
Surgical management of breast cancer-related lymphedema: a narrative review of contemporary practices
Introduction
Lymphedema is a progressive, chronic, and incapacitating disorder resulting from the accumulation of protein-rich fluid in the interstitial space secondary to impaired lymphatic fluid drainage. This pathophysiologic process generates an unremitting cycle of inflammation, adipose tissue deposition, fibrosis, and extensive and progressive cutaneous architectural changes (1,2). Secondary lymphedema is the most common type of lymphedema. It develops when the normal functioning of the lymphatic system is disrupted due to blockages or injuries to the lymphatic vessels caused by inflammation, surgery, medical treatments, infections, or physical trauma (1). In developing countries, filarial infection remains the most prevalent cause of secondary lymphedema, while dissection and excision of lymphatic tissue and radiation are the most common cause of lymphedema in developed countries (1,3).
Improved screening and treatment strategies and higher survival rates in patients with breast cancer have contributed to an increased prevalence of breast cancer-related lymphedema (BCRL) (4-6). Patients with BCRL present with upper limb heaviness, pain, discomfort, pitting edema, decreased range of motion, recurrent episodes of soft tissue infections, cutaneous angiosarcoma, and elephantiasis. Furthermore, patients experience additional psychosocial burdens and a significantly affected quality of life (QoL) associated with anxiety, body image-related disorders, and depression (7,8).
Due to the heterogeneous diagnostic criteria, the incidence of BCRL ranges from less than 5% to more than 50% (4,9,10). In contemporary articles, meta-analytic models of prospective studies have estimated the incidence of unilateral arm lymphoedema after breast cancer at 21.4% (14.9% to 29.8%) (4). The risk of BCRL is associated with axillary lymph node dissection (ALND) and may correlate positively with the number of lymph nodes (LNs) excised (11,12). In secondary studies, the incidence of BCRL has been shown to be four times lower in patients receiving sentinel LN biopsy (5.6%) compared to ALND [19.9%; 95% confidence interval (CI): 13.5% to 28.2%] (4). Likewise, Kilbreath et al. reported that the incidence of BCRL for patients who have had less than five nodes removed was at 3.3%, while for patients with five or more nodes removed, the incidence was 18.2% (13). Loco-regional adjuvant radiation therapy has also been shown to be a significant factor associated with lymphedema (9,13-16). Current secondary studies evaluating the impact of radiation therapy on lymphedema have shown an increased risk of BCRL with the addition of regional nodal irradiation after ALND compared to ALND alone [odds ratio (OR), 2.74; 95% CI: 1.38 to 5.44] (14).
Breast cancer represents the most common oncologic pathology among women, with an age-standardized incidence rate of 65.5 cases per 100,000 women (17,18). BCRL not only represents a colossal burden in terms of health and patient-reported outcomes, but aggravates the stigmatization of cancer (7). Therefore, lymphatic surgical management plays a prominent role in the psychological and physical well-being of women suffering from this aftermath (7). In this study, we present a narrative review of the current surgical management of BCRL and analyze the postoperative surgical results and patient-reported outcomes. We present this article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-292/rc).
Methods
Research selection
We conducted a systematic search across PubMed MEDLINE, Scopus, and Web of Science from database inception through January 2, 2023. We included articles reporting surgical interventions for the management of BCRL or any preventive strategy using prophylactic lymphatic surgery (Figure 1). The following terms were used in different combinations: “lymphedema”, “lymphoedema”, “breast”, and “cancer” (Table 1). We included case reports, case series, longitudinal studies, randomized-controlled trials, meta-analyses, and systematic reviews. We excluded commentaries and editorials. All potential citations were evaluated independently by two reviewers for inclusion (Escandón L & Duarte-Bateman D). Conflicts during articles selection process were solved by a third reviewer (Escandón JM).
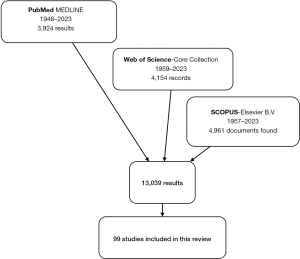
Table 1
| Database | Terms | Search strategy† |
|---|---|---|
| PubMed MEDLINE (NCBI Literature Resources; date of search: January 2nd, 2023; time frame: inception to 01/02/2023) |
#1 | Lymphedema OR “Lymphedema”[Mesh] |
| #2 | Lymphoedema | |
| #3 | Breast | |
| #4 | Cancer | |
| #5 | Neoplasm | |
| #6 | “Breast Cancer Lymphedema”[Mesh] | |
| #7 | #1 OR #2 | |
| #8 | #4 OR #5 | |
| #9 | #7 AND #3 AND #8 | |
| #10 | #9 OR #10 | |
| Web of Science (© 2023 Clarivate; date of search: January 2nd, 2023; time frame: inception to 01/02/2023) | #1 | Lymphedema |
| #2 | Lymphoedema | |
| #3 | Breast | |
| #4 | Cancer | |
| #5 | #1 OR #2 | |
| #6 | #5 AND #2 AND #3 | |
| Scopus (© Elsevier B.V; date of search: January 2nd, 2023; time frame: inception to 01/02/2023) | #1 | Lymphedema |
| #2 | Lymphoedema | |
| #3 | Breast | |
| #4 | Cancer | |
| #5 | #1 OR #2 | |
| #6 | #5 AND #2 AND #3 |
Please note that a narrative review is less methodologically demanding than a systematic review, as it does not require a search of all literature in a field. Therefore, the search strategy summary of a narrative review is mainly used for more transparent reporting. †, one-step selection process conducted by two independent reviewers. NCBI, National Center for Biotechnology Information.
Discussion
The surgical management of lymphedema can be classified into two approaches: (I) physiologic procedures and (II) debulking or ablative procedures (19). While ablative procedures are intended to lessen the symptomatic burden of lymphedema via the removal of pathological tissues (19,20), physiologic procedures are performed to restore the abnormal lymphatic flow by creating bypasses into the venous or lymphatic circulation, or by creating new lymphatic connections by means of lymphangiogenesis (3,19,21,22).
At the present time, there are no solid guidelines for patient selection, timing of intervention, and type of procedure (1). However, physiologic procedures are usually assumed to generate better outcomes in the early stages of lymphedema as there is some residual physiologic flow and vessels are less fibrotic (2,20,23). On the other hand, ablative procedures are regarded to be the best alternative in very advanced lymphedema stages in which reduction of the lymphedematous limb can only be achieved by excising pathologic tissue (24). Due to advancements in imaging, technology, and micro- and super-microsurgery, techniques such as the transposition of pedicled LN flaps or the lymphatico-lymphatic bypass have been abandoned. Lymphaticovenous anastomosis (LVA) and vascularized lymph node transfer (VLNT) are the most common physiologic procedures for lymphedema nowadays (22,25,26).
LVA
LVA is a microsurgical or supermicrosurgical procedure. It involves the connection of a lymphatic vessel onto a neighboring vein to divert lymphatic fluid into the venous circulation, bypassing any area of the lymphatic system that is injured (Table 2) (43,44). Due to the intravascular pressure of veins and the presence of valves, the venous system can easily adjust the extra input from LVAs offering a potent drainage mechanism of lymphatic fluid (45). Regarding the number and site of anastomoses, one or more can be performed in several levels of the affected extremity (19). This is usually guided by diagnostic imaging (e.g., indocyanine lymphography) (46).
