Lung ischemia reperfusion injury: the therapeutic role of dipeptidyl peptidase 4 inhibition
Introduction
Dipeptidyl peptidase 4 (DPP4, CD26) is a cell surface protease with a wide range of biological functions. As a serine-type protease, DPP4 preferentially cleaves off substrates with proline and alanine at the penultimate position. Expression of DPP4 is widespread throughout the body. In the lung, the capillaries may be the main source of DPP4 activity, while submucosal serous glands and alveolar cells also express DPP4 (1).
Several substrates have been identified for DPP4 amongst which the truncation of glucagon-like peptide 1 (GLP-1) is certainly the best illustrated. GLP-1 acts as an incretin by amplifying the meal-induced insulin release and synthesis in a glucose-dependent way. GLP-1 suppresses the secretion of glucagon, is important for basal β cell function and is involved on many levels in the control of glucose in the intestinal tract and peripheral tissues (2). Because of the GLP-1 enhancing actions, DPP4 inhibitors are used in the treatment of diabetes mellitus type 2 (3). DPP4 functions beyond glycemic control include potential roles in cancer, HIV, autoimmunity and immunology (4,5). Increasing evidence suggests a role of DPP4 in ischemia-reperfusion injury (IRI) (6). Especially the role of DPP4 in cardiac IRI has been investigated. The use of DPP4 inhibitors resulted in a decrease of infarct size, improvement of cardiac function and a promotion of myocardial regeneration (7).
Lung ischemia-reperfusion injury (LIRI) gives rise to acute lung injury (ALI), a disorder that is clinically manifested through non-cardiogenic pulmonary edema, respiratory distress and hypoxemia (8). LIRI occurs after lung transplantation, cardiopulmonary bypass cardiac surgery, trauma, pulmonary embolism, and resuscitation after hemorrhagic shock (9). The mechanisms of LIRI are complex as described by den Hengst et al. (10). In summary the pathophysiology is as follows: due to organ ischemia macrophages, endothelial cells, and other immune cells generate reactive oxygen species (ROS), cause uncoupling of calcium/calmodulin-dependent nitric oxide synthases (NOS), activate nuclear factor-κB (NF-κB), activate nicotinamide adenine dinucleotide phosphate oxidase (NOX) and generate pro-inflammatory cytokines. These actions lead to several changes in the microvasculature resulting in an increased pulmonary vascular resistance (PVR), increased microvascular permeability (Pµvasc), and pulmonary edema. After reperfusion, PVR is further increased mainly due to vasoconstriction causing further pulmonary edema formation also during reperfusion. Pµvasc increase during reperfusion is bimodal. A first step depends on factors that are derived from activated pulmonary macrophages, such as IL-8, IL-12, IL-18, TNF-α and platelet-activating factor (PAF). In a later phase Pµvasc is dependent on secretion products from activated neutrophils, such as ROS, IL-8, PAF and TNF-α (10).
This short review will focus the reported roles of DPP4 in LIRI. We will discuss the possible beneficial role of dipeptidyl peptidase 4 inhibition (DPP4i) and describe the potential targets of DPP4i in the mechanism of LIRI.
DPP4and lung ischemia reperfusion injury
Several studies have shown the attenuating effects of DPP4i on LIRI. Zhai et al. (11) used an irreversible DDP4 inhibitor, AB192, in an ex-vivo perfusion model of lung transplantation in rats. In this study they demonstrated an improved oxygenation and reduced pulmonary airway pressure (PAWP). A follow-up study resulted in better lung function, lower PAWP, lower wet-dry ratio (an indication of pulmonary edema), lower myeloperoxidase (MPO) activity (reduced neutrophil activity), less histologic edema and congestion and a significantly vasoactive intestinal peptide (VIP) immunoreactivity in macrophages found in DPP4 inhibited grafts (12). Jungraithmayr et al. (13) used a similar experimental protocol with AB192 in mice. DPP4i resulted in a significant improvement of lung function after 18 hours cold ischemic storage followed by 2 hours reperfusion, as compared to control animals. Normal functional histology was better preserved, a few macrophages were non-activated, and a significant reduction of neutrophil infiltration was seen after treatment. This study also demonstrated a significantly higher VIP-positivity in macrophages after DPP4i. In a similar study with rats undergoing lung transplantation these researchers showed comparable results in lung function, histology, and VIP-positivity in macrophages. They also found that the rejection reaction was less severe after DPP4i (14). VIP diminishes IRI by inhibition of NF-κB, by performing anti-apoptotic actions (via inhibition of caspase activity and upregulation of the anti-apoptotic protein bcl-2), and by preventing excitotoxic glutamate toxicity. Moreover VIP also serves as a free-radical scavenger, plays a protective role against xanthine oxidase, inhibits production of pro-inflammatory cytokines TNF-α and IL-6, and inhibits NO production (15) VIP is also a strong vascular and bronchial dilator (16).
