Proton therapy for tumors of the base of the skull
Introduction
Base of skull tumors are rare tumors whose treatment can pose several challenges not only for neurosurgeons but also for radiation oncologists. These tumors are constantly the subject of research and publication when a new technique arises (1,2). Skull base tumors are challenging lesions because of their anatomical location. Surgical intervention is often the first step in therapeutic management to obtain pathologic sampling and/or improvement of symptoms as well as for optimal cytologic reduction. However, because of the proximity of critical normal structures, surgical intervention to achieve a total removal of the tumor can often come at the price of potentially life-threatening complications that could severely deteriorate vision, hearing, speech or swallowing.
Base of the skull anatomy and symptoms
The tumors arising at the base of the skull are usually indolent and rarely metastasize extracranially; however, these tumors can be locally aggressive. Pathologies include meningioma, pituitary adenoma, schwannoma/acoustic neuroma, chordoma, chondrosarcoma, craniopharyngioma, olfactory neuroblastoma/esthesioneuroblastoma and glomus jugulare/chemodectoma.
Regardless of the definitive pathology of the tumor, the symptoms developed by the patients are always similar. In a recent series of 50 chordomas, Jahangiri et al. described that patients presented mainly headaches (38%), diplopia (36%), and dysphagia (14%) (3). In a large series of base of the skull meningiomas, Noël et al. described varied types of symptoms presented by patients. Concerning visual symptoms, ptosis, 3rd or 6th oculomotor palsies, diplopia, reduced visual acuity and exophthalmos were found in 35%, 37%, 27%, 37%, 55% and 20% of patients, respectively. Headaches, trigeminal sensory loss, motor or vertigo disturbances, facial palsy, 12th palsy and epileptic seizure have been reported in 37%, 37%, 35%, 20%, 14% and 6%, respectively. Patients classically present with headaches, and this symptom can have multiple origins that are not always clearly related to anatomical structure. Twelve variable hypophysis abnormalities were diagnosed in biological exams (4). Cranial nerve numbness or palsies are related to the cranial nerves abutted or invaded by the tumor. Optic difficulties are probably the most frequent group of symptoms. The optic apparatus includes not only optic nerves and chiasm but also cranial nerves and brainstem cranial nuclei. The optic symptoms include ptosis and diplopia. Loss of vision is secondary to suffering of the optic nerves and invasion of the chiasm. The decrease of vision is relatively late in the evolution of the disease because the optic nerves are protected by a bony canal that must first be destroyed and invaded. At the same time, the chiasm is always displaced through a superior position. Exophthalmos is scarcer and is secondary to more advanced tumors or to more anterior lesions. Difficulties with hearing and facial numbness or palsies are common in advanced schwannoma or lateral tumors, such as chondrosarcomas. Dysphagia or difficulty swallowing can be secondary to the inferior location of the tumor, which can track along the posterior pharyngeal musculature and/or impact cranial nerves.
Meningioma
The majority of meningioma (approximately 95%) is benign [World Health Organization (WHO) grade I]. Surgical resection is the referenced treatment for accessible tumors that can be removed with safety. However, with tumors located close to the cavernous sinus, total removal is rarely achieved (5). Incomplete surgical removal is associated with increased risk of progression, but the interval from surgery to progression can be long. Irradiating at time of incomplete surgery or at time of relapse remains questioned. Three-dimensional (3D) conformal radiotherapy, intensity modulation radiotherapy (IMRT) and stereotactic irradiation can result in good local control (LC) rates (6). Compared to other intracranial tumors, skull base meningiomas often have complex shapes (7-10). Particle therapy may be offered to patients with meningioma, especially if they are characterized by long-term survival. Minimization of treatment-related side effects is of high importance, including neurocognitive sequelae (11-13). Moreover, for the same reason, avoiding a low dose spread-distribution is required to decrease the risk of potential radiation-induced secondary malignancy. Therefore, increasing dose conformality and reducing the dose to normal tissue is of high importance.
Proton therapy has been delivered alone or in combination with photon therapy with good results (10,13-16). The numbers of patients in the published series range between 17 and 72. Total doses differed from 56 to 59 Gy relative biological effectiveness (RBE), with classical fractionation of 1.8–2 Gy (RBE) per day for five sessions per week. Proton therapy was approximately 1/3 of the total dose (4). Photon therapy was delivered either by 3D radiotherapy (3DRT) or by intensity modulated radiotherapy (IMRT). With a median follow-up from 37 to 62 months, 4- and 5-year LC rates were approximately 90% and 4- and 5-year overall survival (OS) ranged between 89% and 93% (10,13-16). Likelihood of symptom relief depends on the initial clinical signs and time interval between symptom onset and beginning of the radiation (4). The LC rates were lower for grade II or grade III meningioma, but in the diverse series, the anatomical locations of these meningiomas were not always easy to retrieve and were not always localized in the base of the skull (13,14,17). Some authors reported an increase of the total dose or the use of intensity modulated proton therapy (IMPT), or spot scanning to attempt to improve LC (13,17-19). However, the lack of comparison with conventional treatment (dose, beam) and the short follow-up of these series do not allow definitive conclusions. Nevertheless, some authors concluded that shielding of critical organs is improved with IMPT and spot scanning (13,19).
Proton therapy delivered with hypofractionated stereotactic (HSP) or single-session stereotactic (SSSP) approaches have been used in series of 19 to 50 patients (11,12,20). HSP with three or more fractions reached a 3- and 5-year LC rate of 100% (11) and 91% (12), respectively. Recently, the group of MGH in Boston reported the results of 51 cases of benign meningioma treated with SSSP between 1996 and 2007. Treatments were indicated either as primary exclusive treatment or in adjuvant after incomplete surgery or after post-surgery recurrence. The median delivered dose was 13 Gy (RBE) prescribed to the 90% isodose line. After a median follow-up of 32 months, MRI revealed that 33 meningiomas remained stable, 13 showed a decrease in size, and five worsened. The 3-year tumor control rate was 94%. Symptoms were improved in 47% of patients (20). Regardless of the fractionation of the irradiation, the complication rates have been low and manageable (10,13,15,20).
Pituitary adenoma
Following surgical management of pituitary adenoma, watchful waiting, medical therapy or irradiation is considered. Although slow regrowth is common, the natural evolution of untreated tumors is variable. Conservative follow-up is associated with progression rates of over 40%. The role of radiotherapy in pituitary adenomas is well-established (21), especially when medical and surgical options have been extensively used. The radiotherapeutic intents are to halt of tumor growth, prevent problems from mass effect and normalize excessive hormone secretion. While radiation is highly effective in preventing residual tumor growth, it has long-term side effects (22). For all these reasons, particle treatment can be useful to improve therapeutic ratio by limiting the dose in the critical organs neighboring the tumor. Furthermore, because these patients are often relatively young, reduction in the integral dose in normal tissue can limit the risk of radiation-induced second malignancy. The absent exit dose offered by protons may result in fewer irreversible late sequelae, which is especially important in the cases of benign tumors, when normal life expectancy is predictable. In a recent large series, the most frequent adenoma types were Cushing disease (48% of cases) and growth hormone-secreting adenoma (37% of cases) (23).
