
Primary duodenal embryonal rhabdomyosarcoma in adults: a case report
Introduction
Rhabdomyosarcoma (RMS) is a soft tissue sarcoma that histologically resembles embryonic skeletal muscle. It can occur anywhere in the body, including tissues devoid of skeletal muscles. The cell of origin of RMS remains unknown, however, recent evidence suggests that RMS can originate from aberrant development of non-myogenic cells (1).
Rhabdomyosarcoma is a common malignancy in children; indeed, it accounts for more than 50% of all soft tissue sarcomas in children (2). However, it is a rare malignancy in adults, where soft tissue sarcomas constitute less than 1% of all malignancies, and RMS accounts for only 3% of all soft tissue sarcomas (3). The most common locations in which RMS can be found are the head and the neck (35%), the genitourinary tract (22%), and other extremities (18%) (4). Most cases of gastrointestinal RMS represent a metastatic disease, with primary involvement being extremely rare. Primary RMS has primarily been reported from the oesophagus (5-8) and the stomach (9,10). Here, we report on a case of primary duodenal embryonal RMS in an adult.
Case presentation
A 79-year-old male with a known case of diabetes mellitus, hypertension, and ischemic heart disease from 5 years ago, presented to the emergency room complaining of epigastric pain, nausea, vomiting, and weight loss. The vomiting had become intractable in the week before his presentation. He denied dysphagia, hematemesis, melena, hematochezia, diarrhea, and constipation.
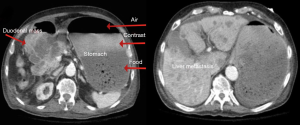
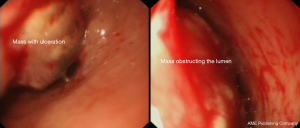
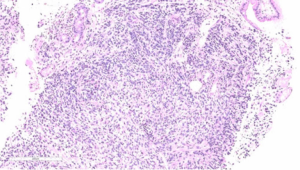
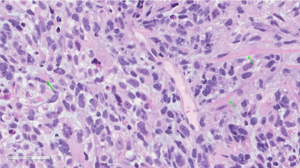
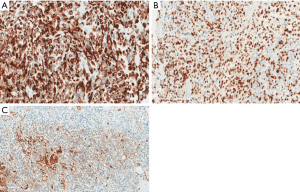
Upon examination, he appeared chronically ill; however, his vital signs were normal. An abdominal exam revealed a soft abdomen with mild fullness and tenderness in the epigastric area, but without palpable masses. Other systems exams were unremarkable. Blood tests revealed only iron deficiency anaemia (haemoglobin 10 g/dL). Tumour markers, including carcinoembryonic antigen (CEA), CA19-9 and alpha-fetoprotein (AFP), were within the normal limits. A computed tomography (CT) scan (Figure 1) revealed a 7 cm × 9 cm mass arising from the duodenal bulb and invading the gastric antrum and pancreatic head. The mass caused high-grade gastric outlet obstruction. Moreover, multiple low-density lymph nodes (LN) were observed at the porta hepatis, peripancreatic, and retroperitoneal regions. The largest LN measured 24 mm at the porta hepatis. The liver showed multiple bilobar variable-sized hypodense lesions, which is suggestive of metastasis. Also, there was moderate free fluid in the abdomen and possible peritoneal metastasis. An esophagogastroduodenoscopy (EGD) revealed a large irregular ulcerated mass originating from the duodenum and growing through the pylorus into the stomach, causing gastric outlet obstruction and preventing further examination of the duodenum (Figure 2). Multiple biopsies were taken for histopathology. A histologic examination revealed diffuse infiltration by a sheet of round, small blue cells (Figure 3). Moreover, areas with spindle-shaped and undifferentiated cells admixed with scattered round, strap, or tadpole-shaped eosinophilic rhabdomyoblasts were also present (Figure 4). Cytoplasmic cross-striation was identified as well. An immunohistochemistry and a molecular study revealed strongly and diffusely positive Desmin (Figure 5A) and Myogenin (Figure 5B). Moreover, smooth muscle actin (SMA) was focally positive (Figure 5C). A molecular study for FOXO1 (13q14) was conducted by fluorescence in situ hybridization (FISH), but no rearrangement of the FOXO1 gene region was identified, precluding the possibility of alveolar-type RMS. Therefore, the final diagnosis was advanced duodenal embryonal RMS. The patient experienced poor functional status and planned for supportive care only. He passed away two weeks after admission, likely due to a massive pulmonary embolism.