Stromal cell-derived factor-1α (SDF-1α) is a very important protein for the recruitment and homing of bone marrow-derived regenerative stem cells, and a known target of DPP4 cleavage. Through its receptor CXCR4 it improves stem-cell engraftment and enhances the chemoattractive response in response to injury and/or hypoxia. An IRI-lung transplant model in mice showed an upregulation of CXCR4, stabilization of SDF-1α, increased homing of regenerative progenitor cells to the transplant, and better recovery from LIRI (17).
Other possible roles of DPP4i in the lung
Oxidative stress
ROS mediate inflammatory reactions by activating alveolar macrophages, with stimulation of the release pro-inflammatory cytokines. A multitude of potential ROS generators should be taken into account, such as mitochondria, xanthine oxidase, NOX, NOS uncoupling and neutrophils (Figure 1) (10).
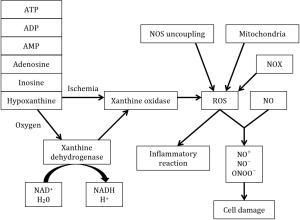
When the lung is deprived of oxygen, anaerobic glycolysis takes over the adenosine triphosphate (ATP) production from the normal oxidative phosphorylation. Because anaerobic glycolysis is unable to replenish the pool of ATP, a deficit of this necessary energy-storing molecule occurs. As a consequence the active transport of ions across the membrane is decreased resulting in an accumulation of sodium followed by cellular edema. Also degradation of ATP to adenosine diphosphate (ADP), adenosine monophosphate (AMP), adenosine, inosine and hypoxanthine occurs during hypoxia (10,18)
Reperfusion is directly related to the formation of ROS by providing oxygen to xanthine dehydrogenase and xanthine oxidase to turn hypoxanthine into xanthine, H2O2 and ROS. NADPH is responsible for converting xanthine dehydrogenase irreversibly into xanthine oxidase. In the lung, ROS have an effect on the activation of inflammatory processes through transcription factors such as NF-κB, and on tissue damage by producing reactive nitrogen species (10,18).
The endothelial form of nitric oxide synthase (eNOS) is present in endothelial cells and can result in generation of superoxide if there is uncoupling of the reduction of molecular oxygen to L-arginine oxidation (19). Under normal conditions, NOS produces nitric oxide (NO) which plays an important role in vascular homeostasis (18) and has important anti-inflammatory functions (10). Inducible nitric oxide synthase (iNOS) may generate high levels of NO in ischemia-reperfusion. When NO reacts with ROS, it forms secondary reactive nitrogen species, such as nitrosonium cation (NO+), nitroxil anion (NO−) and peroxynitrite (ONOO−) (19,20). By reaction of NO with ROS, the physiological and protective action of NO will be lost, resulting in endothelial dysfunction (18).
