
Analysis of the prognostic factors of simultaneous integrated boost-intensity modulated radiation therapy (SIB-IMRT) in 220 cases of locally advanced squamous esophageal cancer: a retrospective cohort study
Highlight box
Key findings
• In the SIB-IMRT era, the survival of ESCC patients treated with radical (chemo)radiotherapy is relatively satisfactory. Gender, N stage, treatment modality, radiotherapy dose, ALC, and GTV were independent factors affecting the prognosis of patients with esophageal cancer.
What is known and what is new?
• Radiotherapy is an effective treatment for locally advanced esophageal cancer.
• All patients in this study received SIB-IMRT, and the patient baseline characteristics, radiotherapy target area size, and hematologic indicators were combined in the data analysis to produce a more comprehensive study result.
What are the implications, and what should change now?
• SIB-IMRT is a better mode of radiotherapy, and a higher dose of radiotherapy and smaller GTV are recommended for patients who can tolerate it. If a more thorough understanding of the relationship between radiotherapy-induced lymphopenia and patient survival is desired, a prospective study could be the next step.
Introduction
Esophageal cancer is a common malignant tumor in China, ranking third in incidence and fourth in mortality among malignant tumors according to a national epidemiological study conducted in 2015 (1). Due to its insidious onset and the lack of specific clinical symptoms in the early stage of the disease, 50–60% of esophageal cancer patients have already progressed to the middle and late stages when they are first diagnosed, and some of them have lost the opportunity for surgical treatment (2). Since more than 90% of the pathological types of esophageal cancer are squamous carcinoma (3), which is sensitive to radiation, radiation therapy is a commonly used treatment for patients with intermediate to advanced esophageal cancer that cannot be treated surgically. Conventional radiation therapy techniques make it difficult to increase the local dose of esophageal lesions due to the dose limitation tolerated by the surrounding normal tissues and organs (4). With the widespread introduction of intensity-modulated radiation therapy, the five-year survival rate for esophageal cancer has improved significantly compared to conventional two-dimensional radiation therapy, and several studies have demonstrated that radical radiotherapy (RT) can yield the same survival rate as surgery (5-7). Simultaneous integrated boost-intensity modulated radiation therapy (SIB-IMRT) is one of the commonly used intensity-modulated RT methods in clinical practice, which can increase the dose to the tumor bed area without increasing the dose to the surrounding normal tissues (8). This can reduce the risk of adverse effects while improving the efficacy of the treatment. However, in non-surgical patients, traditional prognostic factors such as staging tumor, node, metastasis (TNM) staging cannot be accurately determined (9,10). Therefore, there is a need to identify more feasible and effective clinical factors to improve the prognosis prediction of esophageal squamous cell carcinoma (ESCC) patients undergoing radical radiation therapy. The present study summarized the survival of esophageal cancer patients who received SIB-IMRT ± chemotherapy at the First Affiliated Hospital of Bengbu Medical College in recent years and analyzed the prognostic factors to provide an important basis and reference for clinical treatment selection. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6462/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College (approval No. 2021KY032). All the patients included in this study gave their informed consent for the treatment.
Study population
This is a retrospective cohort study. Patients with esophageal squamous carcinoma who were unable or unwilling to undergo surgery and received SIB-IMRT at the Department of Radiotherapy, The First Affiliated Hospital of Bengbu Medical College between January 2017 and December 2018 were collected. Variables including demographic, clinicopathological, and treatment characteristics were obtained from the electronic medical record system. Study variables collected at baseline included age, gender, tumor location, N stage, RT dose, treatment modality, clinical stage, grading, size of gross tumor volume (GTV) at the tumor site, and absolute lymphocyte count (ALC) minimum during RT. Patients with missing follow-up information, imperfect clinical data, Karnofsky Performance Status (KPS) score <70, abnormal lymphocytes in routine blood examination before the start of RT, distant organ metastases at the initial diagnosis, other malignant tumors diagnosed within 5 years before treatment, and less than 3 months of follow-up were excluded, and a total of 220 patients were enrolled in this study.