Data on proton therapy for pituitary adenomas are available both with the schemes of conventional fractionation at a median dose of 54 Gy (RBE) (24) and with SSSP approach at a median dose of 20 Gy (25,26). In a small series of 22 patients treated with proton stereotactic irradiation for persistent acromegaly at a median follow-up of 6.3 years, the biochemical remission of disease was observed in 59% of the patients (25). As in the photon therapy series, time to response was long (42 months) (25). In a retrospective series of 33 patients presenting Cushing’s disease with a median follow-up of 62 months, normalization of plasma and urinary free cortisol was achieved in 52% of the patients, with a time to remission of 18 months (26). However, a larger series was published recently and reported the outcome of 165 patients, and among them, 119 were evaluable for the three following features: imaging, hormonal response status and risk of hypopituitarism (23). SSSP was delivered at a dose of 20 Gy (RBE) in 92% of the patients. With a median follow-up of 52 months, but at least 6 months, the 5-year biochemical complete response ranged from 38% to 75%, depending on the type of hormonal secretion. The median time to obtain complete response ranged from 27 to 62 months (23). The small number of cases treated and the limited follow-up prevent definitive conclusions. Proton therapy provides more conformal dosimetric coverage of the pituitary gland than photon-based treatments; which could be mainly beneficial for pediatric or young adult patients (26-28).
In these series, regardless of the fractionation, the most commonly reported toxicity was new pituitary deficits of one or multiple axes. In the series of Wattson et al., the actuarial 3- and 5-year rates of at least one new axis deficiency requiring replacement were 45% and 62%, respectively. The actuarial median time to hypopituitarism after treatment was 40 months (23). Seizures appeared in less than 2% of the patients and were often correlated with temporal MRI changes. Cases of transient 3rd or 6th nerve palsies have been described (23), whereas no visual defects have been reported. These series have observed no secondary tumors that could be correlated to the proton-irradiated area (23-26). One explanation of the high risk of hypopituitarism in the larger proton series is that the entire gland was included in the target volume (23). It remains to be seen if the use of pencil-beam scanning (PBS) proton therapy could more conformally treat the target volume while shielding sufficient volume of the pituitary gland to preserve pituitary function.
Schwannoma/acoustic neuroma
Acoustic neuroma is the most studied disease to which photon stereotactic irradiation was applied as a standard up-front treatment. Stereotactic irradiation is an effective treatment for acoustic neuroma with high LC and low long-term toxicity rates (29-32). Doses of 12–13 Gy in one fraction resulted in an actuarial 5-year tumor control between 92% and 100% (29). Current evidence supports the use of single-fraction radiosurgery for small to medium sized primary and recurrent vestibular schwannomas. This irradiation approach has also been recommended for adjuvant therapy following subtotal resection, poor surgical candidates, and patients who decline surgery or observation (33).
In the context of the excellent reported results with photons, the role of proton therapy remains to be determined. Notably, the diagnosis of vestibular schwannoma rather than the treatment strategy most significantly impacts quality of life (34). Proton therapy has been used either with conventional fractionation (35) or with stereotactic schemes with a satisfactory level of hearing, facial nerve, and trigeminal nerve preservation and with LC rates of 84–100% (36-38). Weber et al. reported on 88 patients treated at the MGH between 1992 and 2000 with SSSP (38). The median dose was 10–18 Gy (RBE) prescribed to a median isodose line of 70%. At a median follow-up of 38.7 months, the actuarial 2- and 5-year tumor control rates were 95.3% and 93.6%, respectively. Among patients with functional hearing before treatment, 33% maintained efficient hearing. Actuarial 5-year normal facial and trigeminal nerve function preservation rates were 91% and 89%, respectively. Using HSP to a total dose of 26 Gy (RBE) in three fractions in 51 inoperable patients, Vernimmen et al. reported a 5-year LC rate of 98% with a median follow-up of 72 months (37). In these series, the complications rates were low (37,38). However, Weber et al. concluded that a reduction in prescribed dose is associated with a decreased risk of facial neuropathy (38). Interestingly, Niu et al. recently showed a higher probability of tumor growth after radiation therapy with tumors having a faster growth rate before irradiation (39), an observation that should be taken into account when counseling patients regarding treatment.
Craniopharyngioma
The treatment of craniopharyngioma is based on surgical management with transcranial approaches or endoscopic endonasal surgery eventually followed by radiotherapy. Radiotherapy is usually indicated after incomplete or debulking surgery, at the time of first diagnosis or at progression (40). Fractionated radiation regimens are more commonly employed as proximity of tumor to the optic apparatus often renders radiosurgical modalities unsafe. The expected 5- and 10-year LC rates were approximately 80–90% (40).
There are several reasons to favor the use of proton therapy in craniopharyngioma. Craniopharyngioma is a benign tumor arising mainly in children with a long life expectancy of survivors. The incidence of radiation-induced tumors is expected to be reduced using proton therapy given its significant reduction in integral dose. In addition, because of the dose distribution of proton therapy with pencil-beam delivery, organs at risk (OAR) should receive a reduced dose compared to 3DRT, IMRT and passively scattered proton therapy (41-43).
Merchant et al. evaluated 3D imaging and treatment-planning data, including targeted tumor and normal tissues volumes (entire brain, temporal lobes, cochlea, hypothalamus) of ten craniopharyngioma patients. Dose-volume data were compared based on proton and photon treatment modalities using dose-cognitive side effects models. Craniopharyngioma target volume coverage was similar with both treatment modalities. With proton therapy, cochleae, hypothalamus, and normal tissue volumes, such as supratentorial brain or temporal lobes received less of the low and intermediate doses than with photon therapy. This decrease of dose was predicted to translate to a higher IQ score for craniopharyngioma patients treated with protons (44). The first report of cognitive consequences after proton therapy for varied pediatric tumors, including craniopharyngiomas, seems encouraging, with improved cognitive test results compared to those previously reported with photon therapy (44).
Boehling et al. compared 3D conformal proton therapy, IMPT and IMRT plans of ten pediatric patients presenting with craniopharyngioma. The target volume coverage was adequate and comparable for all modalities, but 3D-proton therapy and IMPT reduced the integral dose to critical organs (42). Beltran et al. obtained the same results using similar techniques in 14 craniopharyngioma cases (41).
Preliminary clinical results of 16 craniopharyngioma patients treated with post-operative proton therapy at a total dose of 50.4–59.4 Gy (RBE) were reported by Luu et al. (45). After a mean follow-up of 62 months, LC and OS were 93% and 80%, respectively, and 75% of patients did not develop any late complications. In a series of 15 patients treated with a combination of photons and protons, with a dose reaching 56.9 Gy (RBE), Fitzek et al. reported a 5- and 10-year LC rates of 93% and 85%, respectively and a 10-year OS rate of 72%. Although, no formal neuropsychological testing has been performed, the measures of lifestyle and professional accomplishments appeared to be satisfactory (46). The largest series included a cohort of 52 pediatric patients who were treated between 1996 and 2012, with either proton or photon radiation. At 59.6 months median follow-up, for all patients, the 3-year OS, nodular failure-free and cystic failure-free survival rates were 96%, 95% and 76%, respectively. No survival rates differed between treatment groups. Immediately after therapy, 17 patients developed cyst growth, more commonly in the photon group than in the proton group. Early toxicity profiles were comparable between both groups but follow-up was too short to demonstrate any reduction in late effects (47).
Because secondary neutrons are directly dependent on beam energy, modulation technique, treatment configuration and methodology, improvement towards pencil-beam should dramatically decrease this potentially neutron-induced risk (48-50). This hypothesis was tested in a retrospective planning study involving six pediatric patients previously treated with passive scattered protons (51). This analysis compared passive scattering, IMPT and IMRT. Proton therapy was dosimetrically superior to IMRT, especially at the lower dose region of the dose-volume histograms. Approximately 1.5 to 4 times less volume of soft tissue and 5 to 6.5 times less brain volume were irradiated by protons compared to photons (51).