Table 2
| Author | Methodology | Outcomes |
|---|---|---|
| Yamamoto et al. [2013] (27) | SEATTLE (44 anastomoses); non-SEATTLE (37 anastomoses) | LEL index reduction in SEATTLE: 16.5±14.5 |
| LEL index reduction in non-SEATTLE: 10.9±11.8 (P=0.041) | ||
| Success rate of S-E LVA in SEATTLEL: 95.5% | ||
| Success rate of S-E LVA non-SEATTLE: 81.1% (P=0.040) | ||
| 84.1% of lymph vessels in SEATTLE group were successfully dilated by temporary lymphatic expansion maneuver | ||
| Chen et al. [2015] (28) | LVA with the “octopus” technique in 9 patients (130 anastomoses) | Disease regression: 100% |
| Significant decrease in limb measurements (P=0.0003) | ||
| Severity downstaging in most patients | ||
| Yamamoto et al. [2011] (29) | Lambda-shaped anastomosis assisted by IVS (11 patients); no lambda-shaped anastomosis (9 patients); total: 186 anastomoses |
Duration of the operation: 4.1 hours (range, 3–5 hours) |
| Lambda-shaped number of anastomoses: 10.2±2.3 | ||
| No lambda-shaped number of anastomoses: 8.2±1.4 (P=0.05) | ||
| Ayestaray & Bekara [2014] (30) | π-shaped lymphaticovenular anastomoses (4 per limb) | Venous backflow: 98 LVA (55.7%) |
| Significant postop circumferential reduction: 16 patients (80%) | ||
| Mean volume differential reduction rate: 22.9% (range, 4.9–46.3%) (P<0.001) | ||
| Chang [2010] (31) | Twenty women with stage II or III unilateral UEL; LVA per patient: 3.5 (range, 2–5) | Mean operative: 3 hours (range, 2–5 hours) |
| Postop symptom improvement: 19 patients (95%) | ||
| Postop quantitative improvement: 13 patients (65%) | ||
| Mean volume differential reduction: 36% at 3 months, 39% at 6 months, and 35% at 1 year | ||
| Koshima et al. [2000] (32) | 22 female patients with UEL; continuous bandaging (n=12) versus LVA (n=12) | Mean number of anastomoses: 4.1 (range, 1–7) |
| Mean decrease in circumference with bandaging: 11.7% | ||
| Mean decrease in circumference with LVA: 47.3% | ||
| Engel et al.[2018] (33) | CDT only (n=30); LVA (n=23) | CRR in patients undergoing LVA: 17.3% |
| CRR in patients that had CDT only: 9.8% (P<0.01) | ||
| Chen et al. [2018] (34) | Five patients underwent LVA (42 anastomoses); standard technique: 26 LVA; octopus technique: 16 LVA | 17 LVAs showed “backflow” (40%) |
| After limb compression, 16 of 17 (94%) LVAs that initially demonstrated “backflow” converted to “washout” | ||
| Damstra et al. [2009] (35) | LVA on patients with Campisi stage 3 lymphedema | Post-operative volume measurements: 4.8% reduction of lymphedema at 3 months and a 2% reduction after 1 year |
| Quality of life questionnaires reported minimal improvement | ||
| Chang et al. [2013] (36) | LVA: 89 upper extremities | Symptom improvement (96%) |
| Quantitative improvement (74%) | ||
| Mean volume differential reduction (3 months): 33% | ||
| Mean volume differential reduction (6 months): 36% | ||
| Mean volume differential reduction (12 months): 42% | ||
| Patients with stage 1–2 lymphedema received a higher mean number of bypasses and had significantly better results than patients with stage 3 lymphedema | ||
| Poumellec et al. [2017] (37) | LVA on 31 patients | Wrist CRR: 22.5% |
| Forearm CRR: 21.32% | ||
| Arm CRR: 30.2% | ||
| Functional improvement: 84% (moderate to substantial) | ||
| Lee et al. [2017] (38) | LVA on three patients; Campisi stage II (2 patients) and stage III (1 patients) | Operation time: 222.3±10.8 min |
| Mean volume reduction rate: −4.7%±36% (2-year follow-up) | ||
| Ayestaray et al. [2013] (39) | LVA on 20 patients; Campisi stage: 2.8 (range, 2–5) | Circumferential differential reduction rate: 13.2% (range, 4.2–27.2%) (P<0.001) |
| Better quality of life 6 months after surgery: 90% | ||
| Stopped wearing their elastic stocking and bandage definitively (6 months): 30% | ||
| Gennaro et al. [2016] (40) | 39 patients with UEL (5.3 anastomosis per patient) | Average volume reduction: 50% |
| Most patients (72.5%) were extremely satisfied | ||
| Most patients reported a reduction of compression garments class | ||
| Cornelissen et al. [2017] (41) | 20 women with early-stage BCRL | Lymph-ICF: all the domains and the total score showed improvement postoperatively (P<0.005) |
| Discontinuation of compressive stocking: 85% | ||
| Mean relative volume difference in UEL: preoperative versus postoperative (P=0.58) | ||
| Winters et al. [2017] (42) | LVA on 29 patients | Percentage volume reduction: 33% (1-year follow-up) |
| Increase in volume: one patient | ||
| Overall perceived quality of life improved at a 12-month follow-up (overall QoL, 5.8±1.1 to 7.4±0.7; P<0.001) | ||
| Discontinue the use of compression garments: 53.6% |
SEATTLE, S-E anastomosis through temporary lymphatic expansion; LEL, lower extremity lymphedema; LVA, lymphaticovenous anastomosis; IVS, intravascular stenting; UEL, upper extremity lymphedema; CDT, complex decongestive therapy; CRR, circumference rate reduction; BCRL, breast cancer-related lymphedema; ICF, international classification of functioning; QoL, quality of life.
There are different configurations for the anastomoses depending on the orientation of the vessels, which include the end-to-side, end-to-end, side-to-side, side-to-end, “octopus”, or “lambda” techniques (27-30,47). The outcomes in terms of patency and volume reduction vary depending on the surgeon-specific techniques and patient population. Due to the complexity of the procedure, careful attention must be given when performing the anastomosis to reduce the incidence of venous hypertension and reflux into the lymphatic vessels (19). Some authors such Yamamoto et al. have suggested that a side-to-end and side-to-side configuration achieves the best lymphatic outflow, while an end-to-side type results more often in venous reflux and thrombosis (48).
Initial reports presented the outcomes of a prospective study that included 20 patients with variable lymphedema stages (Campisi stage II and III) undergoing LVA [3.5 (range, 2–5) LVAs per patient]. The mean volume reduction after 12 months was 35% (31). Eighty percent of the patients reported long-term symptoms improvement at an 18-month follow-up (31). In line with these results, studies comparing the superiority of LVA versus compressive therapy demonstrated superior efficacy of LVA for the treatment of lymphedema (32). A 4.1-cm circumference reduction was evident in patients managed with LVA while patients who received compression therapy had a 0.8-cm circumference reduction (P<0.05) (32). Patients who had compression therapy alone had an 11.7% circumference reduction rate (CRR), while patients who underwent LVA had a 47.3% arm CRR (level of evidence: IV) (32). Similarly, Engel et al. reported a 17.3% CRR in patients undergoing lymphovenous bypass (n=23) compared to a 9.8% CRR in patients that had complex decongestive therapy only (n=30, P<0.01) (level of evidence: IV) (33).
The role of compression therapy as an adjunct in the setting of upper limb lymphedema should always be regarded as a valuable asset. Contemporary studies have demonstrated that postoperative compression immediately after LVA does not damage the anastomoses created; conversely, it can augment the lymphatic flow and convert unfavorable retrograde flow to favorable antegrade flow (lymph vessel-to-vein flow) (level of evidence: IV) (34). Forty-two LVAs were performed in 5 patients for an observational study (34). Initially, 60% of the anastomoses demonstrated adequate antegrade flow, while the remaining 40% exhibited “backflow” or retrograde flow (34). After immediate compression was applied, 94% of the LVAs that initially demonstrated backflow transitioned into adequate antegrade flow LVAs (34).
The effect of LVA may be moderate or substandard in patients with more advanced stages of lymphedema (46). Damstra et al. included 10 patients with Campisi stage III lymphedema in a prospective study evaluating the surgical outcomes of lymphatic surgery (35,49). Although 50% of the patients presented subjective relief of their complaints after 12 months following LVA, the volume reduction was 2% (35,49). Furthermore, the authors did not find any significant difference comparing the preoperative and 12-month postoperative lymphatic flow using the Kleinhans transport index (level of evidence: IV) (35,49).
Chang et al. published results on a prospective study of 30 patients with BCRL undergoing LVA (36). After a 1-year follow-up, a mean reduction of 61% in the early-stage group was reported (MD Anderson stage I or II), whereas a mean reduction of 17% in the late-stage group was exhibited (MD Anderson stage III or IV) (36). Overall, 96% of the patients presented subjective improvement of their symptoms (level of evidence: IV) (36). Poumellec et al. published the results of a prospective study with 31 patients who underwent stepped LVAs for BCRL (37). No patients were lost during follow-up (37). Eighty-four percent of the patients experienced a moderate-to-substantial increase in QoL and 93.5% of the patients showed a reduction in limb circumference (37). Remarkably, the mean overall decrease after a 1-year follow-up for all patients was only 24.7% (37). The authors report that patients with a lower Campisi lymphedema stage had better results (level of evidence: IV) (37).
In certain cases, objective parameters using LVA have shown no significant improvement after treatment. Lee et al. performed a retrospective study with a mean follow-up of 2 years employing LVAs (38). Three patients with BCRL were included (38). Two patients with Campisi stage II lymphedema showed an increase in volume and one patient with stage III lymphedema showed a decrease in volume. The mean volume reduction rate was −4.7% after 2 years of observation (level of evidence: IV) (38).
Although variable results using objective parameters have been reported in the literature (e.g., limb CRR, limb volume reduction), physiologic procedures like LVA have been shown to successfully improve the QoL and patient-reported outcomes in patients with BCRL. Ayestaray et al. reported a mean volume reduction of 22.8% in 20 patients undergoing LVA for lymphedema Campisi stage II to V (mean: 2.8) (39). Three out of four patients could discontinue lymph drainage therapy for 4 months and 90% of the patients reported a better QoL 6 months after surgery (level of evidence: IV) (39). Gennaro et al. performed a retrospective study on 69 patients, of which 39 presented with upper limb lymphedema due to breast cancer. An average volume reduction of 50% after LVA was observed (40). Many patients could also stop their decompression therapy and noticed an improvement in subjective symptoms such as reduction in heaviness, hardness, functional impairment, and pain of the affected arm (level of evidence: IV) (40).