Among them, 147 (66.8%) were male and 73 (33.2%) were female; age ranged from 40 to 90 years, with a mean of 72.37 [95% confidence interval (CI): 71.25–73.50] years, including 145 (65.9%) patients ≥70 years old and 75 (34.1%) patients <70 years old. There were 15 patients (6.8%) in the cervical segment, 55 patients (25.0%) in the upper thoracic segment, 97 patients (44.1%) in the middle thoracic segment, and 53 patients (24.1%) in the lower thoracic segment. Regarding the N stage, there were 34 cases (15.5%) in the N0 stage, 162 cases (73.6%) in the N1 stage, and 24 cases (10.9%) in the N2 stage.
The RT doses for the entire cohort ranged from 46 to 66 Gy, with a median of 60 Gy; for the purpose of dose uniformity, the RT doses for the whole cohort were converted according to the equivalent dose of 2 Gy/fraction (EQD2). 153 patients (69.5%) had a RT dose of ≥60.0 Gy in the tumor area, 42 patients (19.1%) had a dose of 50.0–59.9 Gy, and 25 patients (11.4%) had a dose of <50.0 Gy. There were 46 patients (20.9%) in the RT alone group, 154 patients (70.0%) in the chemoradiotherapy (CCRT) group, and 20 patients (9.1%) in the sequential chemoradiotherapy (sCRT) group (patients who did not receive concurrent chemotherapy but received induction chemotherapy and (or) adjuvant chemotherapy were defined as patients receiving sCRT) (Table 1).
Table 1
| Variables | No. of patient (N=220) | % |
|---|---|---|
| Sex | ||
| Male | 147 | 66.8 |
| Female | 73 | 33.2 |
| Age | ||
| <70 years | 75 | 34.1 |
| ≥70 years | 145 | 65.9 |
| Tumor location | ||
| Cervical | 15 | 6.8 |
| Upper | 55 | 25.0 |
| Middle | 97 | 44.1 |
| Lower | 53 | 24.1 |
| N stage | ||
| N0 | 34 | 15.5 |
| N1 | 162 | 73.6 |
| N2 | 24 | 10.9 |
| EQD2 | ||
| 40.0–49.9 Gy | 25 | 11.4 |
| 50.0–59.9 Gy | 42 | 19.1 |
| ≥60.0 Gy | 153 | 69.5 |
| Treatment modality | ||
| RT alone | 46 | 20.9 |
| CCRT | 154 | 70.0 |
| sCRT | 20 | 9.1 |
| ALC | ||
| <0.2×109/L | 44 | 20.9 |
| ≥0.2×109/L | 176 | 79.1 |
| GTV | ||
| <78.5 cm³ | 142 | 64.5 |
| ≥78.5 cm³ | 78 | 35.5 |
EQD2, equivalent dose in 2 Gy; RT, radiotherapy; CCRT, chemoradiotherapy; sCRT, sequential chemoradiotherapy; ALC, absolute lymphocyte count; GTV, gross tumor volume.
Treatment
RT
All patients were treated with RT using the SIB-IMRT technique. The GTV was defined as the primary focus of the esophagus identified by various imaging modalities such as upper gastrointestinal tract imaging and computed tomography (CT).
Chemotherapy
For patients who received concurrent RT, the most common regimens were oral single-agent tegeo (S-1) (n=100, 64.94%) and a two-drug combination of paclitaxel and platinum (n=38, 24.68%), with a few patients also receiving a two-drug combination of tegeo and platinum and single-agent platinum. In contrast, for patients who received sCRT, the most commonly used regimens were single-agent tegeo and paclitaxel + platinum.
Follow-up and evaluation criteria
Patients were followed up by telephone once every 3 months for the first 2 years and every 6 months thereafter, and the number of patients who died was recorded in detail. The study endpoints were overall survival (OS) and progression-free survival (PFS) rates, with OS defined as the time interval between the start of patient treatment and patient death due to any cause, and PFS defined as the time interval between the end of patient treatment and patient tumor recurrence, progression, or death from any cause. Grade 4 lymphopenia was defined as an ALC nadir <0.2×109/L according to the Common Terminology Criteria for Adverse Events version 5.0.
