (I) lumbar levels above L5, with the ability to access thoracolumbar and the thoracic spine with a modified surgical technique; (II) lumbar conditions associated with degeneration of the intervertebral disc including symptomatic DDD or acquired stenosis in DDD, spondylolisthesis and degenerative scoliosis; (III) high demand patients; (IV) higher risk patients can be treated with relative low procedural morbidity [e.g., obese and elderly patients (34,35)]; (V) spondylodiscitis; (VI) iatrogenic conditions, namely adjacent segment treatment as it does not require the revision of posterior instrumentation and in revision of prior anterior or posterior surgeries (fusion or arthroplasty) as the lateral approach avoids scar tissue from prior; (VII) some deformity conditions, especially to reconstruct lordosis and correct sagittal malalignment with ALL resection and placement of hyperlordotic cages to reduce the use of 3-column osteotomies; (VIII) more advanced lumbar and thoracolumbar diseases with the same approach and exposure, including corpectomy for burst fracture, trauma or tumor.
Drawback/limitations
Some relative contraindications follow: (I) patients with an anterior lying plexus (rising or teardrop psoas sign) or posterior lying vasculature support the need for careful attention to preoperative axial MRI at all levels being treated. Those circumstances are more commonly found in transitional anatomy patients (36), grade III or higher degenerative spondylolisthesis, and cases with idiopathic rotational deformity; (II) high iliac crest with collapsed L4–5 disc; (III) severe bony stenosis (particularly lateral recess) or other findings that require direct decompression or in patients with congenitally short pedicles, where direct posterior procedures may be equivalent or preferred; (IV) bilateral retroperitoneal scarring (e.g., kidney surgery; through prior ALIF and LLIF are not typically limitations of the approach); (V) the need to explore the canal or foramina area from the same approach.
Alternative to the traditional transpsoas LLIF technique using advanced neuromonitoring techniques, some surgeons use variations in technique on approaching the disc space.
These variations do not use specialized neuromonitoring. Within them are the shallow-docking (37) and the oblique (38) approaches. The shallow docking technique is still a transpsoas approach and differs from the technique of transpassing the psoas muscle. The retractor is docked on top of the psoas, rather than through it. Under visual dissection through the psoas muscle, the nerves must be avoided. To avoid the lumbar plexus (and specifically the femoral nerve), it is advocated a more anterior passage through the psoas muscle. In oblique approach, the entry point is done in lateral abdominal region, then the psoas muscle is identified and reclined posteriorly to expose the disc space. The adoption of this technique method is adopted to try to avoid lumbar plexus stretching (37), but low-strength evidence shows elevated neural complication rates in non-traditional (39). The first barrier to the adoption for LLIF is that the approach is not regularly taught in training and, as such, the relevant surgical anatomy might be unfamiliar. Next, a side effect of transpsoas approach is that immediate hip flexion weakness occurs in many cases due to muscle trauma with passage through the psoas, but this effect typically recovers rapidly with patient mobilization without intervention (40). Patient counseling preoperatively can help set this expectation as a typical result of the surgical approach. During the approach and procedure, the lumbar plexus is the most vulnerable structure that can be at risk. In order to mitigate these risks, it requires careful attention to the surgical steps, technique, and advanced neuromonitoring. Being reasonably effective in time and amount of retraction can reduce the risk the plexus-related adverse events (41), once nerve neuropraxia seems to occur mainly as result of ischemia. While low, risk to vascular and visceral injuries still exit. Finally, the approach cannot be performed regularly at L5–S1, so for patients with L5–S1 pathology along with L4–5 and above pathology, two approaches or a different one to reach both levels will need to be used.
Description of technique
The LLIF technique was developed in the late 1990s and early 2000s and introduced into the literature as extreme LIF (XLIF®, NuVasive, Inc. San Diego, CA, USA) by Luiz Pimenta in 2006 (42) as a lateral approach for ALIF. LLIF uses a mini-open, 90° off-midline lateral, retroperitoneal, transpsoas approach to reach the lateral aspect of the anterior column. In preparing for this procedure, patient positioning is key. The LLIF procedure is performed in the lateral decubitus with hips and knees flexed and the iliac crest over the table break. The table is slightly bent to open the space between ribs and iliac crest and facilitate access to the lumbar levels. A single or two-incision technique is carried out in the lateral aspect of the body in order to reach the retroperitoneal space. Gentle finger dissection is used to mobilize the retroperitoneal fat and move the peritoneum anteriorly. Digital guidance is used to lead the first dilator to the surface of the psoas muscle. The dilator is then bluntly passed through the psoas muscle under evoked EMG which stimulates in directional orientations and provides discrete threshold responses. Following delivery of the third sequential dilator, a table-mounted, split-blade retractor is placed. Exposure of the disc space is performed by the selective retraction of any one of the cranial, caudal, or posterior retractor blades. The procedure is carried out under direct visualization using standard surgical techniques for annulotomy, discectomy, and endplate preparation. A wide discectomy is performed to prepare endplate and releases contralateral annulus, but with preservation of anterior and posterior longitudinal ligaments (ALL & PLL). Sequential trailing is used to determine implant dimensions and a wide intervertebral graft is placed across the lateral borders of the cortical bone of the ring apophysis. As this is an anterior procedure, relying primarily on indirect decompression of neural elements and ligamentotaxis for segmental realignment, the posterior complex remains intact so that supplemental internal fixation can placed according to surgeon’s preference and the pathologic needs of the patient, including anterolateral plating for single-incision anterior interbody fusion and fixation.