Discussion
Primary malignant tumours of the duodenum represent 0.3% of all gastro-intestinal tract tumours, but can represent up to 50% of small bowel malignancies. The most frequent primary malignant tumour of the duodenum is adenocarcinoma (11). Other primary tumours include lymphomas, leiomyosarcomas, carcinoid tumours, gastrinomas, and stromal tumours. These tumours usually present with bleeding or gastric outlet obstruction.
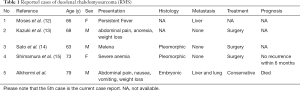
Duodenal RMS is an extremely rare malignancy. Through an extensive search of the relevant literature, we found only four case reports written in English that describe primary duodenal RMS in adults (Table 1). Rhabdomyosarcoma has been classified by the WHO into four histologic subtypes: embryonal, alveolar, pleomorphic, and spindle cell/sclerosing RMS (16). In adults, the most common histologic subtype of RMS is the pleomorphic subtype (17). Our case is the first reported case of embryonal subtype duodenal RMS in an adult.
Full table
Although RMS is considered a single disease, there are important differences in how these tumours behave depending on tumour location, histologic subtype, tumour bulk, patient’s age at the time of diagnosis, and the presence or absence of distant metastasis. Because there are so few reported cases of duodenal RMS, it is not possible to speculate on its behaviour, response to treatment, or prognosis. However, RMS outcomes are considerably less favourable for adults than for children (18). It seems that the pleomorphic subtype carries the worst prognosis (17). Distant metastasis at the time of diagnosis is quite common and the lung is the most common site for metastasis (18). Invasion of the pancreatic head and metastasis to the liver are probably common in RMS arising from the duodenum.
The optimal management of RMS in adults is unknown due to its rarity and the absence of any standard treatment protocol or guidelines. The current treatment standards for adult RMS follow the treatment standards created for children, which were proposed by the Intergroup Rhabdomyosarcoma Studies (IRS) (19). These treatment standards include multimodal therapy (MMT: resection, chemotherapy, and radiation). Surgery is the mainstay of treatment for adult RMS, as it has been correlated with an improved survival rate (20). According to the IRS protocols, all RMS patients should undergo radiotherapy to achieve long-term local control of the tumour (21). The IRS protocols also recommend that all RMS patients receive combination chemotherapy as soon as a diagnosis is made, or after resection, because this significantly improves survival rates. The recommended regimen is a combination chemotherapy approach consisting of vincristine, actinomycin, etoposide or ifosfamide, and cyclophosphamide. The three-year, failure-free survival rate ranges from 78–92% with these regimens (22). The treatment of duodenal RMS should follow the treatment standards of RMS elsewhere in the body until more cases have been evaluated to uncover further clinical characteristics, and thus determine the optimal management for this tumour.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Drummond CJ, Hanna JA, Garcia MR, et al. Hedgehog Pathway Drives Fusion-Negative Rhabdomyosarcoma Initiated From Non-myogenic Endothelial Progenitors. Cancer Cell 2018;33:108-24.e5. [Crossref] [PubMed]
- Pastore G, Peris-Bonet R, Carli M, et al. Childhood soft tissue sarcomas incidence and survival in European children (1978–1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:2136-49. [Crossref] [PubMed]
- Weiss SW, Goldblum J, Weiss SW, et al., editors. Enzinger and Weiss’s Soft Tissue Tumors. 4th ed., St. Louis: CV Mosby; 2001. p. 785-835.