Statistical analysis
SPSS 25.0 software (IBM, USA) was used to perform statistical analysis. The Kaplan-Meier (KM) method was employed to calculate the survival rate, the log-rank test was used for single-factor survival analysis, and the Cox regression model was used for multi-factor survival analysis. The optimal cut-off point (i.e., the point where the sum of sensitivity and specificity is the maximum point of the Jorden index) for esophageal cancer tumor volume was determined by applying the subject receiver operating characteristic (ROC) curve, and the area under the curve (AUC). P<0.05 was considered statistically significant.
Results
Survival
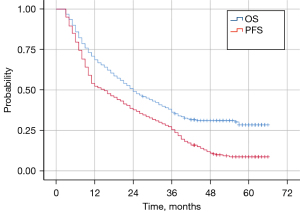
The median follow-up time for the entire cohort was 56.0 months (3.0–66.0 months), with 1-, 2-, and 3-year survival rates of 68.6%, 49.1%, and 36.3%, respectively; and 1-, 2-, and 3-year PFS rates of 52.3%, 37.7%, and 25.5%, respectively. The median OS time was 24 months (95% CI: 19.16–28.84 months) and the median PFS time was 15 months (95% CI: 11.04–18.96 months) (Figure 1).
Univariate and multivariate analysis of prognosis
The univariate analysis results showed that gender, N stage, treatment modality, RT dose, ALC, and GTV were associated with the 3-year OS and 3-year PFS of patients with esophageal cancer (P<0.05, Table 2). Multifactorial analysis showed that gender, RT dose, treatment modality, ALC, and GTV were independent prognostic factors affecting 3-year OS (P<0.05); while gender, N-stage, RT dose, and GTV were independent prognostic factors affecting 3-year PFS (P<0.05, Table 3).
Table 2
| Variables | OS | PFS | |||
|---|---|---|---|---|---|
| 3-year (%) | P | 3-year (%) | P | ||
| Sex | 0.002 | 0.002 | |||
| Male | 30.5 | 19.0 | |||
| Female | 47.9 | 38.4 | |||
| Age | 0.237 | 0.351 | |||
| <70 years | 41.3 | 32.0 | |||
| ≥70 years | 33.6 | 22.1 | |||
| Tumor location | 0.185 | 0.304 | |||
| Cervical | 33.3 | 26.7 | |||
| Upper | 46.9 | 34.5 | |||
| Middle | 35.1 | 25.8 | |||
| Lower | 28.3 | 15.1 | |||
| N stage | 0.002 | 0 | |||
| N0 | 58.8 | 55.9 | |||
| N1 | 35.0 | 21.0 | |||
| N2 | 12.5 | 12.5 | |||
| EQD2 | 0 | 0 | |||
| 40.0–49.9 Gy | 16.0 | 12.0 | |||
| 50.0–59.9 Gy | 25.3 | 11.9 | |||
| ≥60.0 Gy | 42.5 | 31.4 | |||
| Treatment modality | 0.007 | 0.037 | |||
| RT alone | 21.7 | 15.2 | |||
| CCRT | 41.4 | 29.9 | |||
| sCRT | 30.0 | 15.0 | |||
| ALC | 0 | 0 | |||
| <0.2×109/L | 6.8 | 4.5 | |||
| ≥0.2×109/L | 43.7 | 30.7 | |||
| GTV | 0.001 | 0.003 | |||
| <78.5 cm³ | 44.2 | 31.0 | |||
| ≥78.5 cm³ | 21.8 | 15.4 | |||
OS, overall survival; PFS, progression-free survival; EQD2, equivalent dose in 2 Gy; RT, radiotherapy; CCRT, chemoradiotherapy; sCRT, sequential chemoradiotherapy; ALC, absolute lymphocyte count; GTV, gross tumor volume.