Case example
A 56-year-old female presented to the clinical with irradiating leg and low back pain, which was worse with movement. The patient had failed several rounds of non-operative management without result. Preoperative imaging revealed L4–5 degenerative spondylolisthesis. The surgical procedure was an L4–5 LLIF supplemented with bilateral percutaneous pedicle screws. Total surgical duration was 90 minutes with no complications. Patient was discharged on postoperative day one without complication. Case images can be found in Figure 5.
Conclusions
Due to the wide variety of techniques and devices available for the anterior thoracolumbar fusion, it is difficult to develop a definitive algorithm for the treatment of pathologies inherent to the spine. It is mandatory that the surgeon dominate all aspects involved in the surgical indication, from the critical clinical and functional evaluation of the patient, the peculiarities of his pathology, to the available treatment options, adapting them to the characteristic of each type of patients, helping them to choose the most effective and efficient therapeutic option for each case.
Acknowledgements
The authors want to thank Kyle Malone, a direct employee of NuVasive, for his administrative and intellectual support to this work.
Footnote
Conflicts of Interest: A Tohmeh, D Jones, R Amaral, H Bae are NuVasive Inc. Consultants. L Pimenta: Stocks, Consultant and Royalties from NuVasive Inc. The other authors have no conflicts of interest to declare.
References
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Irwin ZN, Hilibrand A, Gustavel M, et al. Variation in surgical decision making for degenerative spinal disorders. Part I: lumbar spine. Spine 2005;30:2208-13. [Crossref] [PubMed]
- Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine 2003;28:S26-35. [Crossref] [PubMed]
- Karikari IO, Isaacs RE. Minimally Invasive Transforaminal Lumbar Interbody Fusion. Spine 2010;35:S294-301. [Crossref] [PubMed]
- Closkey RF, Parsons JR, Lee CK, et al. Mechanics of interbody spinal fusion. Analysis of critical bone graft area. Spine 1993;18:1011-5. [Crossref] [PubMed]
- Matsumura A, Taneichi H, Suda K, et al. Comparative study of radiographic disc height changes using two different interbody devices for transforaminal lumbar interbody fusion: open box vs. fenestrated tube interbody cage. Spine 2006;31:E871-6. [Crossref] [PubMed]
- Tatsumi R, Lee YP, Khajavi K, et al. In vitro comparison of endplate preparation between four mini-open interbody fusion approaches. Eur Spine J 2015;24:372-7. [Crossref] [PubMed]
- Cloward RB. Posterior lumbar interbody fusion updated. Clin Orthop 1985.16-9. [PubMed]
- Roy-Camille R, Saillant G, Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop 1986.7-17. [PubMed]
- Steffee AD, Biscup RS, Sitkowski DJ. Segmental spine plates with pedicle screw fixation. A new internal fixation device for disorders of the lumbar and thoracolumbar spine. Clin Orthop 1986.45-53. [PubMed]
- Okuda S, Miyauchi A, Oda T, et al. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine 2006;4:304-9. [Crossref] [PubMed]
- Santoni BG, Hynes RA, McGilvray KC, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J 2009;9:366-73. [Crossref] [PubMed]
- Pathria M, Sartoris DJ, Resnick D. Osteoarthritis of the facet joints: accuracy of oblique radiographic assessment. Radiology 1987;164:227-30. [Crossref] [PubMed]
- Hibbs RA. A report of fifty-nine cases of scoliosis treated by the fusion operation. By Russell A. Hibbs, 1924. Clin Orthop 1988.4-19. [PubMed]
- Gandhoke GS, Ricks C, Tempel Z, et al. Mini-open anterior lumbar interbody fusion. Neurosurg Focus 2016;41 Video Suppl 1:1.