However, given its high-dose conformality and thus sensitivity to target changes (42), the use of IMPT requires vigorous patient monitoring since 33% of craniopharyngiomas develop cystic changes during treatment (47). Therefore, patients should be closely monitored during treatment by IMPT, typically with weekly or biweekly MRIs (41).
Olfactory neuroblastoma/esthesioneuroblastoma/adenoid cystic carcinoma/neuroendocrine tumors
Olfactory neuroblastoma or esthesioneuroblastoma are relatively uncommon tumors of the frontal skull base believed to originate from olfactory stem cells of neural crest origin. These tumors are often associated with high rates of tumor recurrence and mortality. A meta-analysis written by Dulguerov et al. (52) demonstrated that surgery with radiation is the most frequently used therapeutic approach and achieves the highest cure rates.
Because of the aggressiveness of this tumor and its ability to locally relapse, an increase of radiation dose should be relevant and is often prescribed. However, the anatomic location in close proximity to critical organs limits the curative potential. Proton therapy is an option to improve outcome of these patients. The first report of the use of proton therapy was published in 1997; nine cases were treated with a combination of photons and protons up to 68 Gy (RBE). The radiation was preceded by chemotherapy. All patients but one were responders to chemotherapy and avoided surgery. No patients relapsed with a median follow-up of 14 months. No complications were described (53). Nichols et al. reported the initial experience of ten patients who underwent surgical resection followed by adjuvant proton therapy. The 5-year disease-free and OS rates were 90% and 85.7%, respectively (54). This first analysis was recently updated with 22 patients followed-up at a median time of 73 months (55). Patients were mostly managed with upfront craniofacial resection followed by adjuvant proton therapy. Concurrent chemotherapy was associated in five cases. The median irradiation dose was 66.5 Gy (RBE), and approximately 1/3 of the patients received a part of their irradiation with photons beams in order to prophylactically or curatively irradiate the nodal basins. The 5-year OS and disease-free survival rates were 95.2% and 86.4%, respectively (55). Nishimura et al. reported 14 cases, 7 of which were operated on and then irradiated with proton therapy to a dose of 65 Gy (RBE) with fractions of 2.5 Gy (RBE). With median follow-up of 40 months, 5-year OS, local progression-free survival (PFS) and relapse-free survival rates were 93%, 84%, and 71%, respectively (56). Fitzek et al. reported an original schedule of 69.2 Gy (RBE) using 1.6–1.8 CGE per fraction twice daily in a concomitant boost schedule used in 19 patients with neuroendocrine or neuroblastoma tumors. With a follow-up of 45 months, the 5-year OS and LC rates were 74% and 88%, respectively (57). Pommier et al. reported a series of 23 cases of adenoid cystic carcinoma invading the base of the skull that were treated with proton therapy. The surgery was biopsy or partial resection in 2/3 of the patients. The median irradiation dose was 75.9 Gy (RBE), and some of the patients were irradiated by a combination of photons and protons to cover the nodal basins. With a median follow-up of 66 months, the 5-year LC, disease-free and OS rates were 93%, 56% and 77%, respectively (58).
Childhood and adolescent esthesioneuroblastoma series are rare (59-61). Applying the adult proton therapy approach provides acceptable results (61,62) although the published series reported only eight cases (61). However, the authors recommended that because radiation doses should be chosen on an individual basis, and given the risks of toxicity in children, conservative doses such as 54–59.4 Gy are attractive options for the younger patients. Elective nodal irradiation remains controversial and is largely dependent on upfront Kadish staging (61,63,64).
In a seminal systematic review and meta-analysis of paranasal sinus and nasal cavity malignancies (65), the use of charged particle therapy was associated an improvement in OS as compared to photon irradiation. The majority of the patients in the charged particle therapy cohort were treated with proton therapy, but the cohort also included patients treated with carbon or other ion therapy. In a subgroup analysis, proton therapy was associated with an improvement in 5-year disease-free survival (relative risk, 1.44; P=0.045) with a trend to improvement in 5-year OS (relative risk, 1.39; P=0.057) (65).
Complications are mainly grade 2 optic tracts impairments (53,55-58,61,66) but some patients developed grade 4 unilateral blindness or retinopathy (53,58,66). T1 MRI changes manifesting as seizures controlled by medication have been described (64) but the death of a patient from the toxic effects of radiation-induced brain injury has also been reported (58). Other complications are grade 3 wound healing and infection (56), which may potentially lead to the patient death because of meningitis (58).
Glomus jugulare/chemodectoma
Head and neck paragangliomas are rare tumors of the paraganglia. They consist of chromaffin tissue and are associated with the parasympathetic autonomic nervous system. Due to their location in close proximity to important neurovascular structures, tumor growth may lead to serious morbidity and cranial nerve impairment. However, the majority of head and neck paragangliomas are benign indolent tumors, and a ‘‘wait and scan’’ policy may be judicious in appropriate cases (67). Goals of treatment are to improve symptoms and to obtain relief as long as possible without side effects or complications. Surgery can cure the disease but is associated with a risk of nerve impairment and complications, reported in up to 60% of cases, especially for tumors localized in the base of the skull. Furthermore, arterial side-effects including carotid complications, are not unusual and can be life-threatening. Radiotherapy is an appropriate therapeutic option. Irradiation produces fibrosis and vascular sclerosis rather than eradication of tumor cells. Because of the natural indolence of the tumor, assessment of the radiotherapy efficiency requires long-term follow-up. Radiation techniques are variable; external beam irradiation, IMRT or stereotactic treatment (67-69). Delivered doses were not uniform, and some series have proposed as a reference either 45–50 Gy in classical fractionation or 14 Gy at the periphery of the lesion by monofractionated radiosurgery (68). Radiotherapy can lead to radiographic response in approximately 1/3 of patients and LC in 70–100% of patients (67). With photon irradiation, regardless of the technique, complications were rarely reported. Although radiation is rarely the cause of death, worsening of neurologic problems or the appearance of new cranial nerve palsies can lead to death by swallowing complications (68). Given the effectiveness of conventional radiotherapy approaches, the role of proton therapy and its ability to shield surrounding tissues is limited to malignant paragangliomas requiring dose-escalation for tumor control and in hereditary syndromes involving paragangliomas, in which case proton therapy can have meaningful reduction in the risk of second-cancer induction (70).
Chordoma and chondrosarcomas
Chordoma and chondrosarcoma have been grouped together historically because of their midline presentation, similar radiography, and confusion in initial pathology. However, these lesions are distinct clinicopathological entities and vary significantly in their clinical outcome.
Chordoma
Chordomas are rare tumors arising from the remnants of the notochord, with an incidence rate in the 0.1/1,000,000 per year range (71,72), accounting for 1–4% of all primary malignant bone tumors (73). These tumors arise from the sacrum, skull base, and spine, with base of the skull presentations representing one third of the cases. The median age at diagnosis ranges between 44 and 61 years (74-78), with skull base presentations generally affecting a younger population, including children (79-81), and being more frequent in females (74-76,79). A small number of familial cases of chordoma have been reported, which suggests the potential for genetic predisposition (82-84). Up to 40% of patients referred with the diagnosis of chordoma could be re-classified as chondrosarcomas (77).
The clinical course of the disease is usually slow and local; the median time from initial symptoms to diagnosis is longer than 2 years, with a clinical presentation at onset that varies according to tumor site of origin. Local recurrence is the main problem even in long-term follow-up, but chordoma can also metastasize or relapse in unexpected sites (85,86).