- Meyer WH, Spunt SL. Soft tissue sarcomas of childhood. Cancer Treat Rev 2004;30:269-80. [Crossref] [PubMed]
- Wobbes T, Rinsma SG, Holla AT, et al. Rhabdomyosarcoma of the esophagus. Arch Chir Neerl 1975;27:69-75. [PubMed]
- Batoroev YK, Nguyen GK. Esophageal rhabdomyosarcoma: report of a case diagnosed by imprint cytology. Acta Cytol 2006;50:213-6. [Crossref] [PubMed]
- Chetty R, Learmonth GM, Price SK, et al. Primary oesophageal rhabdomyosarcoma. Cytopathology 1991;2:103-8. [Crossref] [PubMed]
- Gandhi JS, Pasricha S, Gupta G, et al. Synchronous Embryonal Rhabdomyosarcoma (NOS) of the Mid-oesophagus and Stomach. J Gastrointest Cancer 2012;43 Suppl 1:S217-20. [Crossref] [PubMed]
- Fox KR, Moussa SM, Mitre RJ, et al. Clinical and pathologic features of primary gastric rhabdomyosarcoma. Cancer 1990;66:772-8. [Crossref] [PubMed]
- Palermo M, Mastronardi LM, García RH, et al. Primary gastric rhabdomyosarcoma. Case report. Acta Gastroenterol Latinoam 2012;42:131-4. [PubMed]
- Wang Z, Ding Z, Huang S, et al. Experience in clinical diagnosis and treatment of duodenal tumors. Mol Clin Oncol 2016;5:731-9. [Crossref] [PubMed]
- Moses I, Coodley EL. Rhabdomyosarcoma of duodenum. Am J Gastroenterol 1969;51:48-54. [PubMed]
- Yamada K, Douglass HO, Holyoke D. Rhabdomyosarcoma of the duodenum with sinus tract formation into the gastric wall visualized by gastroduodenoscopy. Am J Dig Dis 1975;20:871-5. [Crossref] [PubMed]
- Sato A, Hashimoto M, Moriyama J, et al. Rhabdomyosarcoma of the duodenum: report of a case. Surg Today 2014;44:378-82. [Crossref] [PubMed]
- Shimamura Y, Omata F, Nakamura K, et al. Adult Duodenal Pleomorphic Rhabdomyosarcoma. ACG Case Rep J 2014;2:11-2. [Crossref] [PubMed]
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. World Health Organization Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC Press; 2013.
- Furlong MA, Mentzel T, Fanburg-Smith JC. Pleomorphic rhabdomyosarcoma in adults: A clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol 2001;14:595-603. [Crossref] [PubMed]
- Khosla D, Sapkota S, Kapoor R, et al. Adult rhabdomyosarcoma: Clinical presentation, treatment, and outcome. J Cancer Res Ther 2015;11:830-34. [Crossref] [PubMed]
- Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with non-metastatic disease. J Clin Oncol 2001;19:3091-102. [Crossref] [PubMed]
- Ferrari A, Dileo P, Casanova M, et al. Rhabdomyosarcoma in adults. A retrospective analysis of 171 patients treated at a single institution. Cancer 2003;98:571-80. [Crossref] [PubMed]
- Crist W, Gehan EA, Ragab AH, et al. The third intergroup Rhabdomyosarcoma study. J Clin Oncol 13:610-30. [Crossref] [PubMed]
- Breneman JC, Lyden E, Pappo AS, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma-A report from the intergroup Rhabdomyosarcoma Study IV. J Clin Oncol 2003;21:78-84. [Crossref] [PubMed]
Cite this article as: Alkhormi AM, Alqifari A, Aljarbou OZ, Alqarni M. Primary duodenal embryonal rhabdomyosarcoma in adults: a case report. AME Case Rep 2019;3:29.