- Rao PJ, Loganathan A, Yeung V, et al. Outcomes of anterior lumbar interbody fusion surgery based on indication: a prospective study. Neurosurgery 2015;76:7-23; discussion 23-4. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Thayaparan GK, et al. Anterior Lumbar Interbody Fusion as a Salvage Technique for Pseudarthrosis following Posterior Lumbar Fusion Surgery. Global Spine J 2016;6:14-20. [Crossref] [PubMed]
- Mobbs RJ, Loganathan A, Yeung V, et al. Indications for anterior lumbar interbody fusion. Orthop Surg 2013;5:153-63. [Crossref] [PubMed]
- Marchi L, Abdala N, Oliveira L, et al. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal 2012;2012:456346. [PubMed]
- Jiang SD, Chen JW, Jiang LS. Which procedure is better for lumbar interbody fusion: anterior lumbar interbody fusion or transforaminal lumbar interbody fusion? Arch Orthop Trauma Surg 2012;132:1259-66. [Crossref] [PubMed]
- Uribe EV, Amaral R, Marchi L, et al. Immediate Reciprocal Changes at Adjacent Level Following Single-Level ALIF. Coluna/Columna 2015;14:286-9. [Crossref]
- Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 17: bone growth stimulators and lumbar fusion. J Neurosurg Spine 2005;2:737-40. [Crossref] [PubMed]
- Crock HV. Anterior lumbar interbody fusion: indications for its use and notes on surgical technique. Clin Orthop 1982.157-63. [PubMed]
- Garg J, Woo K, Hirsch J, et al. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg 2010;51:946-50. [Crossref] [PubMed]
- Lindley EM, McBeth ZL, Henry SE, et al. Retrograde ejaculation after anterior lumbar spine surgery. Spine 2012;37:1785-9. [Crossref] [PubMed]
- Brau SA. Mini-open approach to the spine for anterior lumbar interbody fusion: description of the procedure, results and complications. Spine J 2002;2:216-23. [Crossref] [PubMed]
- Thaler M, Mayr E, Liebensteiner M, et al. Injury of the right and left inferior epigastric artery during the implantation of a stand-alone ALIF cage through a left retroperitoneal approach: a case report. Arch Orthop Trauma Surg 2010;130:31-5. [Crossref] [PubMed]
- Rothenfluh DA, Koenig M, Stokes OM, et al. Access-related complications in anterior lumbar surgery in patients over 60 years of age. Eur Spine J 2014;23 Suppl 1:S86-92. [Crossref] [PubMed]
- Quraishi NA, Konig M, Booker SJ, et al. Access related complications in anterior lumbar surgery performed by spinal surgeons. Eur Spine J 2013;22 Suppl 1:S16-20. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Daly D, et al. Approach-Related Complications of Anterior Lumbar Interbody Fusion: Results of a Combined Spine and Vascular Surgical Team. Global Spine J 2016;6:147-54. [Crossref] [PubMed]
- Spondylolisthesis Capener N. Br J Surg 1932;19:374-86. [Crossref]
- Elowitz EH, Yanni DS, Chwajol M, et al. Evaluation of indirect decompression of the lumbar spinal canal following minimally invasive lateral transpsoas interbody fusion: radiographic and outcome analysis. Minim Invasive Neurosurg 2011;54:201-6. [Crossref] [PubMed]
- Oliveira L, Marchi L, Coutinho E, et al. A Radiographic Assessment of the Ability of the Extreme Lateral Interbody Fusion Procedure to Indirectly Decompress the Neural Elements. Spine Phila Pa 1976 2010;35:S331-7. [Crossref] [PubMed]
- Rodgers WB, Cox CS, Gerber EJ. Early Complications of Extreme Lateral Interbody Fusion in the Obese. J Spinal Disord Tech 2010;23:393-7. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Rodgers JA. Lumbar Fusion in Octogenarians. Spine 2010;35:S355-60. [Crossref] [PubMed]
- Smith WD, Youssef JA, Christian G, et al. Lumbarized Sacrum as a Relative Contraindication for Lateral Transpsoas Interbody Fusion at L5-6. J Spinal Disord Tech 2012;25:285-91. [Crossref] [PubMed]
- Acosta FL Jr, Drazin D, Liu JC. Supra-Psoas Shallow Docking in Lateral Interbody Fusion. Neurosurgery 2013;73:ons48-51; discussion ons52.
- Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and Morbidities of Mini-open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lumbar Interbody Fusion in 179 Patients. Asian Spine J 2012;6:89-97. [Crossref] [PubMed]
- Lehmen JA, Gerber EJ. MIS lateral spine surgery: a systematic literature review of complications, outcomes, and economics. Eur Spine J 2015;24 Suppl 3:287-313. [Crossref] [PubMed]
- Tohmeh AG, Rodgers WB, Peterson MD. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine 2011;14:31-7. [Crossref] [PubMed]
- Uribe JS, Isaacs RE, Youssef JA, et al. Can triggered electromyography monitoring throughout retraction predict postoperative symptomatic neuropraxia after XLIF? Results from a prospective multicenter trial. Eur Spine J 2015;24 Suppl 3:378-85. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]