Table 3
| Variables | OS | PFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Sex | |||||
| Male | 1 | 1 | |||
| Female | 0.678 (0.468–0.982) | 0.04 | 0.646 (0.472–0.886) | 0.007 | |
| N stage | |||||
| N0 | 1 | 1 | |||
| N1 | 1.630 (0.941–2.821) | 0.081 | 1.871 (1.198–2.922) | 0.006 | |
| N2 | 1.962 (0.969–3.973) | 0.061 | 2.072 (1.123–3.824) | 0.02 | |
| EQD2 | |||||
| ≥60.0 Gy | 1 | 1 | |||
| 50.0–59.9 Gy | 1.591 (1.054–2.400) | 0.027 | 1.963 (1.356–2.843) | 0 | |
| 40.0–49.9 Gy | 1.953 (1.198–3.185) | 0.007 | 1.519 (0.948–2.433) | 0.082 | |
| Treatment modality | |||||
| RT alone | 1 | 1 | |||
| CCRT | 0.577 (0.392–0.851) | 0.006 | 0.741 (0.516–1.065) | 0.105 | |
| sCRT | 1.014 (0.559–1.839) | 0.963 | 1.350 (0.773–2.355) | 0.291 | |
| ALC | |||||
| <0.2×109/L | 1 | 1 | |||
| ≥0.2×109/L | 0.581 (0.392–0.863) | 0.007 | 0.777 (0.535–1.128) | 0.184 | |
| GTV | |||||
| <78.5 cm³ | 1 | 1 | |||
| ≥78.5 cm³ | 1.603 (1.132–2.271) | 0.008 | 1.551 (1.139–2.110) | 0.005 | |
OS, overall survival; PFS, progression-free survival; EQD2, equivalent dose in 2 Gy; RT, radiotherapy; CCRT, chemoradiotherapy; sCRT, sequential chemoradiotherapy; ALC, absolute lymphocyte count; GTV, gross tumor volume.
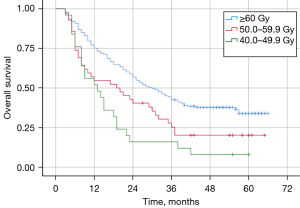
Effect of different RT doses on survival
The median OS of patients in the EQD2 (equivalent dose in 2 Gy/fraction) ≥60.0 Gy, 50.0–59.9 Gy, and 40.0–49.9 Gy groups were 30.0, 19.0, and 13.0 months, respectively (Figure 2). The median OS of patients in the ≥60.0 Gy group was better than those in the 50.0–59.9 Gy (P=0.016) and 40.0–49.9 Gy (P=0.000) groups. There was no statistically significant difference in median OS between patients in the 50.0–59.9 and 40.0–49.9 Gy groups (P=0.128).
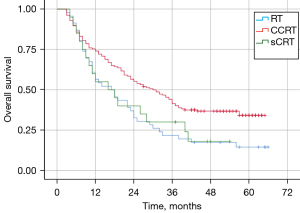
Impact of different treatment modalities on survival
The median OS was 30.0, 17.0, and 16.0 months for patients in the CCRT, RT alone, and sCRT groups, respectively (Figure 3). The prognosis of patients who received CCRT was significantly better than that of patients who received RT alone (P=0.005); the survival curve was also superior to that of patients who received sCRT but it was not statistically significant (P=0.085). In contrast, the survival of patients in the sCRT group tended to be higher than that in the RT-alone group but the difference was not statistically significant (P=0.802).