Chordomas are low-grade malignancies with low metastatic potential, divided into conventional, chondroid, and dedifferentiated histopathologic categories. Conventional (typical) chordomas are the most common; the aggressive “dedifferentiated” variety can occur in a minority of patients with a less benign evolution (87). Histological differentiation from chordomas is often difficult and must include immunohistochemical staining (88,89). Chordoma is immunopositive for epithelial markers, such as cytokeratin and endothelial membrane antigen (EMA), and can also be positive for S-100 and vimentin (90). Brachyury was recognized as the diagnostic hallmark for chordoma and is helpful for distinction of chordoma from histological entities with similar morphological or immunophenotypic features (84).
Classically, treatment is local and requires surgery followed by dose-escalated radiotherapy. Objectives of surgery are pathologic analysis and the maximally safe tumor removal. Removal is often if not always incomplete because of the infiltrative nature of the tumor in bone and soft tissues and its proximity to adjacent base of the skull anatomical structures. The difficulty in achieving complete removal leads to a high likelihood of residual tumor after surgery (91) and a relatively high risk of relapse. While there is no direct relation between size of residual tumor and relapse risk (3), the last international recommendations concluded that the quality and extent of surgical removal are important determinants of therapeutic outcome (91). Adjuvant radiotherapy provides an acceptable tumor control if the dose of radiotherapy exceeded 70 Gy. However, this dose can be difficult to reach given the radiobiologic sensitivity of adjacent critical OAR. Particle therapy has been used in combination or in instead of photons to improve this therapeutic ratio. The principal rationale for the use of protons has been to reduce the dose to the brainstem, optic apparatus and temporal lobes, and proton therapy should be considered as monotherapy or in combination with photon therapy to permit safer dose-escalation to the primary tumor and improved tumor control and survival (91,92).
In addition to multiple articles reporting successively updated series, the literature includes seven articles (74-79,93,94) and among them, two reported pediatric cases (79,80) (Tables 1,2). Most of the studies are retrospective analyses (77), and the series of Noël et al. reported 100 patients, with ten patients with upper cervical chordoma (78). Others series presented fewer cases. Multiple overlaps of the series from the same institution do not allow for the inter-comparison or cumulative assessment of the series (92).
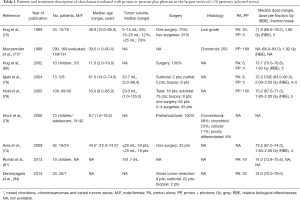
Full table
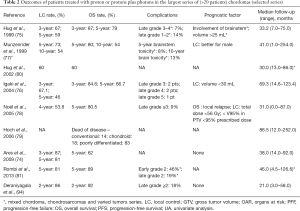
Full table
The sex ratio (M/F) was not significant (female to male, at 1:1.07). The median follow-up duration was between 29 and 86.5 months. The mean total dose ranged from 66 to 83 Gy (RBE), and at least 1/3 of the patients received a combination of radiation and proton therapy. The last international recommendations propose a dose of 74 Gy with a conventional fractionation and a combination of photons and protons (91). All studies, except two (93,95), used a passive scattering beam to deliver treatment; patients who were not treated with passive scattering received irradiation by spot scanning treatment (pencil-beam irradiation).
Outcomes were reported at different time points. Five-year LC ranged between 46% and 73% whereas 5-year OS rates ranged from 66.7% to 80.5%. The largest published study reported 10-year OS and LC rates of 54% (77).
Chondrosarcomas
Chondrosarcomas occur most commonly at the petroclival junction and comprise in 0.15% and 6% of all intracranial and skull-base tumors, respectively (96-99). A chondrosarcoma is a rare malignant bone tumor and represents a heterogeneous group of neoplasms with tumor cells producing a cartilage matrix, originating from endochondral bones. At the base of the skull, common sites of involvement are usually represented by the temporo occipital junction, parasellar area, spheno-ethmoid complex, and clivus (89). Chondrosarcomas tend to arise from the off-axis part of the skull base in contrast to chordomas (100). According to the WHO categories, there are three classes: grade I (well-differentiated), grade II (moderately differentiated) and grade III (dedifferentiated). The classes determine the outcome of the patients: the lower-grade tumors are usually indolent and have minimal malignant potential regardless of their location and stage of presentation; the less frequent dedifferentiated and mesenchymal subtypes exhibit both an anaplastic appearance and more aggressive behavior (101) and are associated with the lowest survival rates (97). There are also three histological subgroups: classic, mesenchymal and myxoid (89). The mesenchymal type has more aggressive growth behaviour and is associated with a poorer prognosis. Immunhistochemical staining shows some particular features of chondrosarcomas, negative for cytokeratin and EMA, but positive for S-100 and vimentin (90). Specific associations with Ollier disease or Maffucci syndrome or Paget disease and malignant transformation of giant cell tumors have been described (102,103).
Skull base chondrosarcomas are slow-growing tumors that gradually progress in the base of the skull structures from abutting or encasing to subsequently invading critical organs. Most patients are asymptomatic, or develop symptoms at a late stage of the disease. Consequently, at diagnosis, the tumor infiltrates adjacent critical structures, making complete removal difficult or even impossible. Therefore, diagnosed treatment requires a multidisciplinary approach (104,105). Because of this loco-regional evolution, durable LC can be challenging.
Surgery remains the key to the treatment. Maximal surgery is required in the chondrosarcomas. However, to achieve an optimal surgery, several interventions are often required. Consequently, the risk of complications increases. Because of this high risk of complications associated with wide resection, limited removal is usually proposed. A large debulking allows for safer irradiation away from surrounding critical organs. Adjuvant radiation therapy is also prescribed with excellent results compared to surgery alone (97).
Concerning radiation therapy, the efficacy of treatment is difficult to analyze for varied reasons: the rareness of the tumors, the long history of the disease, beneficing of several treatment procedures with updated techniques with time and finally the series mix of chordoma and chondrosarcomas (75,77,93,95,106-109) (Tables 3,4). Nevertheless, the required doses to control the disease significantly exceed the dose constraints for critical organs. Proton therapy is considered the optimal method for dose-gradient irradiation to irregularly shaped targets closely juxtaposed to critical organs. Proton therapy have been used mainly with conventional fractionation at doses between 67 and 83 Gy (RBE), even to large target volumes with 3- and 5-year LC rates ranges between 85% and 100% and between 75% and 99%, respectively (75,76,89,93,112). The single series with an extended follow-up of 10 years reported a LC rate of 87–98% (77,89,111). Three- and 5-year OS ranged between 93.8% and 100% and 99–100%, respectively. These rates remained equivalent at 10 years (75,77,89,93,111,112). However, inferior outcomes have been observed in mesenchymal subtypes of chondrosarcoma (113). In these series, almost all patients were treated with a traditional passive scattering technique with brass apertures, compensators, and range shifter wheels using a “patching” strategy to overcome the risk of overdosing critical normal tissues in tumors with complex shapes. Three series of 5 to 15 adults patients with chordomas or chondrosarcomas (93,95) and a series of 26 children (81) were treated with a spot-scanning beam technique. The largest series of PBS proton therapy for skull base chondrosarcomas demonstrated 8-year LC and OS of 90% and 94%, respectively, with large tumor size (>25 cc) and compression of adjacent brainstem or optic apparatus associated with inferior LC. Grade 3 or higher toxicity was observed in 8% of patients (110).