Cornelissen et al. prospectively performed a study assessing improvements in QoL after LVA in women with BCRL (41). Although the authors did not find a significant improvement in the mean relative volume difference between preoperative and postoperative measurements, a statistically significant improvement in QoL after 1 year of follow-up using a validated questionnaire was evident [lymphedema international classification of functioning (Lymph-ICF) questionnaire, Dutch version] (41). Moreover, 85% of the patients discontinued compressive stockings (level of evidence: IV) (41). Winter et al. performed a retrospective study on the efficacy of LVA in BCRL on 29 patients (42). After 1-year follow-up, the percentage volume reduction was 33% (42). Only one patient showed an increase in volume (42). The overall perceived QoL and scores for other domains were significantly improved at a 12-month follow-up (overall QoL, 5.8±1.1 to 7.4±0.7; P<0.001). Fifteen patients (53.6%) were able to discontinue the use of compression garments (level of evidence: III) (42).
VLNT
Auto-transplantation of vascularized LNs from an unaffected donor site as a free flap and placing them into an affected lymphedematous extremity using a microsurgical approach is denominated VLNT (Table 3) (21,44,61). Although the therapeutic mechanism of VLNT remains an area of investigation, this surgical technique has gained popularity and has been shown to provide encouraging outcomes for patients with upper extremity lymphedema (1). On one side, after reconstituting perfusion and physiologic flow, LN flaps seem to release endothelial growth factors [e.g., vascular endothelial growth factor (VEGF)] that promote lymphangiogenesis and flow from distal non-functional lymphatic channels into healthy proximal lymphatic vessels (22,62-64). VLNT may also promote lymphatic fluid drainage by redirecting the excess fluid into the vascular system and acting as a “pump” (22,63,65). Certainly, the pre-existing intra-tissue lymphovenous communications within flaps or the formation of new lymphovenous connections after VLNT, can also play a role in the therapeutic effect of this surgical modality (66,67).
Table 3
| Author | Methodology | Outcomes |
|---|---|---|
| Manrique et al. [2020] (50) | Gastroepiploic VLNT; middle buried (n=7); distal (n=7) | No difference in excess volume reduction rate between middle (23.3%±5.1%) or distal inset (22%±10%, P=0.207) |
| Shorter length of stay (P=0.0013) and early return to daily activities (P=0.003) with middle buried VLNT | ||
| Becker et al. [2006] (51) | Groin VLNT; 24 patients: stage I (n=6) and stage II (n=18) | Downstaging: 12 patients (50%) |
| Complete resolution: 10 patients (41.7%) | ||
| No improvement: 2 patients (8.3%) | ||
| Activity of the VLNT lymphoscintigraphy: 31% (5/16) | ||
| Lin et al. [2009] (52) | Groin VLNT; 13 consecutive patients | Reduction in the arm’s circumference: 92.3% |
| Mean reduction rate: 50.55%±19.26% (range, 0–71%). | ||
| Incidence of cellulitis: decreased in 11 patients | ||
| Liu et al. [2018] (53) | Orthotopic groin VLNT; thirty consecutive patients | Reduction in limb circumference: 70% (21 patients) |
| Mean CRR: 47.1%±27.9% (range, 0–100%) | ||
| Radiological improvement (lymphoscintigraphy): 37% | ||
| Gharb et al. [2011] (54) | Patients with early stage II UEL; Groin VLNT-standard (n=11); Groin VLNT with hilar perforators (n=10) | Differences between preop and postop measurements: better in the perforator-based group at the levels below elbow, wrist, and mid-palm (P=0.004, 0.002, and 0.007, respectively) |
| Patients treated with standard VLNT had higher requirements of secondary procedures (P=0.03) | ||
| Granzow et al. [2014] (55) | Groin VLNT + MBR with DIEP (8 patients) | Decreased incidence of cellulitis/infection (P=0.009) |
| Decreased requirements of compression garments (P=0.009) | ||
| Decreased requirements of lymphedema therapy (P=0.009) | ||
| Patel et al. [2015] (56) | Groin (87%) and submental VLNT (13%); UEL stage II–IV (15 patients) | Significant CRR after VLNT: 24.4%±14.7% (P=0.03) |
| Reduction in cellulitis rate: preop, 3.5±3.3; postop, 0.7±0.9 | ||
| Improvement in overall quality of life and each specific subdomain of LYMQOL after VLNT (P<0.001) | ||
| Dionyssiou et al. [2016] (57) | VLNT and postoperative physiotherapy (n=18) | Mean LVR: greater with VLNT (57%) versus conservative treatment (18%) |
| Conservative management with the physiotherapy (n=18) | Functional nodes on lymphoscintigraphy: 72% of patients undergoing VLNT | |
| Reduced infection episodes with VLNT versus conservative treatment (P=0.001) | ||
| VLNT was estimated as cost effective compared to conservative treatment alone | ||
| Gratzon et al. [2017] (58) | Groin VLNT; ISL stage 1–2 | Median percent reduction rate: 42.73% (P=0.052) |
| Scores for all domains of quality of life significantly improved compared to preoperative values | ||
| Maruccia et al. [2019] (59) | VLNT + scar release with fat grafting (n=18); VLNT alone (n=21); stage II–III | Improved CRR at above elbow level was observed at 3 and 6 months of follow-up in the VLNT + scar release compared to VLNT alone (P<0.01) |
| LYMQOL: significantly better scores (P<0.01) in all domains at all follow-up visits in the VLNT + scar release group | ||
| Aljaaly et al. [2019] (60) | Submental VLNT; Cheng’s grade II or higher; wrist inset: (I) dorsal wrist (n=7, 46.7%); (II) volar wrist (n=8, 53.3%) | Cellulitis: preop—6 per year; postop—0.3 per year (P=0.04). No difference between the dorsal and volar groups |
| Greater circumferential difference and reduction rates in the dorsally placed flaps: above-elbow and below-elbow (P=0.04) | ||
| Better patient-reported outcome (LYMQOL) with dorsal inset compared to a volar inset (P=0.04) |
VLNT, vascularized lymph node transfer; CRR, circumference reduction rate; UEL, upper extremity lymphedema; MBR, microvascular breast reconstruction; DIEP, deep inferior epigastric perforator; preop, preoperative; postoperative; LYMQOL, lymphoedema quality-of-life study; LVR, limb volume reduction; ISL, International Society of Lymphology.
Several locations have been reported to be useful as donor sites for VLNT (68-70). These include the groin, lateral thoracic, supraclavicular, and submental regions. Additionally, intraabdominal LNs from the appendicular, ileocolic, and omental areas have been also reported (19,68-70). In this setting, microsurgeons are provided with several possibilities to harvest LNs but a thorough selection is still required to minimize morbidity and avoid the risk of iatrogenic donor site lymphedema (19). On this basis, the intra-abdominal region is of special consideration; more specifically, the right gastroepiploic (GE) LNs (50,68).
In a recent meta-analysis of eight studies that evaluated quantitative outcomes of the effect of VLNT on upper limb lymphedema (33,52,54,56,57,60,71,72), the authors found that the mean reduction in the volume difference between the healthy and affected extremities was 40.31% after surgery (95% CI: 31.44% to 49.17%) (level of evidence: II) (73). Despite the heterogeneity in follow-up between studies, most articles displayed a moderate (20.1% to 33.2%) to elevated (49.2% to 57.1%) mean reduction in the volume difference between the healthy and affected limb (73).
Several individual studies have shown improvements in the circumferential or volumetric measurements of upper lymphedematous extremities following VLNT. Twenty-four patients with BCRL were managed with groin-VLNT in a retrospective study reported by Becker et al., and were followed for 8.3 years on average (51). Patients with lymphedema International Society of Lymphology (ISL) stage III were excluded (51). While downstaging was observed in 12 patients (50%) and complete resolution was evident in 10 patients (41.7%), two patients did not show any improvement (8.3%) (level of evidence: IV) (51). In 31% of the cases, lymphoscintigraphy demonstrated the effectiveness of VLNT (5/16 limbs) (51). After surgery, complete remission of soft tissue infections occurred in 17 patients (58.3%) and only one episode happened in 7 patients during the follow-up (29.2%) (51).
Likewise, Lin et al. reported 13 consecutive patients with BCRL managed with groin-VLNT (52). Re-exploration was required in one case and no donor-site morbidity occurred (52). At a mean follow-up of 4.7±2.26 years, reduction in the arm’s circumference was found in 92.3% of the patients (52). The mean reduction rate was 50.55%±19.26% (range, 0–71%) (level of evidence: IV) (52). Two patients with prolonged lymphedema required ablative procedures (e.g., wedge excision or liposuction) to improve symptoms. Postoperative lymphoscintigraphy exhibited decreased stasis and improved clearance of the radiotracer through deep lymphatic vessels or collateral channels (level of evidence: IV) (52).