GLP-1 affects the production of free radicals in several ways and may therefore attenuate LIRI. Firstly, GLP-1 inhibits the production of free radicals by influencing NADPH oxidase through the cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) pathway (21,22). Inhibition of DPP4, significantly reduces vascular oxidative stress via multiple mechanisms. Attenuation of xanthine oxidase activity, expression of eNOS, and expression of NOX1, and NOX2 isoforms of NADPH oxidase was achieved through DPP4i in a lipopolysaccharide (LPS)-septic model. In the same model, DPP4i also preserved vascular function (23). DPP4i was reported to inhibit iNOS activity and iNOS-dependent NO production in a GLP-1 receptor dependent way in an LPS-septic model. The same study showed an inhibition of the NOX2-isoform of NAPDH oxidase, with attenuation of ROS generation (24). DPP4i proved to reduce the inflammatory reaction by attenuation of the NF-κB p65 nuclear translocation and reduction of the release of inflammatory cytokines. DD4i further protects against oxygen radicals by increasing the expression of free radical scavengers, such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and thioredoxin (Trx) (25).
Signal transduction pathways
The phosphatidylinositol 3'-kinase (PI3K)/Akt pathway, Janus kinase 2/signal transducer and activator of transcription 3 (JAK/STAT3) pathway, and p38 mitogen-activated protein kinase (MAPK) pathway play pivotal roles in cell signal transduction, inflammation, stress, apoptosis, cell cycle, and cell growth (26). Akt is a serine/threonine kinase that acts downstream of PI3K. Akt has a variety of functions in apoptosis, proliferation, and differentiation (26). Akt and MAPK activate redox-sensitive transcription factor NF-E2-related factor 2 (NrF2), which is essential for protection against oxidative stress in different forms of ALI. NrF2 binds with the antioxidant responsive element (ARE) to promote gene expression of several antioxidant enzymes (27-29). The latter might play an important role in protection against ROS in LIRI. However to our knowledge no LIRI studies were performed to characterize the role of NrF2 in this disorder. In ventilator-induced lung injury (VILI) silencing of PI3K/Akt turned out to attenuate injury and to increase glutathione concentrations (8).
MAPK activation can promote production of inflammatory cytokines (e.g., TNF-α, IL-8) in mononuclear macrophages, and mediate the activation of neutrophils (26). TNF-α regulates several important pro-inflammatory, damage-inducing actions. Intercellular adhesion molecule-1 (ICAM-1) on endothelial cells will be upregulated, which will facilitate leukocyte extravasation into the tissue. It will further upregulate the expression of P-selectin on endothelial cells and CD-18 on neutrophils. TNF-α will cause degranulation of adherent neutrophils and an increase of ROS (30). TNF-α activation of ICAM-1 is regulated through Akt/MAPK/c-Jun N-terminal kinase (JNK) phosphorylation and NF-κB (31) Hwang et al. (32) showed that DPP4i prevents injury by attenuating NF-κB via an Akt dependent pathway. Also thrombin-induced activation of NF-κB and subsequent expression of IL-8 was reported to be regulated through the PI3K/Akt pathway in the lungs (33).
Non-cardiac edema is a clinical outcome of ALI. Fluid volume in the lungs is determined by alveolar fluid clearance (AFC), which is related to the balance of trans-epithelial Na+ transport. AFC is impaired in ALI. The epithelial sodium channel (ENaC) is the primary determinant of AFC. ENaC can be activated by insulin through the PI3K/Akt pathway. Insulin further reduced ALI by attenuating the increase of TNF-α, IL-6, MPO activity and reducing the number of neutrophils (34).
DD4i may influence the previously mentioned signal transduction pathways by several mechanisms. The first mechanism might be through stabilization of GLP-1. GLP-1 has been shown to activate the cAMP-PKA pathway which in turn activate PI3K/Akt and MAPK. These actions, combined with reduction of oxidative stress and attenuation of neutrophil activation, was shown to lead to reduction of infarct size in certain cardiac ischemia-reperfusion models (35). Secondly, SDF-1α may play an important role in these pathways. The actions of SDF-1α through CXCR4 receptors were linked to MAPK and PI3K/Akt (36-38). In ischemic myocytes, SDF-1α/CXCR4/MAPK and SDF-1α/CXCR4/Akt interactions were important for protection against IRI (37). The same results were seen in cardiomyocytes where DPP4i reduces the concentrations of pro-inflammatory cytokines and restored SDF-1α to normal levels (39). These mechanisms might also play a role in the ischemic lung. Upregulation of SDF-1α and its receptor CXCR4 is seen after DPP4i in lung transplants (17). Thirdly, insulin is increased by DPP4i through inhibition of DPP4 cleavage of GLP-1, pituitary adenylate cyclase-activating peptide, VIP, glucose-dependent insulinotropic peptide, and neuropeptide Y (1). Therefore increased insulin levels might improve clearance of lung edema through ENaC and alleviate the symptoms of ALI.