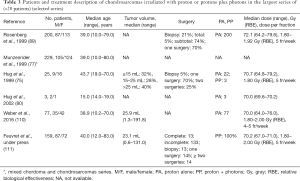
Full table
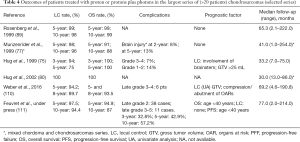
Full table
Complications are rare. During treatment almost patients described one or several of the following side effects: asthenia, loss of appetite, transitory temporal and/or fronto-parietal alopecia, mild erythema and nausea (107). In the most recent series (111) there were 11 cases of grades 3–5 late complications, leading to 3-, 5- and 10-year grades 3–5 toxicity rates of 6.4%, 10%, and 10%, respectively. Studying quality of life in patients with varied tumors of the base of the skull, Srivastava et al. concluded that apart from global health status and physical functioning scores, there was no change in most patients when comparing scores prior to and at the completion of radiotherapy. When comparing the clinically important differences, the results were heterogeneous, with some patients improving and others deteriorating (108). At the end of treatment, improvement of the mini mental test was the rule, although in some cases a transient deterioration was found (109).
Complications of base of the skull proton irradiation
For early side effects (up to 6 months after irradiation completion) of the brain or the base of the skull, Combs et al. reported a list of side effects in 157 patients treated with particle therapy (proton therapy or other ion therapy) for base of the skull or brain tumors. The most frequent were hair loss (37% of the patients), visual deficits (28% of patients), headaches, motor deficits and fatigue (27% each). These outcomes were similar to those observed with photon irradiation (114). In the setting of chordoma and chondrosarcoma of the skull base, where dose-escalation is required, important planning constraints have been proposed based on available published literature for brainstem, optic apparatus, temporal lobes and spinal cord (90,97).
The most frequently described complications are pituitary gland insufficiency, ocular pathway damage, sensorineural nearing loss and temporal lobe necrosis. De Marzi et al. proposed a generalized equivalent uniform dose (gEUD)-based NTCP to predict the risk of pituitary complications (115). A pretreatment analysis of the gland function was proposed for all patients because some deficiencies exist before irradiation (4). For internal ear and optic pathways, dose limits are well established, however, some exceptional complications can appear at lower doses (112).
In a series of 66 patients, McDonald et al. showed that the risk of radionecrosis was related to the dose-volume histogram of the temporal dose by multivariate analysis. In the EC50 model, all dose levels from 10 to 70 Gy (RBE) were highly correlated with radiation necrosis, with a 15% 3-year risk of any-grade temporal lobe radiation necrosis when the absolute volume of a temporal lobe receiving 60 Gy (RBE) or V60 Gy (RBE) exceeded 5.5 cm3 or a V70 Gy (RBE) >1.7 cm3 (116). In a previous series of 62 patients, Pehlivan et al. showed that temporal necrosis was highly correlated with the generalized equivalent uniform dose (117). Weber et al. demonstrated that although total dose was not a predictor of the efficacy of radiation, it was prognostic of the late complication, mainly for patients who received more than 70 Gy (RBE) (110). Igaki et al. reported three cases of necrosis grades 4–5 for patients who received between 92.8 and 113.3 Gy (76).
Pencil-beam system
Because of the physical characteristics of radiation deposition, known as Bragg peak deposition, protons offer a steeper dose gradient to the OARs close to the target volume compared with non-particle radiotherapy. Historically, and in the majority of treatment rooms, proton therapy has been delivered most commonly by passive scattering techniques, in which customized metal apertures and lucent compensators are used to shape the lateral and distal aspects of individual proton beams. In contrast, with spot scanning proton therapy, a small original proton beam is magnetically scanned to cover the lateral aspects of the target. This technique, PBS, can dynamically position the Bragg peaks throughout the target volume. The depth of the dose is controlled by the use of different proton beam energies as well as range shifters. PBS has the ability to conform to the target dose 3-dimensionally within a single field. The scanning beam allows greater control over the proximal properties of the beam and potentially improved conformality of high-dose regions (118). Moreover, similar to up-to-date photon therapy techniques, the optimized proton dose distribution can be achieved with increased conformality, and the scanning proton beam permits multiple target volumes to be treated to separate doses through the use of a simultaneous integrated boost (SIB) technique (95). The superposition of multiple pencil-proton beamlets with near mono-energetic Bragg peaks constitutes the treated volume (118). One other advantage of PBS over passive delivery systems is that the neutron production, resulting when protons hit material (range shifter, modulation wheel, aperture) and potentially associated with cancer induction, is substantially reduced (119).
Moreover, unlike passive scattering, the ability of either single-field optimization (SFO) or multi-field optimization (MFO) to modulate the proximal aspect of the individual beam frequently afforded some sparing of the normal tissue when target volumes were at a greater depth (95). By comparing passive scattering and scanning beam, Grosshans et al. showed that in 15 cases of chordomas and chondrosarcomas, the primary target was similarly covered with both techniques. However, the brainstem maximum dose was non-statistically lower when using the scanning beam; the opposite was observed for the chiasma. However, the volume of brainstem receiving at least 60 Gy and the volume of temporal lobes receiving at least 70 Gy were statistically lower with scanning beam compared to passive scattering (95).
Conclusions
The role of proton therapy for skull base tumors is variable by histology. For malignancies of the paranasal sinuses and nasal cavity and for chordomas and chondrosarcomas, the ability of proton therapy to escalate radiation dose close to critical and radiosensitive OAR such as the optic apparatus and brainstem has been associated with improved tumor control and, in the case of malignancies of the paranasal sinus and nasal cavity, improved OS as compared to photon irradiation. For benign tumors of the skull base, such as pituitary adenomas, craniopharyngiomas, and benign meningiomas, proton therapy can more effectively shield the remaining normal brain parenchyma in a manner that can lower radiation-induced secondary malignancy risk and potentially cognitive effects. The emergence of PBS technologies has the potential to further enhance these benefits of proton therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: V Gondi has received speaking honoraria from prIME Oncology and US Oncology. None of these activities are related to this paper. Dr. Noel has no conflicts of interest to declare.