Discussion
In China, most patients with esophageal cancer have advanced disease at the time of initial diagnosis, with only 20–30% of these patients being operable at the time of initial diagnosis according to statistics. Therefore, non-surgical treatment, mainly radiation therapy, is particularly important in Chinese patients with esophageal cancer. SIB-IMRT is a method of irradiation with different split doses for different dose requirements in the same field, which can achieve additional local dose irradiation for large tumors in the same plan without increasing the dose to normal tissues, and its dosimetric advantages have been confirmed by previous study (11). The OS rates at 1 and 3 years in this study were 68.6% and 36.3%, which are consistent with the results of Lan et al., who utilized SIB-IMRT for esophageal cancer and reported OS rates of 65.2% and 36.2% at 1 and 3 years, respectively (12). In addition, the survival outcome of patients in the RT-alone group in this study was satisfactory, with a 3-year OS of 21.7%, which is higher than previously reported in the 2D/3D RT era, which may be related to recent advances in radiation therapy and imaging techniques. These results suggest that in the era of SIB-IMRT, single radiation therapy can still lead to promising survival outcomes for patients who cannot tolerate CCRT.
Previous study has shown that the combination of chemotherapy and RT can increase the sensitivity of malignant tumors to RT, while RT can increase the cytotoxicity of chemotherapy drugs (13). The combined application can enhance the effect of inactivating tumor cells, thus avoiding the accelerated re-proliferation of tumor cells after RT to affect the effect of RT . In a phase 3 randomized clinical trial of older patients with esophageal cancer by Ji et al., the CCRT (combined with S-1) group had a higher 2-year OS rate (53.2% versus 35.8%; hazard ratio (HR): 0.63; 95% CI: 0.47–0.85; P=0.002), with a significant benefit compared with RT alone (14). The 3-year survival rate in the CCRT group in this study was 41.4%, including 113 patients (73.38%) who received the S-1 chemotherapy regimen, and although the OS rate was lower than that reported by Ji et al. (14), the results were still consistent. The survival outcome of the CCRT group was better than that of the RT-alone group (median OS 30 vs. 17 months, P=0.005), indicating that CCRT remains the best treatment option in the SIB-IMRT era for patients with esophageal cancer who can tolerate concurrent chemotherapy.
The value of sequential chemotherapy for esophageal cancer is controversial; to date, there are no large prospective clinical trials to confirm the efficacy of sequential chemotherapy in patients with esophageal cancer after RT, and published retrospective studies have come to different conclusions (15,16). In our analysis, patients receiving sequential chemotherapy did not show a better survival outcome than patients receiving radiation alone (median OS: 16.0 vs. 17.0 months, P=0.802). However, due to the small number of patients in the sCRT group (20 cases, 9.1%), no further exploration was performed.
In terms of radiation dose, the majority (69.5%) of patients used a radical radiation dose of ≥60 Gy, which is the current radical dose commonly used in most Asian countries. This dose is different from the current dose (50.4 Gy) recommended by most guidelines. After Radiation Therapy Oncology Group (RTOG) 85-01 (17) and RTOG 94-05 (18) in the RTOG, 50.4 Gy became the standard dose for definitive RT; however, these two prospective clinical trials were conducted in the era of 2 dimensional RT. A study in recent years has confirmed that local area control using such doses is very ineffective (19). In a phase III multicenter randomized clinical trial of 60 versus 50 Gy CCRT for inoperable esophageal squamous carcinoma, the survival endpoints of the 60 Gy group were similar to those of the 50 Gy group, except that the incidence of severe pneumonia was higher in the 50 Gy group (20). In this study, we observed that patients receiving EQD2 ≥60 Gy had better OS than those receiving 50.0–59.9 Gy or 40.0–49.9 Gy (median OS: 42.5 vs. 25.3 vs. 16.0 months, P=0.000). This difference may be because all patients in Xu et al.’s study (20) completed CCRT, whereas patients in the 60 Gy group in this study had a higher rate of concurrent chemotherapy (72.55%) and a larger patient base. On the other hand, there are still many large retrospective studies that suggest that higher radiation doses may provide better local control and survival benefits for patients with advanced esophageal cancer, especially in patients with ESCC (21-23). These results support the idea that patients with esophageal squamous carcinoma may indeed require a higher radical radiation dose.
However, higher radiation doses necessarily entail higher therapeutic toxicity. For some patients, complete pathological regression (PCR) was achieved even after irradiation doses as low as 40–50 Gy (24). Therefore, we believe that it would be useful in the future to be able to correctly predict the sensitivity of patients to radiation therapy and to stratify the radiation dose according to the sensitivity of patients.