As previously shown, radiologic findings may not strictly correlate with a reduction of the extremity volume in some instances. In a study including 30 patients by Liu et al., 70% of patients showed limb circumference reduction while 30% had no reduction (no worsened edema) (53). Postoperative lymphoscintigraphy showed no radiological improvement in 19 patients (63%) and radiological improvement in 11 patients (37%). Of the patients exhibiting no radiologic improvement, 10 had circumference reduction and 9 had no clinical improvement (level of evidence: IV) (53).
Contemporary studies have shown a reduction of volumetric measurements of lymphedematous limbs and rates of episodes of cellulitis, and important improvement in QoL following VLNT. Gharb et al. treated 25 patients with early-stage II lymphedema after mastectomy and axillary dissection with groin-VLNT (54). Subjectively, all patients reported symptomatologic improvement (54). The authors compared the standard groin flap containing LNs (LNs are randomly included in the flap) to groin-VLNT based on the hilar perforators, where a dominant pedicle provided direct physiologic blood supply to the LNs and the cutaneous portion of the flap. Improved outcomes for the difference between preoperative and postoperative measures below the elbow (P=0.004), wrist (P=0.002), and mid-palm (P=0.007) were found in the hilar perforator-based VLNT group (level of evidence: IV) (54). Likewise, the rate of secondary ablative procedures was lower in patients managed with VLNT based on the hilar perforators compared to the standard approach (P=0.03) (54).
In another series of 8 patients experiencing lymphedema symptoms for an average period of 3.8 years, patients undergoing combined microvascular breast reconstruction (MBR) and groin-VLNT for BCRL exhibited a decrease in daily requirements of compression garments (P=0.009) and physiotherapy sessions (P=0.009) at a 32-month follow-up after surgery (level of evidence: IV) (55). One patient required drainage of seroma at the axillary recipient site (55). Patel et al. presented a prospective series of 15 patients treated with groin-VLNT flap and submental VLNT for upper extremity lymphedema stage II–IV using a modified lymphedema grading system (56). The authors reported a significant reduction in limb difference at a 12-month follow-up (preoperative, 18.1±4.2; postoperative 12.1±5.3; P=0.03) and a reduction in the rate of cellulitis (preoperative, 3.5±3.3; postoperative, 0.7±0.9) over an observation period of 25.4±8.4 months (level of evidence: IV) (56). Patients exhibited a significant improvement in overall QoL using the lymphoedema quality-of-life tool (LYMQOL) (P<0.001), and a significant improvement of each specific subdomain after VLNT (function, appearance, symptom, and mood domains) (level of evidence: IV) (56).
When compared to conservative treatment, VLNT has been shown to offer a significant therapeutic effect in patients with BCRL. Dionyssiou et al. evaluated the effect of groin-VLNT and physiotherapy in patients with BCRL ISL stage II versus no surgical treatment (6-month physiotherapy regimen) (57). Both groups were re-evaluated 18 months after initiating treatment (57). Postoperative imaging with lymphoscintigraphy indicated functional LNs in 72% of patients undergoing VLNT (57). The mean difference in volume, infection rate, pain scale scores, heaviness scale scores, and overall function scores of patients undergoing VLNT with 6 months of physiotherapy compared favorably against a 6-month physiotherapy regimen without lymphatic surgery (P<0.001 for all comparisons) (level of evidence: III) (57).
Conflicting outcomes regarding the effectiveness of VLNT have been also reported. Gratzon et al. reported the outcomes of groin-VLNT in a prospective study with ISL stage 1–2 BCRL patients (58). Although the median percent reduction rate was 42.73% in patients that reached a 12-month follow-up, it did not reach statistical significance (P=0.052) (level of evidence: IV) (58). Conversely, scores for all domains of QoL significantly improved at a 12-month follow-up compared to preoperative values (58). Donor site complications included superficial wound dehiscence (6%), seroma (12%), and infection (12%); while recipient site complications included infection (6%), non-healing wound (2%), minor superficial wound dehiscence (2%), bleeding (2%), and hematoma (2%) (58).
The role of scar release at the time of VLNT has been hypothesized to offer an additional therapeutic benefit in patients with BCRL during the early postoperative period and may offer a higher degree of symptomatic relief. In previous retrospective studies, we assessed the effect of combined VLNT and scar release with fat grafting (n=18) in patients with BCRL stage II and III compared to VLNT alone (n=21) (59). Right GE LN flaps and groin LN flaps were used (59). All flaps survived and no donor site complications occurred (59). All cases demonstrated improved lymphatic flow on lymphoscintigraphy after surgery (59). Although CRRs were higher in patients with combined VLNT and scar release at 3 and 6 months after surgery compared to VLNT alone, comparable CRR for both groups were reported at 12 and 24 months after surgery (59). Remarkably, LYMQOL scores were significantly better in patients who had adjunct scar release (level of evidence: IV) (59).
Current evidence has not shown superior or inferior outcomes when comparing heterotopic (cubital fossa or wrist) versus orthotopic (axilla) inset of LN flaps for BCRL (74). In previous studies, we have determined that the location of flap inset (middle versus distal inset) did not significantly affect the postoperative outcomes of GE VLNT in terms of mean excess volume reduction at a 6-month follow-up (middle inset 23.3% versus distal inset 22.0%) or in terms of physical, psychosocial, and functional outcomes using Lymphedema Life Impact Scale-v2 (LLISv2) scores to assess QoL (level of evidence: IV) (50). Furthermore, in a recent meta-analysis evaluating the effect of the recipient site for VLNT on BCRL, comparable outcomes were found comparing the wrist and the axilla as recipient sites. The CRR (wrist, 42.1% versus axilla, 51.5%) or excess volume reduction rates were comparable between groups (wrist 35.6% and axilla 48.8%) (level of evidence: II) (74). Likewise, similar outcomes were found for the rate of postoperative discontinuation of compression garments, reduction rate of infection episodes per year, and overall pooled complication rates when comparing the different recipient sites (level of evidence: II) (74). Of note, when selecting the wrist as the recipient site, a dorsal placement seemed to generate a significant reduction in limb circumference at 12 months (P=0.04), improved venous outflow (P<0.0001), and better patient-reported outcomes using the LYMQOL (P=0.04) compared to a volar inset (level of evidence: IV) (60).
MBR and VLNT
In patients with upper limb lymphedema requiring breast reconstruction, simultaneous MBR and VLNT have been shown to be a safe therapeutic alternative without an increased risk of VLNT failure. Saaristo et al. reported a study of nine patients who underwent reconstruction with modified lower abdominal free flaps [deep inferior epigastric perforator (DIEP) flap, muscle-sparing transverse rectus abdominis myocutaneous (msTRAM) flap, muscle-sparing transverse rectus abdominis flap] containing LN from the groin area (72). When compared to a standard DIEP/msTRAM flap, the combined LN-DIEP/LN-msTRAM added 35 minutes to the operation on average (72). One patient (11.1%) required seroma drainage of the donor site while 22.2% had seroma drainage of the axillary region after LN-DIEP/LN-msTRAM flap transfer (72). Delayed wound healing of the abdominal wound was present in 22.2% of the patients who had LN-DIEP/LN-msTRAM flaps in comparison to 7.7% of the patients who received a standard abdominal flap (72). After surgery, recurrent episodes of erysipelas subsided in all patients (100%) (72). A reduction of limb circumferences was detected in 77.7% of patients at 3 and 6 months postoperatively (level of evidence: IV) (72). Postoperative lymphoscintigraphy demonstrated at least minimal improvement of lymphatic drainage compared to preoperative results in 5 of 6 patients (72). No donor site lymphedema was reported (72).
In a prospective study, Nguyen et al. reported a series of 29 consecutive patients who underwent MBR and groin-VLNT (75). Seven patients had hemi-abdominal flaps with ipsilateral VLNT (24%) while 22 patients received abdominal flaps (flaps crossing the midline) with VLNT (75). For abdominal flaps with VLNT, the LNs were anastomosed to the recipient vessels in the axilla and the abdominal flaps were connected to the internal mammary vessels (75). Of the seven patients who received a hemi-abdominal flap and VLNT, three utilized the recipient vessels in the axilla for the LN and hemi-abdominal flap due to limited length. The remaining four used the internal mammary vessels as recipient vessels for the LN-DIEP flap (75). All flaps survived (75). Although one patient presented with lower extremity swelling, which improved after healing from donor site wound dehiscence, no patients had iatrogenic donor site lymphedema (75). Seventy-nine percent of the patients reported symptom improvement at a mean follow-up of 11 months, with a trend toward gradual improvement with time (level of evidence: IV) (75). No patient reported symptomatologic deterioration following reconstruction (75).