Discussion
The serine-type protease, DPP4 has potential roles in cancer, HIV, autoimmunity and immunology (4,5) but is also involved in the inflammatory reaction caused by ischemia and subsequent reperfusion (6). As shown in Figure 2, LIRI is a complex process in with several possible target sites for DPP4i. Macrophages and neutrophils are determining for the development of LIRI (10). ROS play a central role in the development of LIRI. During ischemia the degradation of ATP to hypoxanthine will cause production of ROS through xanthine oxidase. During reperfusion hypoxanthine will be converted in the presence of oxygen, by xanthine dehydrogenase and ultimately will cause a spike of ROS (10). DPP4i has proven to normalize the activity of xanthine oxidase in an LPS-sepsis model (23) but this has not been validated yet in a LIRI-model. VIP has an important protective role against xanthine oxidase(15) and might be responsible for the reduction of xanthine oxidase levels in LIRI. Several LIRI studies indeed showed higher VIP-activity (12-14), which gives an incentive to further investigate the potential of VIP-induced xanthine oxidase inhibition during ischemia-reperfusion in the lung.
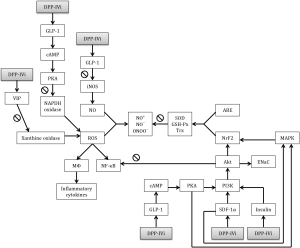
DPP4i reduces ROS production by NADPH oxidase production through the GLP-1/cAMP/PKA pathway in LPS-models (21,23,24). As NAPDPH oxidase plays only a small role in the pathophysiology of LIRI (10), the attenuating potential role of DPP4i on ROS production might be limited with respect to this possible cause of ALI.
ROS produce secondary reactive products such as NO+, NO−, and ONOO− through a reaction with NO (19). In lungs, NO is physiologically produced by NOS but is generated in high levels during ischemia-reperfusion by iNOS. DPP4i proved to inhibit iNOS activity and NO production in a GLP-1 dependent manner in an LPS-model (24). Due to the importance of iNOS for LIRI, this mechanism could be an important target for further investigation in LIRI models.
Free radical scavengers such as SOD, GSH-Px, and Trx are natural protectors against excessive ROS production. DPP4i has been shown to increase the production of SOD, GSH-Px, and Trx in an atherosclerosis model (25). The possible mechanisms of this upregulation are higher levels of GLP-1 and/or via SDF-1α stimulation of the PI3K/Akt/NrF2 and MAPK/NrF2 pathways (27,28). After VILI however, PI3K/Akt inhibition caused attenuation of injury and increased GSH activity (8). Jungraithmayr et al. (17) showed an upregulation of the CXCR4 receptor for SDF-1α and stabilization of SDF-1α itself in a lung transplant model giving rise to speculations about the role of these pathways in LIRI. The role of NrF2 activation in LIRI remains uncertain and needs further investigation.
Conclusions
DPP4i might have an important therapeutic potential for lung ischemia reperfusion injury. We showed an incentive for the protective role of the DPP4 substrates VIP, GLP-1 and SDF-1α in lung ischemia reperfusion injury. The protection could be attributed to the anti-inflammatory actions of VIP, glucagon-like peptide-1, and SDF-1α such as attenuation of oxidative stress, neutrophil activation, NF-κB activation, cytokine release, and anti-apoptotic and regenerative functions.