The different prognoses of patients with the same stage of esophageal cancer given the same treatment plan may be related to the differences in immune function and nutritional status among individuals. RT can reduce the number of lymphocytes, which is associated with a state of radiation-induced immunosuppression, and a study of RT for esophageal cancer reported grade 4 lymphopenia in about 31% of patients during CCRT (25). Davuluri et al. found that the probability of decreased grade 1, 2, 3, and 4 lymphocytes during CRT in esophageal cancer was 2%, 12%, 59%, and 27%, respectively, but only decreased grade 4 ALC was associated with poorer OS time (26). One study reported significantly shorter OS in patients who developed grade 4 lymphopenia during CRT than in patients who did not (median OS 34.7 vs. 63.1 months) (27).
In this study, KM survival analysis showed that the OS time was significantly lower in the group with grade 4 ALC reduction (ALC <0.2×109/L) than in the group without grade 4 ALC reduction (median OS 14 vs. 29 months, P=0.000). Yet, grade 4 ALC reduction was not an independent prognostic factor for PFS in the multifactorial analysis, suggesting an independent association between grade 4 ALC reduction and decreased OS in esophageal cancer. However, the relationship with short-term outcomes needs to be further investigated. Also, the presence or absence of concurrent chemotherapy during RT was not significantly associated with lymphocyte changes in patients with esophageal cancer, which is consistent with the findings of van Rossum et al. (27). In further analysis, patients who developed grade 4 lymphocytopenia had greater GTVs and higher irradiation doses compared with those who did not; the mean dose to both lungs and the mean dose to the body of patients in the ALC <0.2×109/L group were significantly higher than those with esophageal cancer in the ALC ≥0.2×109/L group (P<0.05). However, there was no significant difference between the two groups in the comparison between myelosuppression, radiation pneumonia, and radiation esophagitis (P>0.05). Intensity-modulated radiation therapy decreases the total number of lymphocytes in patients with esophageal cancer, and although the lymphocytes improve to some extent upon completion of radiation therapy, they remain lower than the pre-RT level in the short term. The presence of lymphopenia during RT is one of the predictors of OS time in patients with esophageal cancer. Therefore, optimizing the RT schedule to reduce the dose of irradiation to normal organs and enhancing nutritional support for esophageal cancer patients during RT (as a way of mitigating the impact on the body’s immune function) can reduce the effect of RT on lymphocytes and potentially improve the prognosis of patients with esophageal cancer.
At present, the clinical staging standards for non-surgical treatment of esophageal cancer are not uniform but most are based on the thickness of the esophageal wall without considering the length of the tumor. The main judgment indexes of the current clinical staging of esophageal cancer include the maximum diameter of the lesion shown in a chest CT scan, the relationship between the lesion and surrounding tissues and organs in CT, and the length of the lesion in a barium meal esophagogram. GTV is a comprehensive display of the lesion length and cross-sectional diameter, which is clinically advantageous for reflecting the overall condition of a local tumor. Larger tumor volumes reflect a greater tumor cell load, more frequent intratumor blood flow disorders, and a greater lack of oxygen cells; these factors can affect the treatment outcome, leading to an uncontrolled local area or recurrence after treatment, which in turn affects long-term survival. Tumor volume factors are closely related to tumor treatment outcomes such as the local tumor control rate and long-term patient survival. In the study by Chen et al., GTV accurately predicted the prognosis of patients with esophageal squamous carcinoma without distant metastases (28).
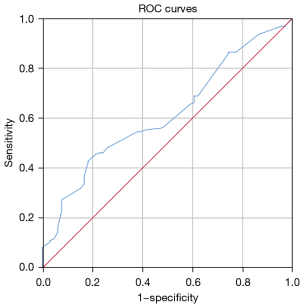
In this study, ROC curve analysis was performed to determine the cut-off value of the GTV (78.5 cm³) taking mortality status as an endpoint, which was used to divide the patients into two groups (Table 4, Figure 4). KM survival analysis showed that patients in the GTV ≥78.5 cm³ group had a significantly shorter survival time than those in the GTV <78.5 cm³ group (median OS 31 vs. 13 months, P=0.001). This result suggests that GTV may become a clinical staging criterion for the non-surgical treatment of esophageal cancer in the future, thus predicting the prognosis of patients.