Akita et al. evaluated the postoperative outcomes of patients with advanced-stage BCRL undergoing VLNT-DIEP flap (n=13) and groin-VLNT alone (n=14) (76). Overall, 33.3% of the patients had improvement of dermal backflow pattern after surgery (≥75% reduction of dermal backflow) and were able to entirely discontinue compression garments (76). The scores of upper extremity index were comparable between patients undergoing VLNT-DIEP flap versus VLNT alone (13.9±4.1 versus 13.2±1.5, P=0.75) (76). Nonetheless, the rates of patients presenting with improved lymphatic function and patients discontinuing compression garments were higher in patients who had MBR and VLNT compared to VLNT alone (77% versus 21.4%, P=0.04) (76).
De Brucker et al. evaluated outcomes of QoL using the Upper Limb Lymphedema-27 questionnaire (ULL-27) in 25 patients who underwent VLNT for the treatment of BCRL (77). Three patients had groin-VLNT while 22 underwent VLNT and MBR with combined DIEP-VLNT (77). Compared to VLNT alone (total time: 155 minutes), 210 and 360 additional minutes were required for unilateral and bilateral MBR, respectively, when combined with VLNT (77). One patient suffered complete flap loss secondary to an infection of the axilla (4%) (77). A significant improvement was evident for the scores of the ULL-27 questionnaire after VLNT (preoperative, 44±18; postoperative, 26±16; P<0.001) (level of evidence: IV) (77). Forty-four percent of patients were able to stop physiotherapy and 60% discontinued compression garments after surgery (77). The authors did not identify an association between the scores of the ULL-27 questionnaire and BCRL risk factors [e.g., body mass index (BMI), age, smoking, or time from symptom onset to VLNT] (level of evidence: IV) (77).
Despite the evident success of combined VLNT and MBR in most studies, some studies have shown that this combined procedure may reduce the performance of VLNT if performed simultaneously. Engel et al. evaluated 124 patients with BCRL using three different therapeutic modalities with and without MBR (e.g., compressive therapy, LVA, or VLNT) (33). VLNT alone exhibited a significant improvement in reduction rates (P=0.004) and circumferential difference (P=0.004) compared to combined VLNT and MBR (33). No significant difference was found in terms of the rate of cellulitis (level of evidence: IV) (33). On the other hand, the authors reported that any form of lymphatic microsurgery (LVA and VLNT) significantly improved the rate of episodes of cellulitis, the circumferential difference, and the reduction rate compared to compressive therapy in patients with or without combined MBR (P<0.05) (33).
Although VLNT has been practical in reducing the rate of episodes of cellulitis per year, limb volume, and limb circumference difference, some authors have suggested that the combination of two physiologic procedures can potentiate the surgical outcomes of lymphatic surgery and MBR. Chang et al. (78) presented a retrospective study of patients who underwent combined LVA, groin-VLNT, and autologous MBR in 38 patients with BCRL. On average, 1.53 (range, 1–4) LVAs were performed per patient (78). None of the patients had postoperative cellulitis and 81.6% of patients exhibited volume reduction at a mean follow-up of 19.1 months (78). As both techniques have different therapeutic mechanisms, authors suggested VLNT provides a delayed benefit as lymphangiogenesis requires some time, while LVA provides immediate relief of the excess fluid (78).
Debulking or ablative procedures
Procedures like VLNT and LVA are hypothesized to generate a significant success rate for early-stage limb lymphedema. Nonetheless, these procedures require expertise; a significant surgical effort and time; can generate important donor-site morbidity; and when use as a single procedure, they have a limited therapeutic effect in patients with late-stage BCRL (19,23,46). On the other hand, ablative procedures such as the Charles’ procedure, or the Sistrunk and Thompson procedures achieve radical elimination of pathologic fibrotic and subcutaneous tissue deposition by means of direct excision (20,22). Considering the morbidity of the aforementioned procedures, suction-assisted lipectomy (SAL) and radical reduction with preservation of perforators (RRPP) have gained popularity for the management of upper limb lymphedema (23,44,79-81).
Since it was introduced by O’Brien in 1989, SAL has been implemented for the treatment of lymphedema (23,82,83). As monotherapy, SAL has been shown to provide a 117–118% mean reduction in excess volume in patients with BCRL (level of evidence: IV) (84,85). Furthermore, besides providing a 109% mean excess volume reduction of the upper extremity in other series (P<0.001), SAL has demonstrated an optimal therapeutic effect by significantly reducing the episodes of cellulitis per year by 87% (preoperative, 0.47 bouts; postoperative, 0.06 bouts; P<0.001) (level of evidence: IV) (86). In a recent cost-effective analysis comparing power-assisted lipectomy versus compression garments and physical therapy in patients with fat-dominant BCRL, debulking procedures compared favorably against conservative management in terms of quality-adjusted life years demonstrating higher clinical effectiveness (level of evidence: IV). The relative cost reduction was determined to be $74,487 with SAL (87). Remarkably, although SAL may be able to provide symptomatic relief in a very expeditious way, patients usually require lifelong complex compression therapy as lipectomy does not reconstitute the integrity of the lymphatic system; therefore, it does not address lymphostasis (level of evidence: III) (19,23,84).
When used in combination with ablative procedures, physiologic surgical interventions such as LVA or VLNT are hypothesized to yield better outcomes in patients with advanced-stage BCRL (88). LVA or VLNT can improve lymphatic drainage while direct excision allows complete removal of pathologic tissue affected by irreversible histologic changes (level of evidence: III) (23,46,83,89). For instance, when using fibro-lipo-lymph-aspiration with a lymph vessel-sparing procedure in patients with previous multiple LVAs, this staged procedure demonstrated optimal response (90). Symptoms such as redness, papillomatosis, and hyperkeratosis improved in all cases (90). A reduction of the excess volume of the affected limb was evident after SAL without an increased risk of lymphatic complications (preoperative, 20.19%; postoperative, 2.68%; P<0.001) (level of evidence: IV) (90).
Likewise, previous studies have also demonstrated the superiority of combined VLNT-SAL, as a simultaneous procedure or as a staged procedure, over VLNT alone (81,91-93). Ghazaleh et al. evaluated the effect of combined water jet-assisted liposuction (WAL) and VLNT compared to VLNT alone in patients with stage II and III BCRL (94). Comparable rates of postoperative complications and scores for patient satisfaction were reported between groups (P=0.323) (94). WAL added 71.2 minutes to VLNT on average (P=0.0003) (94). Although the mean difference of circumferences between lymphedematous and unaffected arms was not significantly different between groups after surgery, preoperative differences in circumferences were significantly higher in the WAL-VLNT group. In this setting, WAL-VLNT achieved optimal outcomes taking into consideration that more advanced stages of lymphedema being present in patients who underwent WAL-VLNT compared to VLNT alone (level of evidence: IV) (94).
In our recent study, we evaluated patients undergoing physiologic procedures (VLNT or LVA) in patients with BCRL ISL stage II and combined procedures (SAL-LVA or SAL-VLNT) in stage III patients (46). When compared to SAL-LVA (85%±10.5%) and to SAL-VLNT (75%±8.5%), a more moderate CRR was evident in patients undergoing LVA alone (56.5%±8.4%), VLNT alone (54.4%±10.2%), or combined VLNT-DIEP flap (56.5%±3.9%) despite having a reduced disease severity (level of evidence: IV) (46). A comparable rate of complications was evident between groups (46).
Besides SAL, RRPP has demonstrated outstanding results in patients with more advanced stages of BCRL. This procedure is intended to reduce the bulk of pathologic tissue while preserving adequate blood supply to the skin in order to minimize complications. Salgado et al. reported the outcomes of eleven patients with ISL stage IIB BCRL who underwent RRPP of the forearm with simultaneous SAL (n=2) or wedge resection (n=9) of the affected arm (95). The hand was not treated (95). At a 24-month follow-up, although the authors did not find a significant circumference reduction at the level of the wrist (P=0.8) and hand (P=0.5), a significant circumference reduction was found above (P=0.048) and below the elbow (P=0.02) (level of evidence: IV) (95). No complications were reported (0%) and all patients were satisfied with the cosmesis of the procedure (100%) (95). Hyperesthesia occurred in one patient (9.1%) and numbness occurred in 4 patients (36.4%) (95).
In a previous study, we performed six combined double VLNT and modified RRPP for advanced stage BCRL (79). All flaps survived and the CRR was 70.8%±5.9% (range, 62% to 84%) at a follow-up of 14.83 months (79). Four patients reported preoperative episodes of cellulitis. After surgery, none of the patients had episodes of soft tissue infection (79). One patient reported paresthesia (16.7%) and one reported numbness after surgery (16.7%) (79). Lymphoscintigraphy exhibited significant postoperative improvement of lymphatic drainage compared to preoperative imaging (79). Global QoL scores showed a 2.72-fold improvement using the LYMQOL questionnaire (P<0.01) (level of evidence: IV) (79).