References
- Combs SE, Ellerbrock M, Haberer T, et al. Heidelberg Ion Therapy Center (HIT): Initial clinical experience in the first 80 patients. Acta Oncol 2010;49:1132-40. [Crossref] [PubMed]
- Debus J, Schulz-Ertner D, Schad L, et al. Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int J Radiat Oncol Biol Phys 2000;47:591-6. [Crossref] [PubMed]
- Jahangiri A, Chin AT, Wagner JR, et al. Factors predicting recurrence after resection of clival chordoma using variable surgical approaches and radiation modalities. Neurosurgery 2015;76:179-85; discussion 185-6. [Crossref] [PubMed]
- Noël G, Bollet MA, Calugaru V, et al. Functional outcome of patients with benign meningioma treated by 3D conformal irradiation with a combination of photons and protons. Int J Radiat Oncol Biol Phys 2005;62:1412-22. [Crossref] [PubMed]
- Sindou M, Nebbal M, Guclu B. Cavernous sinus meningiomas: imaging and surgical strategy. Adv Tech Stand Neurosurg 2015;42:103-21. [Crossref] [PubMed]
- Pechlivanis I, Wawrzyniak S, Engelhardt M, et al. Evidence level in the treatment of meningioma with focus on the comparison between surgery versus radiotherapy. A review. J Neurosurg Sci 2011;55:319-28. [PubMed]
- Cusimano MD, Sekhar LN, Sen CN, et al. The results of surgery for benign tumors of the cavernous sinus. Neurosurgery 1995;37:1-9; discussion 9-10. [Crossref] [PubMed]
- Glaholm J, Bloom HJ, Crow JH. The role of radiotherapy in the management of intracranial meningiomas: the Royal Marsden Hospital experience with 186 patients. Int J Radiat Oncol Biol Phys 1990;18:755-61. [Crossref] [PubMed]
- Newman SA. Meningiomas: a quest for the optimum therapy. J Neurosurg 1994;80:191-4. [Crossref] [PubMed]
- Noël G, Habrand JL, Mammar H, et al. Highly conformal therapy using proton component in the management of meningiomas. Preliminary experience of the Centre de Protonthérapie d'Orsay. Strahlenther Onkol 2002;178:480-5. [Crossref] [PubMed]
- Gudjonsson O, Blomquist E, Nyberg G, et al. Stereotactic irradiation of skull base meningiomas with high energy protons. Acta Neurochir (Wien) 1999;141:933-40. [Crossref] [PubMed]
- Vernimmen FJ, Harris JK, Wilson JA, et al. Stereotactic proton beam therapy of skull base meningiomas. Int J Radiat Oncol Biol Phys 2001;49:99-105. [Crossref] [PubMed]
- Weber DC, Schneider R, Goitein G, et al. Spot scanning-based proton therapy for intracranial meningioma: long-term results from the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys 2012;83:865-71. [Crossref] [PubMed]
- Wenkel E, Thornton AF, Finkelstein D, et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys 2000;48:1363-70. [Crossref] [PubMed]
- Slater JD, Loredo LN, Chung A, et al. Fractionated proton radiotherapy for benign cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 2012;83:e633-7. [Crossref] [PubMed]
- Mendenhall WM, Friedman WA, Amdur RJ, et al. Management of benign skull base meningiomas: a review. Skull Base 2004;14:53-60; discussion 61. [Crossref] [PubMed]
- Boskos C, Feuvret L, Noel G, et al. Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys 2009;75:399-406. [Crossref] [PubMed]
- Chan AW, Bernstein KD, Adams JA, et al. Dose escalation with proton radiation therapy for high-grade meningiomas. Technol Cancer Res Treat 2012;11:607-14. [PubMed]
- Kosaki K, Ecker S, Habermehl D, et al. Comparison of intensity modulated radiotherapy (IMRT) with intensity modulated particle therapy (IMPT) using fixed beams or an ion gantry for the treatment of patients with skull base meningiomas. Radiat Oncol 2012;7:44. [Crossref] [PubMed]
- Halasz LM, Bussière MR, Dennis ER, et al. Proton stereotactic radiosurgery for the treatment of benign meningiomas. Int J Radiat Oncol Biol Phys 2011;81:1428-35. [Crossref] [PubMed]
- Loeffler JS, Shih HA. Radiation therapy in the management of pituitary adenomas. J Clin Endocrinol Metab 2011;96:1992-2003. [Crossref] [PubMed]
- Greenman Y, Stern N. Optimal management of non-functioning pituitary adenomas. Endocrine 2015;50:51-5. [Crossref] [PubMed]
- Wattson DA, Tanguturi SK, Spiegel DY, et al. Outcomes of proton therapy for patients with functional pituitary adenomas. Int J Radiat Oncol Biol Phys 2014;90:532-9. [Crossref] [PubMed]
- Ronson BB, Schulte RW, Han KP, et al. Fractionated proton beam irradiation of pituitary adenomas. Int J Radiat Oncol Biol Phys 2006;64:425-34. [Crossref] [PubMed]
- Petit JH, Biller BM, Coen JJ, et al. Proton stereotactic radiosurgery in management of persistent acromegaly. Endocr Pract 2007;13:726-34. [Crossref] [PubMed]
- Petit JH, Biller BM, Yock TI, et al. Proton stereotactic radiotherapy for persistent adrenocorticotropin-producing adenomas. J Clin Endocrinol Metab 2008;93:393-9. [Crossref] [PubMed]
- Chemaitilly W, Sklar CA. Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer 2010;17:R141-59. [Crossref] [PubMed]
- Mostoufi-Moab S, Grimberg A. Pediatric brain tumor treatment: growth consequences and their management. Pediatr Endocrinol Rev 2010;8:6-17. [PubMed]
- Murphy ES, Suh JH. Radiotherapy for vestibular schwannomas: a critical review. Int J Radiat Oncol Biol Phys 2011;79:985-97. [Crossref] [PubMed]
- Nakaya K, Niranjan A, Kondziolka D, et al. Gamma knife radiosurgery for benign tumors with symptoms from brainstem compression. Int J Radiat Oncol Biol Phys 2010;77:988-95. [Crossref] [PubMed]
- Sughrue ME, Yang I, Rutkowski MJ, et al. Preservation of facial nerve function after resection of vestibular schwannoma. Br J Neurosurg 2010;24:666-71. [Crossref] [PubMed]
- Yang I, Sughrue ME, Han SJ, et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg 2010;112:851-9. [Crossref] [PubMed]
- Conley GS, Hirsch BE. Stereotactic radiation treatment of vestibular schwannoma: indications, limitations, and outcomes. Curr Opin Otolaryngol Head Neck Surg 2010;18:351-6. [Crossref] [PubMed]
- Carlson ML, Tveiten OV, Driscoll CL, et al. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg 2015;122:833-42. [Crossref] [PubMed]
- Bush DA, McAllister CJ, Loredo LN, et al. Fractionated proton beam radiotherapy for acoustic neuroma. Neurosurgery 2002;50:270-3; discussion 273-5. [PubMed]
- Harsh GR, Thornton AF, Chapman PH, et al. Proton beam stereotactic radiosurgery of vestibular schwannomas. Int J Radiat Oncol Biol Phys 2002;54:35-44. [Crossref] [PubMed]
- Vernimmen FJ, Mohamed Z, Slabbert JP, et al. Long-term results of stereotactic proton beam radiotherapy for acoustic neuromas. Radiother Oncol 2009;90:208-12. [Crossref] [PubMed]