Table 4
| Variable | Area | 95% CI | Sensitivity | Specificity | Asymptotic Sig. |
|---|---|---|---|---|---|
| GTV | 0.616 | 0.539–0.693 | 0.429 | 0.818 | 0.006 |
ROC, receiver operating characteristic; GTV, gross tumor volume; CI, confidence interval; Sig., significance.
Conclusions
In summary, there is a significant improvement in survival for patients with esophageal cancer receiving SIB-IMRT compared with previous RT techniques. CCRT remains the best option for the non-surgical treatment of patients who are clinically evaluated to tolerate concurrent RT, with a recommended radiation dose of ≥60.0 Gy to the tumor area, a smaller GTV associated with better survival outcomes, and lymphopenia due to RT associated with patient survival. Prospective studies are still needed to obtain approaches that can alleviate severe lymphopenia, which may eventually translate into survival benefits.
Limitations
This study has some limitations that should be noted. Firstly, this was a single-institution retrospective study with a relatively small sample size. Also, we could only include a limited number of items for analysis and lacked information on several detailed variables (i.e., performance status, nutritional status, comorbidities, treatment-related side effects, quality of life, etc.), which affected our ability to further investigate these factors in this study, so our findings may not be generalizable to other clinical settings. Therefore, prospective studies with larger sample sizes are needed to explore the survival and prognosis of patients with esophageal squamous carcinoma treated with SIB-IMRT.
Acknowledgments
The authors appreciate the academic support from the AME Esophageal Cancer Collaborative Group.
Funding: This study was supported by the Anhui Province Key Laboratory of Translational Cancer Research (Bengbu Medical College, No. KFDX202204) and the Natural Science Foundation of Anhui Province (No. KJ2021A0699).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6462/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6462/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6462/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College (Approval No. 2021KY032). All the patients included in this study gave their informed consent for the treatment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Li C, Wang X, Wang L, et al. Clinical practice and outcome of radiotherapy for advanced esophageal squamous cell carcinoma between 2002 and 2018 in China: the multi-center 3JECROG Survey. Acta Oncol 2021;60:627-34. [Crossref] [PubMed]
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- Gao HJ, Wei YC, Gong L, et al. Role of radiation therapy in node-negative esophageal cancer: A propensity-matched analysis. Thorac Cancer 2020;11:2820-9. [Crossref] [PubMed]
- Liu J, Ladbury C, Tam A, et al. Current landscape of radiation oncology in esophageal cancer: a narrative review. J Thorac Dis 2022;14:4494-505. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Gao LR, Wang X, Han W, et al. A multicenter prospective phase III clinical randomized study of simultaneous integrated boost intensity-modulated radiotherapy with or without concurrent chemotherapy in patients with esophageal cancer: 3JECROG P-02 study protocol. BMC Cancer 2020;20:901. [Crossref] [PubMed]
- Xiao B, Peng J, Wang Y, et al. Prognostic value of tumor infiltrating lymphocytes combined with PD-L1 expression for patients with solitary colorectal cancer liver metastasis. Ann Transl Med 2020;8:1221. [Crossref] [PubMed]
- Inada M, Nishimura Y, Ishikawa K, et al. Comparing the 7th and 8th editions of the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system for esophageal squamous cell carcinoma treated by definitive radiotherapy. Esophagus 2019;16:371-6.