Personal approach
Our current approach takes into consideration if patients with BCRL desire MBR. In these cases, a DIEP or msTRAM flap is performed simultaneously at the time of lymphatic surgery (46,96). All patients are evaluated using indocyanine green (ICG)-lymphography and the initial lymphatic surgery involves a physiologic procedure, either LVA or VLNT (46). LVAs are performed when ICG-lymphography demonstrates the presence of viable lymphatic vessels; more specifically, areas of linear pattern interrupted by some areas of dermal backflow (46). In case suitable lymphatic channels are not evident on ICG-lymphography and some areas of segmental dermal backflow are encountered, VLNT using the GE LN flap is performed (21,70,97). With GE-VLNT, we avoid harvesting LNs from the extremities, completely reducing the risk of iatrogenic lymphedema, and avoid additional skin incisions from another donor site if MBR with DIEP flap is planned (68,70). Furthermore, as the GE-VLNT is not attached to the abdominal flap, there are no restrictions or limitations for inset of VLNT compared to composite DIEP/transverse rectus abdominis myocutaneous (TRAM)-groin LN flaps.
If monotherapy with a single physiologic procedure is regarded insufficient due to an advanced progression of the disease (stage III), a combined procedure is performed with physiologic and ablative surgical modalities (46). RRPP is preferred when the lymphedematous limbs are predominantly fibrotic (70,79,80), while SAL is selected when the exceeding tissue is mostly adipose in nature (23). If substandard results are evident 12 months after the initial reconstructive procedure using VLNT or LVA, an excisional procedure is performed. Conversely, if an excisional procedure fails to optimize the patient’s symptoms, another type of excisional procedure is performed (46).
Primary prevention
With contemporary evidence indicating physiologic procedures provide optimal outcomes to treat lymphedema, researchers have focused on risk reduction with prophylactic lymphatic surgery in patients with breast cancer (98,99). If ALND is performed, current literature favors the implementation of LVA as a simultaneous procedure in order to restore the function of the lymphatic system by means of bypasses onto axillary veins (99). These connections create a direct pathway for lymphatic fluid outflow from the vessels draining the arm into the central circulation (99). In a recent meta-analytic model performed by our group, the pooled rate of upper limb cancer-related lymphedema following ALND and preventive lymphatic surgery was 5.15% (95% CI: 2.9% to 7.5%, P<0.001) (99). When compared to no intervention, the same study demonstrated that preventive lymphatic surgery reduced the rate of upper limb BCRL following ALND by 18.7% [risk difference (RD): 95% CI: 29.5% to 7.9%; P<0.001] (level of evidence: III) (99). Nonetheless, some remarks were made regarding a high risk of bias in several articles involving issues with randomization or blinding, and underreported methods for subject allocation (99).
Due to the success of prophylactic LVA for the primary prevention of BCRL, some patients may be candidates for prophylactic VLNT and simultaneous breast reconstructions in patients requiring immediate autologous breast reconstruction and patients undergoing ALND (3). In a recent case, we performed simultaneous MBR with the DIEP flap and immediate lymphatic reconstruction with GE-VLNT. The flap was harvested via a subxiphoid 8-cm midline incision at the time of DIEP flap harvest (3). No significant difference was found between preoperative and 2- or 3-year postoperative circumferences (3). Furthermore, ICG-lymphography exhibited a linear pattern without evidence of stardust or splash pattern at 1, 2, and 3 years after surgery, objectively demonstrating physiologic lymphatic function (3).
Limitations
While this was an exhaustive review of over 99 included studies, given the heterogeneity between and within studies, there was insufficient evidence to form any clear recommendations for the surgical management of BCRL. Outcomes regarding conservative management were not evaluated in this review. Most included studies had a level of evidence of IV. Despite conducting a comprehensive search, the manuscript in question is a narrative review, which means its methodology lacks replicability and verifiability. Since this narrative review did not follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria, it may possess methodological deficiencies that could introduce bias into the interpretation and conclusions. We did not conduct any pooled analyses using the data from the studies summarized. Although a critical appraisal of the risk of bias is usually not included in narrative reviews, we evaluated the level of evidence of the results presented in the included studies.
Conclusions
Lymphedema is a common complication of breast cancer treatment with an extensive incidence range. In the scientific literature, outcomes after surgical management are heterogeneous despite most studies indicating favorable results. This indicates the need for further large-scale, randomized primary studies in this field, and high-quality systematic reviews and meta-analyses. Understanding the physiology and anatomy of the lymphatic system, and the pathophysiology of lymphedema, is critical for the decision-making and patient selection process. A thorough assessment of specific anatomic locations of surgery and staging using different diagnostic tools should guide the surgical management of BCRL.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine for the series “Breast Reconstruction”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-292/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-292/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-292/coif). The series “Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. O.J.M. served as the unpaid Guest Editor of the series, and serves as an unpaid editorial board member of Annals of Translational Medicine from July 2022 to June 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Manrique OJ, Bustos SS, Ciudad P, et al. Overview of Lymphedema for Physicians and Other Clinicians: A Review of Fundamental Concepts. Mayo Clin Proc 2022;97:1920-35. [Crossref] [PubMed]
- Matsui C, Yamamoto T, Tsukuura R, et al. Intraoperative monitoring of lymphatic vessel characteristics using video-capillaroscopy in the procedure of lymphaticovenular anastomosis. Microsurgery 2022;42:850-1. [Crossref] [PubMed]
- Ciudad P, Escandón JM, Manrique OJ, et al. Lymphedema prevention and immediate breast reconstruction with simultaneous gastroepiploic vascularized lymph node transfer and deep inferior epigastric perforator flap: A case report. Microsurgery 2022;42:617-21. [Crossref] [PubMed]
- DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500-15. [Crossref] [PubMed]
- Kavola H, Suominen S. Breast Cancer-Related Lymphedema. In: Wyld L, Markopoulos C, Leidenius M, et al. editors. Breast Cancer Management for Surgeons: A European Multidisciplinary Textbook. Springer International Publishing; 2018:689-99.
- Escandón JM, Christiano JG, Gooch JC, et al. Two-Stage Implant-Based Breast Reconstruction Using Intraoperative Fluorescence Imaging: A Propensity Score-Matched Analysis. Plast Reconstr Surg 2023; Epub ahead of print. [Crossref]
- Marchica P, D'Arpa S, Magno S, et al. Integrated Treatment of Breast Cancer-related Lymphedema: A Descriptive Review of the State of the Art. Anticancer Res 2021;41:3233-46. [Crossref] [PubMed]
- Soran A, Polat AK, Mager LG. Breast Cancer-Related Lymphedema (BCRL). In: Aydiner A, İgci A, Soran A. editors. Breast Disease: Management and Therapies. Springer International Publishing; 2016:853-76.
- Tsai RJ, Dennis LK, Lynch CF, et al. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol 2009;16:1959-72. [Crossref] [PubMed]
- Shah C, Vicini FA. Breast cancer-related arm lymphedema: incidence rates, diagnostic techniques, optimal management and risk reduction strategies. Int J Radiat Oncol Biol Phys 2011;81:907-14. [Crossref] [PubMed]
- Kim M, Kim SW, Lee SU, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys 2013;86:498-503. [Crossref] [PubMed]
- McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008;26:5213-9. [Crossref] [PubMed]
- Kilbreath SL, Refshauge KM, Beith JM, et al. Risk factors for lymphoedema in women with breast cancer: A large prospective cohort. Breast 2016;28:29-36. [Crossref] [PubMed]
- Shaitelman SF, Chiang YJ, Griffin KD, et al. Radiation therapy targets and the risk of breast cancer-related lymphedema: a systematic review and network meta-analysis. Breast Cancer Res Treat 2017;162:201-15. [Crossref] [PubMed]
- Gärtner R, Jensen MB, Kronborg L, et al. Self-reported arm-lymphedema and functional impairment after breast cancer treatment--a nationwide study of prevalence and associated factors. Breast 2010;19:506-15. [Crossref] [PubMed]
- Warren LE, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys 2014;88:565-71. [Crossref] [PubMed]
- Ginsburg O, Bray F, Coleman MP, et al. The global burden of women's cancers: a grand challenge in global health. Lancet 2017;389:847-60. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Ciudad P, Escandón JM, Manrique OJ, et al. Lessons Learnt from an 11-year Experience with Lymphatic Surgery and a Systematic Review of Reported Complications: Technical Considerations to Reduce Morbidity. Arch Plast Surg 2022;49:227-39. [Crossref] [PubMed]
- Ciudad P, Agko M, Huang TCT, et al. Comprehensive multimodal surgical treatment of end-stage lower extremity lymphedema with toe management: The combined Charles,' Homan's, and vascularized lymph node transfer (CHAHOVA) procedures. J Surg Oncol 2019;119:430-8. [Crossref] [PubMed]
- Ciudad P, Huayllani MT, Forte AJ, et al. Vascularized Lymph Node Transfer for the Treatment of Posttraumatic Lower Extremity Lymphedema: A Preliminary Report. Indian J Plast Surg 2022;55:97-101. [Crossref] [PubMed]
- Brahma B, Yamamoto T. Breast cancer treatment-related lymphedema (BCRL): An overview of the literature and updates in microsurgery reconstructions. Eur J Surg Oncol 2019;45:1138-45. [Crossref] [PubMed]
- Ciudad P, Manrique OJ, Bustos SS, et al. Single-stage VASER-assisted liposuction and lymphatico-venous anastomoses for the treatment of extremity lymphedema: a case series and systematic review of the literature. Gland Surg 2020;9:545-57. [Crossref] [PubMed]
- Carl HM, Walia G, Bello R, et al. Systematic Review of the Surgical Treatment of Extremity Lymphedema. J Reconstr Microsurg 2017;33:412-25. [Crossref] [PubMed]
- Kung TA, Champaneria MC, Maki JH, et al. Current Concepts in the Surgical Management of Lymphedema. Plast Reconstr Surg 2017;139:1003e-1013e. [Crossref] [PubMed]
- Escandón JM, Ciudad P, Mayer HF, et al. Free flap transfer with supermicrosurgical technique for soft tissue reconstruction: A systematic review and meta-analysis. Microsurgery 2023;43:171-84. [Crossref] [PubMed]
- Yamamoto T, Yoshimatsu H, Yamamoto N, et al. Side-to-end Lymphaticovenular anastomosis through temporary lymphatic expansion. PLoS One 2013;8:e59523. [Crossref] [PubMed]
- Chen WF, Yamamoto T, Fisher M, et al. The "Octopus" Lymphaticovenular Anastomosis: Evolving Beyond the Standard Supermicrosurgical Technique. J Reconstr Microsurg 2015;31:450-7. [Crossref] [PubMed]
- Yamamoto T, Narushima M, Kikuchi K, et al. Lambda-shaped anastomosis with intravascular stenting method for safe and effective lymphaticovenular anastomosis. Plast Reconstr Surg 2011;127:1987-92. [Crossref] [PubMed]
- Ayestaray B, Bekara F. π-shaped lymphaticovenular anastomosis: the venous flow sparing technique for the treatment of peripheral lymphedema. J Reconstr Microsurg 2014;30:551-60. [Crossref] [PubMed]
- Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg 2010;126:752-8. [Crossref] [PubMed]
- Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 2000;16:437-42. [Crossref] [PubMed]
- Engel H, Lin CY, Huang JJ, et al. Outcomes of Lymphedema Microsurgery for Breast Cancer-related Lymphedema With or Without Microvascular Breast Reconstruction. Ann Surg 2018;268:1076-83. [Crossref] [PubMed]
- Chen WF, Bowen M, Ding J. Immediate Limb Compression Following Supermicrosurgical Lymphaticovenular Anastomosis – Is It Helpful or Harmful? International Microsurgery Journal 2018;2:1.