Acknowledgements
Funding: This work was supported by a GOA BOF 2015 grant (No. 30729) of the University of Antwerp.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lambeir AM, Durinx C, Scharpé S, et al. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 2003;40:209-94. [Crossref] [PubMed]
- Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology 2002;122:531-44. [Crossref] [PubMed]
- Kim SH, Yoo JH, Lee WJ, et al. Gemigliptin: An Update of Its Clinical Use in the Management of Type 2 Diabetes Mellitus. Diabetes Metab J 2016;40:339-53. [Crossref] [PubMed]
- Thompson MA, Ohnuma K, Abe M, et al. CD26/dipeptidyl peptidase IV as a novel therapeutic target for cancer and immune disorders. Mini Rev Med Chem 2007;7:253-73. [Crossref] [PubMed]
- Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013;495:251-4. [Crossref] [PubMed]
- Matheeussen V, Jungraithmayr W, De Meester I. Dipeptidyl peptidase 4 as a therapeutic target in ischemia/reperfusion injury. Pharmacol Ther 2012;136:267-82. [Crossref] [PubMed]
- Wang XM, Yang YJ, Wu YJ. The emerging role of dipeptidyl peptidase-4 inhibitors in cardiovascular protection: current position and perspectives. Cardiovasc Drugs Ther 2013;27:297-307. [Crossref] [PubMed]
- Li LF, Liu YY, Yang CT, et al. Improvement of ventilator-induced lung injury by IPS cell-derived conditioned medium via inhibition of PI3K/Akt pathway and IP-10-dependent paracrine regulation. Biomaterials 2013;34:78-91. [Crossref] [PubMed]
- Zhang W, Zhang JQ, Meng FM, et al. Dexmedetomidine protects against lung ischemia-reperfusion injury by the PI3K/Akt/HIF-1α signaling pathway. J Anesth 2016;30:826-33. [Crossref] [PubMed]
- den Hengst WA, Gielis JF, Lin JY, et al. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol 2010;299:H1283-99. [Crossref] [PubMed]
- Zhai W, Cardell M, De Meester I, et al. Ischemia/reperfusion injury: The role of CD26/dipeptidyl-peptidase-IV-inhibition in lung transplantation. Transplant Proc 2006;38:3369-71. [Crossref] [PubMed]
- Zhai W, Jungraithmayr W, De Meester I, et al. Primary graft dysfunction in lung transplantation: the role of CD26/dipeptidylpeptidase IV and vasoactive intestinal peptide. Transplantation 2009;87:1140-6. [Crossref] [PubMed]
- Jungraithmayr W, De Meester I, Matheeussen V, et al. Inhibition of CD26/DPP IV attenuates ischemia/reperfusion injury in orthotopic mouse lung transplants: the pivotal role of vasoactive intestinal peptide. Peptides 2010;31:585-91. [Crossref] [PubMed]
- Jungraithmayr W, Oberreiter B, De Meester I, et al. The effect of organ-specific CD26/DPP IV enzymatic activity inhibitor-preconditioning on acute pulmonary allograft rejection. Transplantation 2009;88:478-85. [Crossref] [PubMed]
- Said SI, Dickman KG. Pathways of inflammation and cell death in the lung: modulation by vasoactive intestinal peptide. Regul Pept 2000;93:21-9. [Crossref] [PubMed]
- Groneberg DA, Springer J, Fischer A. Vasoactive intestinal polypeptide as mediator of asthma. Pulm Pharmacol Ther 2001;14:391-401. [Crossref] [PubMed]
- Jungraithmayr W, De Meester I, Matheeussen V, et al. CD26/DPP-4 inhibition recruits regenerative stem cells via stromal cell-derived factor-1 and beneficially influences ischaemia-reperfusion injury in mouse lung transplantation. Eur J Cardiothorac Surg 2012;41:1166-73. [Crossref] [PubMed]
- Ferrari RS, Andrade CF. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid Med Cell Longev 2015;2015:590987.