- Cai P, Yang Y, Li DJ. Efficacy and Prognostic Analysis of 315 Stage I-IVa Esophageal Cancer Patients Treated with Simultaneous Integrated Boost-Intensity-Modulated Radiation Therapy. Cancer Manag Res 2021;13:6969-75. [Crossref] [PubMed]
- Lan W, Lihong L, Chun H, et al. Comparison of efficacy and safety between simultaneous integrated boost intensity-modulated radiotherapy and standard-dose intensity-modulated radiotherapy in locally advanced esophageal squamous cell carcinoma: a retrospective study. Strahlenther Onkol 2022;198:802-11. [Crossref] [PubMed]
- Rades D, Manig L, Janssen S, et al. Concurrent Chemotherapy Improves the Overall Survival of Patients Irradiated for Locally Recurrent Bladder Cancer. Anticancer Res 2017;37:1485-8. [Crossref] [PubMed]
- Ji Y, Du X, Zhu W, et al. Efficacy of Concurrent Chemoradiotherapy With S-1 vs Radiotherapy Alone for Older Patients With Esophageal Cancer: A Multicenter Randomized Phase 3 Clinical Trial. JAMA Oncol 2021;7:1459-66. [Crossref] [PubMed]
- Chen M, Shen M, Lin Y, et al. Adjuvant chemotherapy does not benefit patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Radiat Oncol 2018;13:150. [Crossref] [PubMed]
- Koh HK, Park Y, Koo T, et al. Adjuvant Chemotherapy and Dose Escalation in Definitive Concurrent Chemoradiotherapy for Esophageal Squamous Cell Carcinoma. Anticancer Res 2020;40:1771-8. [Crossref] [PubMed]
- Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Jeong H, Im HS, Bang Y, et al. Analysis of clinical outcomes and prognostic factors in patients treated with definitive chemoradiotherapy for oesophageal squamous cell carcinoma. Cancer Med 2021;10:1745-58. [Crossref] [PubMed]
- Xu Y, Dong B, Zhu W, et al. A Phase III Multicenter Randomized Clinical Trial of 60 Gy versus 50 Gy Radiation Dose in Concurrent Chemoradiotherapy for Inoperable Esophageal Squamous Cell Carcinoma. Clin Cancer Res 2022;28:1792-9. [Crossref] [PubMed]
- Chow R, Lock M, Lee SL, et al. Esophageal Cancer Radiotherapy Dose Escalation Meta Regression Commentary: "High vs. Low Radiation Dose of Concurrent Chemoradiotherapy for Esophageal Carcinoma With Modern Radiotherapy Techniques: A Meta-Analysis". Front Oncol 2021;11:700300. [Crossref] [PubMed]
- Chang CL, Tsai HC, Lin WC, et al. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother Oncol 2017;125:73-9. [Crossref] [PubMed]
- Chen CY, Li CC, Chien CR. Does higher radiation dose lead to better outcome for non-operated localized esophageal squamous cell carcinoma patients who received concurrent chemoradiotherapy? A population based propensity-score matched analysis. Radiother Oncol 2016;120:136-9. [Crossref] [PubMed]
- Rice TW, Chen LQ, Hofstetter WLWorldwide Esophageal Cancer Collaboration, et al. pathologic staging data. Dis Esophagus 2016;29:724-33. [Crossref] [PubMed]
- Zhou XL, Zhu WG, Zhu ZJ, et al. Lymphopenia in Esophageal Squamous Cell Carcinoma: Relationship to Malnutrition, Various Disease Parameters, and Response to Concurrent Chemoradiotherapy. Oncologist 2019;24:e677-86. [Crossref] [PubMed]
- Davuluri R, Jiang W, Fang P, et al. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2017;99:128-35. [Crossref] [PubMed]
- van Rossum PSN, Deng W, Routman DM, et al. Prediction of Severe Lymphopenia During Chemoradiation Therapy for Esophageal Cancer: Development and Validation of a Pretreatment Nomogram. Pract Radiat Oncol 2020;10:e16-26. [Crossref] [PubMed]
- Chen J, Lin Y, Cai W, et al. A new clinical staging system for esophageal cancer to predict survival after definitive chemoradiation or radiotherapy. Dis Esophagus 2018; [Crossref]
(English Language Editor: A. Kassem)