- Damstra RJ, Voesten HG, van Schelven WD, et al. Lymphatic venous anastomosis (LVA) for treatment of secondary arm lymphedema. A prospective study of 11 LVA procedures in 10 patients with breast cancer related lymphedema and a critical review of the literature. Breast Cancer Res Treat 2009;113:199-206. [Crossref] [PubMed]
- Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305-14. [Crossref] [PubMed]
- Poumellec MA, Foissac R, Cegarra-Escolano M, et al. Surgical treatment of secondary lymphedema of the upper limb by stepped microsurgical lymphaticovenous anastomoses. Breast Cancer Res Treat 2017;162:219-24. [Crossref] [PubMed]
- Lee KT, Park JW, Mun GH. Serial two-year follow-up after lymphaticovenular anastomosis for the treatment of lymphedema. Microsurgery 2017;37:763-70. [Crossref] [PubMed]
- Ayestaray B, Bekara F, Andreoletti JB. Patent blue-enhanced lymphaticovenular anastomosis. J Plast Reconstr Aesthet Surg 2013;66:382-9. [Crossref] [PubMed]
- Gennaro P, Gabriele G, Mihara M, et al. Supramicrosurgical lymphatico-venular anastomosis (LVA) in treating lymphoedema: 36-months preliminary report. Eur Rev Med Pharmacol Sci 2016;20:4642-53.
- Cornelissen AJM, Kool M, Lopez Penha TR, et al. Lymphatico-venous anastomosis as treatment for breast cancer-related lymphedema: a prospective study on quality of life. Breast Cancer Res Treat 2017;163:281-6. [Crossref] [PubMed]
- Winters H, Tielemans HJP, Hameeteman M, et al. The efficacy of lymphaticovenular anastomosis in breast cancer-related lymphedema. Breast Cancer Res Treat 2017;165:321-7. [Crossref] [PubMed]
- Forte AJ, Sisti A, Huayllani MT, et al. Lymphaticovenular anastomosis for breast cancer-related upper extremity lymphedema: a literature review. Gland Surg 2020;9:539-44. [Crossref] [PubMed]
- Pappalardo M, Starnoni M, Franceschini G, et al. Breast Cancer-Related Lymphedema: Recent Updates on Diagnosis, Severity and Available Treatments. J Pers Med 2021;11:402. [Crossref] [PubMed]
- Scaglioni MF, Meroni M, Fritsche E. Lymphaticovenous anastomosis (LVA) for breast cancer-related lymphedema treatment. Transl Cancer Res 2020;9:3167-71. [Crossref] [PubMed]
- Ciudad P, Bolletta A, Kaciulyte J, et al. The breast cancer-related lymphedema multidisciplinary approach: Algorithm for conservative and multimodal surgical treatment. Microsurgery 2023;43:427-36. [Crossref] [PubMed]
- Chen WF. How to Get Started Performing Supermicrosurgical Lymphaticovenular Anastomosis to Treat Lymphedema. Ann Plast Surg 2018;81:S15-20. [Crossref] [PubMed]
- Yamamoto T. Comment: Selection of anastomosis type for lymphaticovenular anastomosis. J Plast Reconstr Aesthet Surg 2013;66:207-8. [Crossref] [PubMed]
- Chang DW, Dayan J, Greene AK, et al. Surgical Treatment of Lymphedema: A Systematic Review and Meta-Analysis of Controlled Trials. Results of a Consensus Conference. Plast Reconstr Surg 2021;147:975-93. [Crossref] [PubMed]
- Manrique OJ, Bustos SS, Kapoor T, et al. Gastroepiploic vascularized lymph node transfer for the treatment of extremity lymphedema: comparison between middle and distal inset. Gland Surg 2020;9:528-38. [Crossref] [PubMed]
- Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313-5. [Crossref] [PubMed]
- Lin CH, Ali R, Chen SC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265-75. [Crossref] [PubMed]
- Liu HL, Pang SY, Lee CC, et al. Orthotopic transfer of vascularized groin lymph node flap in the treatment of breast cancer-related lymphedema: Clinical results, lymphoscintigraphy findings, and proposed mechanism. J Plast Reconstr Aesthet Surg 2018;71:1033-40. [Crossref] [PubMed]
- Gharb BB, Rampazzo A, Spanio di Spilimbergo S, et al. Vascularized lymph node transfer based on the hilar perforators improves the outcome in upper limb lymphedema. Ann Plast Surg 2011;67:589-93. [Crossref] [PubMed]
- Granzow JW, Soderberg JM, Kaji AH, et al. An effective system of surgical treatment of lymphedema. Ann Surg Oncol 2014;21:1189-94. [Crossref] [PubMed]
- Patel KM, Lin CY, Cheng MH. A Prospective Evaluation of Lymphedema-Specific Quality-of-Life Outcomes Following Vascularized Lymph Node Transfer. Ann Surg Oncol 2015;22:2424-30. [Crossref] [PubMed]
- Dionyssiou D, Demiri E, Tsimponis A, et al. A randomized control study of treating secondary stage II breast cancer-related lymphoedema with free lymph node transfer. Breast Cancer Res Treat 2016;156:73-9. [Crossref] [PubMed]
- Gratzon A, Schultz J, Secrest K, et al. Clinical and Psychosocial Outcomes of Vascularized Lymph Node Transfer for the Treatment of Upper Extremity Lymphedema After Breast Cancer Therapy. Ann Surg Oncol 2017;24:1475-81. [Crossref] [PubMed]
- Maruccia M, Elia R, Ciudad P, et al. Postmastectomy upper limb lymphedema: Combined vascularized lymph node transfer and scar release with fat graft expedites surgical and patients' related outcomes. A retrospective comparative study. J Plast Reconstr Aesthet Surg 2019;72:892-901. [Crossref] [PubMed]
- Aljaaly HA, Fries CA, Cheng MH. Dorsal Wrist Placement for Vascularized Submental Lymph Node Transfer Significantly Improves Breast Cancer-Related Lymphedema. Plast Reconstr Surg Glob Open 2019;7:e2149. [Crossref] [PubMed]
- Lo Torto F, Kaciulyte J, Mori FL, et al. Microsurgical lymphedema treatment: An objective evaluation of the quality of online information. J Plast Reconstr Aesthet Surg 2021;74:637-40. [Crossref] [PubMed]
- Becker C, Vasile JV, Levine JL, et al. Microlymphatic surgery for the treatment of iatrogenic lymphedema. Clin Plast Surg 2012;39:385-98. [Crossref] [PubMed]
- Allen RJ Jr, Cheng MH. Lymphedema surgery: Patient selection and an overview of surgical techniques. J Surg Oncol 2016;113:923-31. [Crossref] [PubMed]
- Chang EI, Masià J, Smith ML. Combining Autologous Breast Reconstruction and Vascularized Lymph Node Transfer. Semin Plast Surg 2018;32:36-41. [Crossref] [PubMed]
- Cheng MH, Huang JJ, Wu CW, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg 2014;133:192e-198e. [Crossref] [PubMed]
- Miranda Garcés M, Pons G, Mirapeix R, et al. Intratissue lymphovenous communications in the mechanism of action of vascularized lymph node transfer. J Surg Oncol 2017;115:27-31. [Crossref] [PubMed]
- Gasteratos K, Morsi-Yeroyannis A, Vlachopoulos NC, et al. Microsurgical techniques in the treatment of breast cancer-related lymphedema: a systematic review of efficacy and patient outcomes. Breast Cancer 2021;28:1002-15. [Crossref] [PubMed]
- Manrique OJ, Bustos SS, Kuruoglu D, et al. Gastroepiploic Lymph Node Flap Harvest for Patients With Lymphedema: Minimally Invasive Versus Open Approach. Ann Plast Surg 2020;85:S87-91. [Crossref] [PubMed]