- Weber DC, Chan AW, Bussiere MR, et al. Proton beam radiosurgery for vestibular schwannoma: tumor control and cranial nerve toxicity. Neurosurgery 2003;53:577-86; discussion 586-8. [Crossref] [PubMed]
- Niu NN, Niemierko A, Larvie M, et al. Pretreatment growth rate predicts radiation response in vestibular schwannomas. Int J Radiat Oncol Biol Phys 2014;89:113-9. [Crossref] [PubMed]
- Kortmann RD. Different approaches in radiation therapy of craniopharyngioma. Front Endocrinol (Lausanne) 2011;2:100. [Crossref] [PubMed]
- Beltran C, Roca M, Merchant TE. On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int J Radiat Oncol Biol Phys 2012;82:e281-7. [Crossref] [PubMed]
- Boehling NS, Grosshans DR, Bluett JB, et al. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys 2012;82:643-52. [Crossref] [PubMed]
- Merchant TE, Hua CH, Shukla H, et al. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer 2008;51:110-7. [Crossref] [PubMed]
- Pulsifer MB, Sethi RV, Kuhlthau KA, et al. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys 2015;93:400-7. [Crossref] [PubMed]
- Luu QT, Loredo LN, Archambeau JO, et al. Fractionated proton radiation treatment for pediatric craniopharyngioma: preliminary report. Cancer J 2006;12:155-9. [PubMed]
- Fitzek MM, Linggood RM, Adams J, et al. Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at Harvard Cyclotron Laboratory and Massachusetts General Hospital. Int J Radiat Oncol Biol Phys 2006;64:1348-54. [Crossref] [PubMed]
- Bishop AJ, Greenfield B, Mahajan A, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys 2014;90:354-61. [Crossref] [PubMed]
- Schneider U, Agosteo S, Pedroni E, et al. Secondary neutron dose during proton therapy using spot scanning. Int J Radiat Oncol Biol Phys 2002;53:244-51. [Crossref] [PubMed]
- Farah J, Sayah R, Martinetti F, et al. Secondary neutron doses in proton therapy treatments of ocular melanoma and craniopharyngioma. Radiat Prot Dosimetry 2014;161:363-7. [Crossref] [PubMed]
- Amsbaugh MJ, Zhu XR, Palmer M, et al. Spot scanning proton therapy for craniopharyngioma. Pract Radiat Oncol 2012;2:314-8. [Crossref] [PubMed]
- Moteabbed M, Yock TI, Paganetti H. The risk of radiation-induced second cancers in the high to medium dose region: a comparison between passive and scanned proton therapy, IMRT and VMAT for pediatric patients with brain tumors. Phys Med Biol 2014;59:2883-99. [Crossref] [PubMed]
- Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol 2001;2:683-90. [Crossref] [PubMed]
- Bhattacharyya N, Thornton AF, Joseph MP, et al. Successful treatment of esthesioneuroblastoma and neuroendocrine carcinoma with combined chemotherapy and proton radiation. Results in 9 cases. Arch Otolaryngol Head Neck Surg 1997;123:34-40. [Crossref] [PubMed]
- Nichols AC, Chan AW, Curry WT, et al. Esthesioneuroblastoma: the massachusetts eye and ear infirmary and massachusetts general hospital experience with craniofacial resection, proton beam radiation, and chemotherapy. Skull Base 2008;18:327-37. [Crossref] [PubMed]
- Herr MW, Sethi RK, Meier JC, et al. Esthesioneuroblastoma: an update on the massachusetts eye and ear infirmary and massachusetts general hospital experience with craniofacial resection, proton beam radiation, and chemotherapy. J Neurol Surg B Skull Base 2014;75:58-64. [PubMed]
- Nishimura H, Ogino T, Kawashima M, et al. Proton-beam therapy for olfactory neuroblastoma. Int J Radiat Oncol Biol Phys 2007;68:758-62. [Crossref] [PubMed]
- Fitzek MM, Thornton AF, Varvares M, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer 2002;94:2623-34. [Crossref] [PubMed]
- Pommier P, Liebsch NJ, Deschler DG, et al. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg 2006;132:1242-9. [Crossref] [PubMed]
- Eich HT, Müller RP, Micke O, et al. Esthesioneuroblastoma in childhood and adolescence. Better prognosis with multimodal treatment? Strahlenther Onkol 2005;181:378-84. [Crossref] [PubMed]
- El Kababri M, Habrand JL, Valteau-Couanet D, et al. Esthesioneuroblastoma in children and adolescent: experience on 11 cases with literature review. J Pediatr Hematol Oncol 2014;36:91-5. [Crossref] [PubMed]
- Lucas JT Jr, Ladra MM, MacDonald SM, et al. Proton therapy for pediatric and adolescent esthesioneuroblastoma. Pediatr Blood Cancer 2015;62:1523-8. [Crossref] [PubMed]
- Keole SR. Proton therapy for esthesioneuroblastoma: Can we shed some light on a murky topic? Pediatr Blood Cancer 2015;62:1514-5. [Crossref] [PubMed]
- Demiroz C, Gutfeld O, Aboziada M, et al. Esthesioneuroblastoma: is there a need for elective neck treatment? Int J Radiat Oncol Biol Phys 2011;81:e255-61. [Crossref] [PubMed]
- Monroe AT, Hinerman RW, Amdur RJ, et al. Radiation therapy for esthesioneuroblastoma: rationale for elective neck irradiation. Head Neck 2003;25:529-34. [Crossref] [PubMed]
- Patel SH, Wang Z, Wong WW, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol 2014;15:1027-38. [Crossref] [PubMed]
- Morimoto K, Demizu Y, Hashimoto N, et al. Particle radiotherapy using protons or carbon ions for unresectable locally advanced head and neck cancers with skull base invasion. Jpn J Clin Oncol 2014;44:428-34. [Crossref] [PubMed]
- van Hulsteijn LT, Corssmit EP, Coremans IE, et al. Regression and local control rates after radiotherapy for jugulotympanic paragangliomas: systematic review and meta-analysis. Radiother Oncol 2013;106:161-8. [Crossref] [PubMed]
- Dupin C, Lang P, Dessard-Diana B, et al. Treatment of head and neck paragangliomas with external beam radiation therapy. Int J Radiat Oncol Biol Phys 2014;89:353-9. [Crossref] [PubMed]
- Galland-Girodet S, Maire JP, De-Mones E, et al. The role of radiation therapy in the management of head and neck paragangliomas: impact of quality of life versus treatment response. Radiother Oncol 2014;111:463-7. [Crossref] [PubMed]
- Hankinson TC, Ogden AT, Canoll P, et al. Intraorbital and intracranial soft-tissue glomus tumor in an 8-year-old child. Case report. J Neurosurg Pediatr 2008;1:389-91. [Crossref] [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43-66. [Crossref] [PubMed]
- Newton HB. Chordomas. In: Raghavan D, Brecher ML, Johnson DH, et al. editors. Textbook of uncommon cancer, 3rd ed. Chichester: Wiley, 2006:614-25.
- Mirra J, Nelson S, Della Rocca C. Chordoma. In: Fletcher CD, Unni KK, Mertens F. editors. Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press, 2002:316-7.