- Ovechkin AV, Lominadze D, Sedoris KC, et al. Lung ischemia-reperfusion injury: implications of oxidative stress and platelet-arteriolar wall interactions. Arch Physiol Biochem 2007;113:1-12. [Crossref] [PubMed]
- Gielis JF, Lin JY, Wingler K, et al. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Radic Biol Med 2011;50:765-76. [Crossref] [PubMed]
- Hendarto H, Inoguchi T, Maeda Y, et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012;61:1422-34. [Crossref] [PubMed]
- Hirano T, Yamashita S, Takahashi M, et al. Anagliptin, a dipeptidyl peptidase-4 inhibitor, decreases macrophage infiltration and suppresses atherosclerosis in aortic and coronary arteries in cholesterol-fed rabbits. Metabolism 2016;65:893-903. [Crossref] [PubMed]
- Kröller-Schön S, Knorr M, Hausding M, et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res 2012;96:140-9. [Crossref] [PubMed]
- Steven S, Jurk K, Kopp M, et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br J Pharmacol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Matsubara J, Sugiyama S, Sugamura K, et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol 2012;59:265-76. [Crossref] [PubMed]
- Zhao YR, Wang D, Liu Y, et al. The PI3K/Akt, p38MAPK, and JAK2/STAT3 signaling pathways mediate the protection of SO2 against acute lung injury induced by limb ischemia/reperfusion in rats. J Physiol Sci 2016;66:229-39. [Crossref] [PubMed]
- Deng X, Rui W, Zhang F, Ding W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol Toxicol 2013;29:143-57. [Crossref] [PubMed]
- Zhang R, Chae S, Lee JH, et al. The cytoprotective effect of butin against oxidative stress is mediated by the up-regulation of manganese superoxide dismutase expression through a PI3K/Akt/Nrf2-dependent pathway. J Cell Biochem 2012;113:1987-97. [Crossref] [PubMed]
- Kweon MH. The novel antioxidant 3-O-caffeoyl-1-methylquinic acid induces Nrf2-dependent phase II detoxifying genes and alters intracellular glutathione redox. Free Radic Biol Med 2006;40:1349-61. [Crossref] [PubMed]
- Chiang CH. Effects of anti-tumor necrosis factor-alpha and anti-intercellular adhesion molecule-1 antibodies on ischemia/reperfusion lung injury. Chin J Physiol 2006;49:266-74. [PubMed]
- Sung HC, Liang CJ, Lee CW, et al. The protective effect of eupafolin against TNF-α-induced lung inflammation via the reduction of intercellular cell adhesion molecule-1 expression. J Ethnopharmacol 2015;170:136-47. [Crossref] [PubMed]
- Hwang HJ, Chung HS, Jung TW, et al. The dipeptidyl peptidase-IV inhibitor inhibits the expression of vascular adhesion molecules and inflammatory cytokines in HUVECs via Akt- and AMPK-dependent mechanisms. Mol Cell Endocrinol 2015;405:25-34. [Crossref] [PubMed]
- Lin CH, Cheng HW, Ma HP, et al. Thrombin induces NF-kappaB activation and IL-8/CXCL8 expression in lung epithelial cells by a Rac1-dependent PI3K/Akt pathway. J Biol Chem 2011;286:10483-94. [Crossref] [PubMed]
- Deng W, Li CY, Tong J, et al. Regulation of ENaC-mediated alveolar fluid clearance by insulin via PI3K/Akt pathway in LPS-induced acute lung injury. Respir Res 2012;13:29. [Crossref] [PubMed]
- Chinda K, Chattipakorn S, Chattipakorn N. Cardioprotective effects of incretin during ischaemia-reperfusion. Diab Vasc Dis Res 2012;9:256-69. [Crossref] [PubMed]
- Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells 2005;23:879-94. [Crossref] [PubMed]
- Hu X, Dai S, Wu WJ, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation 2007;116:654-63. [Crossref] [PubMed]
- Delgado-Martín C, Escribano C, Pablos JL, et al. Chemokine CXCL12 uses CXCR4 and a signaling core formed by bifunctional Akt, extracellular signal-regulated kinase (ERK)1/2, and mammalian target of rapamycin complex 1 (mTORC1) proteins to control chemotaxis and survival simultaneously in mature dendritic cells. J Biol Chem 2011;286:37222-36. [Crossref] [PubMed]
- Hwang HJ, Jung TW, Ryu JY, et al. Dipeptidyl petidase-IV inhibitor (gemigliptin) inhibits tunicamycin-induced endoplasmic reticulum stress, apoptosis and inflammation in H9c2 cardiomyocytes. Mol Cell Endocrinol 2014;392:1-7. [Crossref] [PubMed]