- Ciudad P, Manrique OJ, Bustos SS, et al. Comparisons in long-term clinical outcomes among patients with upper or lower extremity lymphedema treated with diverse vascularized lymph node transfer. Microsurgery 2020;40:130-6. [Crossref] [PubMed]
- Ciudad P, Agko M, Perez Coca JJ, et al. Comparison of long-term clinical outcomes among different vascularized lymph node transfers: 6-year experience of a single center's approach to the treatment of lymphedema. J Surg Oncol 2017;116:671-82. [Crossref] [PubMed]
- Montag E, Okada AY, Arruda EGP, et al. Influence of vascularized lymph node transfer (VLNT) flap positioning on the response to breast cancer-related lymphedema treatment. Rev Col Bras Cir 2019;46:e2156. [Crossref] [PubMed]
- Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg 2012;255:468-73. [Crossref] [PubMed]
- Winters H, Tielemans HJP, Paulus V, et al. A systematic review and meta-analysis of vascularized lymph node transfer for breast cancer-related lymphedema. J Vasc Surg Venous Lymphat Disord 2022;10:786-795.e1. [Crossref] [PubMed]
- Chocron Y, Azzi AJ, Bouhadana G, et al. Axilla versus Wrist as the Recipient Site in Vascularized Lymph Node Transfer for Breast Cancer-Related Lymphedema: A Systematic Review and Meta-Analysis. J Reconstr Microsurg 2022;38:539-48. [Crossref] [PubMed]
- Nguyen AT, Chang EI, Suami H, et al. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol 2015;22:2919-24. [Crossref] [PubMed]
- Akita S, Tokumoto H, Yamaji Y, et al. Contribution of Simultaneous Breast Reconstruction by Deep Inferior Epigastric Artery Perforator Flap to the Efficacy of Vascularized Lymph Node Transfer in Patients with Breast Cancer-Related Lymphedema. J Reconstr Microsurg 2017;33:571-8. [Crossref] [PubMed]
- De Brucker B, Zeltzer A, Seidenstuecker K, et al. Breast Cancer-Related Lymphedema: Quality of Life after Lymph Node Transfer. Plast Reconstr Surg 2016;137:1673-80. [Crossref] [PubMed]
- Chang EI, Schaverien MV, Hanson SE, et al. Evolution in Surgical Management of Breast Cancer-related Lymphedema: The MD Anderson Cancer Center Experience. Plast Reconstr Surg Glob Open 2020;8:e2674. [Crossref] [PubMed]
- Ciudad P, Manrique OJ, Adabi K, et al. Combined double vascularized lymph node transfers and modified radical reduction with preservation of perforators for advanced stages of lymphedema. J Surg Oncol 2019;119:439-48. [Crossref] [PubMed]
- Ciudad P, Vargas MI, Bustamante A, et al. Combined radical reduction with preservation of perforators and distal lymphaticovenular anastomosis for advanced lower extremity lymphedema. Microsurgery 2020;40:417-8. [Crossref] [PubMed]
- Nicoli F, Constantinides J, Ciudad P, et al. Free lymph node flap transfer and laser-assisted liposuction: a combined technique for the treatment of moderate upper limb lymphedema. Lasers Med Sci 2015;30:1377-85. [Crossref] [PubMed]
- O'Brien BM, Khazanchi RK, Kumar PA, et al. Liposuction in the treatment of lymphoedema; a preliminary report. Br J Plast Surg 1989;42:530-3. [Crossref] [PubMed]
- Forte AJ, Huayllani MT, Boczar D, et al. Lipoaspiration and Lymph Node Transfer for Treatment of Breast Cancer-related Lymphedema: A Systematic Review. Cureus 2019;11:e6096. [Crossref] [PubMed]
- Damstra RJ, Voesten HG, Klinkert P, et al. Circumferential suction-assisted lipectomy for lymphoedema after surgery for breast cancer. Br J Surg 2009;96:859-64. [Crossref] [PubMed]
- Hoffner M, Ohlin K, Svensson B, et al. Liposuction Gives Complete Reduction of Arm Lymphedema following Breast Cancer Treatment-A 5-year Prospective Study in 105 Patients without Recurrence. Plast Reconstr Surg Glob Open 2018;6:e1912. [Crossref] [PubMed]
- Lee D, Piller N, Hoffner M, et al. Liposuction of Postmastectomy Arm Lymphedema Decreases the Incidence of Erysipelas. Lymphology 2016;49:85-92.
- Bloom JA, Granoff M, Karlsson T, et al. Power-assisted Liposuction for Lymphedema: A Cost-utility Analysis. Plast Reconstr Surg Glob Open 2022;10:e4671. [Crossref] [PubMed]
- Brorson H. Liposuction in Lymphedema Treatment. J Reconstr Microsurg 2016;32:56-65. [Crossref] [PubMed]
- Hoffner M, Bagheri S, Hansson E, et al. SF-36 Shows Increased Quality of Life Following Complete Reduction of Postmastectomy Lymphedema with Liposuction. Lymphat Res Biol 2017;15:87-98. [Crossref] [PubMed]
- Campisi CC, Ryan M, Boccardo F, et al. Fibro-Lipo-Lymph-Aspiration With a Lymph Vessel Sparing Procedure to Treat Advanced Lymphedema After Multiple Lymphatic-Venous Anastomoses: The Complete Treatment Protocol. Ann Plast Surg 2017;78:184-90. [Crossref] [PubMed]
- Leppäpuska IM, Suominen E, Viitanen T, et al. Combined Surgical Treatment for Chronic Upper Extremity Lymphedema Patients: Simultaneous Lymph Node Transfer and Liposuction. Ann Plast Surg 2019;83:308-17. [Crossref] [PubMed]
- Agko M, Ciudad P, Chen HC. Staged surgical treatment of extremity lymphedema with dual gastroepiploic vascularized lymph node transfers followed by suction-assisted lipectomy-A prospective study. J Surg Oncol 2018;117:1148-56. [Crossref] [PubMed]
- Granzow JW, Soderberg JM, Dauphine C. A novel two-stage surgical approach to treat chronic lymphedema. Breast J 2014;20:420-2. [Crossref] [PubMed]
- Ghazaleh AA, Handschin TM, Buckowiecki J, et al. Combining reconstructive and ablative surgical treatment of chronic breast cancer-related lymphedema (BCRL): safe and effective. Breast Cancer Res Treat 2023;197:83-92. [Crossref] [PubMed]
- Salgado CJ, Sassu P, Gharb BB, et al. Radical reduction of upper extremity lymphedema with preservation of perforators. Ann Plast Surg 2009;63:302-6. [Crossref] [PubMed]
- Ciudad P, Manrique OJ, Bustos SS, et al. Combined microvascular breast and lymphatic reconstruction with deep inferior epigastric perforator flap and gastroepiploic vascularized lymph node transfer for postmastectomy lymphedema patients. Gland Surg 2020;9:512-20. [Crossref] [PubMed]
- Ciudad P, Agko M, Patel KM, et al. A single-stage triple-inset vascularized gastroepiploic lymph node transfers for the surgical treatment of extremity lymphedema. Microsurgery 2021;41:97-9. [Crossref] [PubMed]
- Faisal M, Sayed MG, Antonious K, et al. Prevention of lymphedema via axillary reverse mapping for arm lymph-node preservation following breast cancer surgery: a randomized controlled trial. Patient Saf Surg 2019;13:35. [Crossref] [PubMed]
- Ciudad P, Escandón JM, Bustos VP, et al. Primary Prevention of Cancer-Related Lymphedema Using Preventive Lymphatic Surgery: Systematic Review and Meta-analysis. Indian J Plast Surg 2022;55:18-25. [Crossref] [PubMed]