- Ares C, Hug EB, Lomax AJ, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys 2009;75:1111-8. [Crossref] [PubMed]
- Hug EB, Loredo LN, Slater JD, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg 1999;91:432-9. [Crossref] [PubMed]
- Igaki H, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys 2004;60:1120-6. [Crossref] [PubMed]
- Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol 1999;175 Suppl 2:57-63. [Crossref] [PubMed]
- Noël G, Feuvret L, Calugaru V, et al. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol 2005;44:700-8. [Crossref] [PubMed]
- Hoch BL, Nielsen GP, Liebsch NJ, et al. Base of skull chordomas in children and adolescents: a clinicopathologic study of 73 cases. Am J Surg Pathol 2006;30:811-8. [Crossref] [PubMed]
- Hug EB, Sweeney RA, Nurre PM, et al. Proton radiotherapy in management of pediatric base of skull tumors. Int J Radiat Oncol Biol Phys 2002;52:1017-24. [Crossref] [PubMed]
- Rombi B, Ares C, Hug EB, et al. Spot-scanning proton radiation therapy for pediatric chordoma and chondrosarcoma: clinical outcome of 26 patients treated at paul scherrer institute. Int J Radiat Oncol Biol Phys 2013;86:578-84. [Crossref] [PubMed]
- Bhadra AK, Casey AT. Familial chordoma. A report of two cases. J Bone Joint Surg Br 2006;88:634-6. [Crossref] [PubMed]
- Kelley MJ, Shi J, Ballew B, et al. Characterization of T gene sequence variants and germline duplications in familial and sporadic chordoma. Hum Genet 2014;133:1289-97. [Crossref] [PubMed]
- Yang XR, Ng D, Alcorta DA, et al. T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat Genet 2009;41:1176-8. [Crossref] [PubMed]
- Vogin G, Calugaru V, Bolle S, et al. Investigation of ectopic recurrent skull base and cervical chordomas: The Institut Curie's proton therapy center experience. Head Neck 2016;38 Suppl 1:E1238-46. [Crossref] [PubMed]
- Iloreta AM, Nyquist GG, Friedel M, et al. Surgical pathway seeding of clivo-cervical chordomas. J Neurol Surg Rep 2014;75:e246-50. [Crossref] [PubMed]
- Barnes L, Kapadia SB. The biology and pathology of selected skull base tumors. J Neurooncol 1994;20:213-40. [Crossref] [PubMed]
- Heffelfinger MJ, Dahlin DC, MacCarty CS, et al. Chordomas and cartilaginous tumors at the skull base. Cancer 1973;32:410-20. [Crossref] [PubMed]
- Rosenberg AE, Nielsen GP, Keel SB, et al. Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol 1999;23:1370-8. [Crossref] [PubMed]
- Lanzino G, Dumont AS, Lopes MB, et al. Skull base chordomas: overview of disease, management options, and outcome. Neurosurg Focus 2001;10:E12. [Crossref] [PubMed]
- Stacchiotti S, Sommer J, Chordoma Global Consensus Group. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol 2015;16:e71-83. [Crossref] [PubMed]
- Amichetti M, Cianchetti M, Amelio D, et al. Proton therapy in chordoma of the base of the skull: a systematic review. Neurosurg Rev 2009;32:403-16. [Crossref] [PubMed]
- Weber DC, Rutz HP, Pedroni ES, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: the Paul Scherrer Institut experience. Int J Radiat Oncol Biol Phys 2005;63:401-9. [Crossref] [PubMed]
- Deraniyagala RL, Yeung D, Mendenhall WM, et al. Proton therapy for skull base chordomas: an outcome study from the university of Florida proton therapy institute. J Neurol Surg B Skull Base 2014;75:53-7. [Crossref] [PubMed]
- Grosshans DR, Zhu XR, Melancon A, et al. Spot scanning proton therapy for malignancies of the base of skull: treatment planning, acute toxicities, and preliminary clinical outcomes. Int J Radiat Oncol Biol Phys 2014;90:540-6. [Crossref] [PubMed]
- Koch BB, Karnell LH, Hoffman HT, et al. National cancer database report on chondrosarcoma of the head and neck. Head Neck 2000;22:408-25. [Crossref] [PubMed]
- Bloch OG, Jian BJ, Yang I, et al. A systematic review of intracranial chondrosarcoma and survival. J Clin Neurosci 2009;16:1547-51. [Crossref] [PubMed]
- Lee SY, Lim YC, Song MH, et al. Chondrosarcoma of the head and neck. Yonsei Med J 2005;46:228-32. [Crossref] [PubMed]
- Oghalai JS, Buxbaum JL, Jackler RK, et al. Skull base chondrosarcoma originating from the petroclival junction. Otol Neurotol 2005;26:1052-60. [Crossref] [PubMed]
- Rapidis AD, Archondakis G, Anteriotis D, et al. Chondrosarcomas of the skull base: review of the literature and report of two cases. J Craniomaxillofac Surg 1997;25:322-7. [Crossref] [PubMed]
- Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer 1977;40:818-31. [Crossref] [PubMed]
- Noël G, Feuvret L, Calugaru V, et al. Chondrosarcomas of the base of the skull in Ollier's disease or Maffucci's syndrome--three case reports and review of the literature. Acta Oncol 2004;43:705-10. [Crossref] [PubMed]
- Noël G, Ben Jelloun H, Feuvret L, et al. Giant cell tumor of the base of the skull: a report of two cases and review of the literature. Cancer Radiother 2006;10:175-84. [PubMed]
- Crockard HA, Cheeseman A, Steel T, et al. A multidisciplinary team approach to skull base chondrosarcomas. J Neurosurg 2001;95:184-9. [Crossref] [PubMed]
- Lohnstein PU, Schipper J, Tatagiba M, et al. Skull base chondrosarcoma. An interdisciplinary challenge. HNO 2006;54:287-93. [Crossref] [PubMed]
- Fuji H, Nakasu Y, Ishida Y, et al. Feasibility of proton beam therapy for chordoma and chondrosarcoma of the skull base. Skull Base 2011;21:201-6. [Crossref] [PubMed]
- Noël G, Habrand JL, Jauffret E, et al. Radiation therapy for chordoma and chondrosarcoma of the skull base and the cervical spine. Prognostic factors and patterns of failure. Strahlenther Onkol 2003;179:241-8. [Crossref] [PubMed]
- Srivastava A, Vischioni B, Fiore MR, et al. Quality of life in patients with chordomas/chondrosarcomas during treatment with proton beam therapy. J Radiat Res 2013;54 Suppl 1:i43-8. [Crossref] [PubMed]
- Tuan J, Vischioni B, Fossati P, et al. Initial clinical experience with scanned proton beams at the Italian National Center for Hadrontherapy (CNAO). J Radiat Res 2013;54 Suppl 1:i31-42. [Crossref] [PubMed]
- Weber DC, Badiyan S, Malyapa R, et al. Long-term outcomes and prognostic factors of skull-base chondrosarcoma patients treated with pencil-beam scanning proton therapy at the Paul Scherrer Institute. Neuro Oncol 2016;18:236-43. [Crossref] [PubMed]
- Feuvret L, Bracci S, Calugaru V, et al. Efficacy and safety of adjuvant proton therapy combined with surgery for chondrosarcoma of the skull base: A retrospective, population-based study. Int J Radiat Oncol Biol Phys 2016;95:312-21. [Crossref] [PubMed]
- Noël G, Feuvret L, Ferrand R, et al. Radiotherapeutic factors in the management of cervical-basal chordomas and chondrosarcomas. Neurosurgery 2004;55:1252-60; discussion 1260-2. [PubMed]
- Bloch O, Parsa AT. Skull base chondrosarcoma: evidence-based treatment paradigms. Neurosurg Clin N Am 2013;24:89-96. [Crossref] [PubMed]
- Combs SE, Kessel K, Habermehl D, et al. Proton and carbon ion radiotherapy for primary brain tumors and tumors of the skull base. Acta Oncol 2013;52:1504-9. [Crossref] [PubMed]
- De Marzi L, Feuvret L, Boulé T, et al. Use of gEUD for predicting ear and pituitary gland damage following proton and photon radiation therapy. Br J Radiol 2015;88:20140413. [Crossref] [PubMed]
- McDonald MW, Linton OR, Calley CS. Dose-volume relationships associated with temporal lobe radiation necrosis after skull base proton beam therapy. Int J Radiat Oncol Biol Phys 2015;91:261-7. [Crossref] [PubMed]
- Pehlivan B, Ares C, Lomax AJ, et al. Temporal lobe toxicity analysis after proton radiation therapy for skull base tumors. Int J Radiat Oncol Biol Phys 2012;83:1432-40. [Crossref] [PubMed]
- Lomax AJ. Physics of treatment planning using scanned beams. In: Paganetti H. editor. Proton Therapy Physics. Boca Raton, FL: CRC Press Inc., 2011:335-80.
- Hälg RA, Besserer J, Boschung M, et al. Measurements of the neutron dose equivalent for various radiation qualities, treatment machines and delivery techniques in radiation therapy. Phys Med Biol 2014;59:2457-68. [Crossref] [PubMed]


