Prognostic value of the expression of estrogen receptor β in patients with non-small cell lung cancer: a meta-analysis
Introduction
Lung cancer is the leading cause of death in both men and women throughout the world (1). The cause of high mortality of lung cancer is mainly caused by the diagnosis of patients with advanced stage of cancer, at this time the tumor cells have already occurred in the local or distant invasion and metastasis, and another important aspect is the lack of effective treatment. In addition to the traditional tumor radiotherapy, chemotherapy, gene mutation detection technology has become another popular therapeutic tool. For the majority advanced non-small cell lung cancer (NSCLC) patients, especially who with adenocarcinoma, targeted therapy became increasingly important in the treatment (2). Through a large number of data contrast tests (3-6), it can be seen that the treatment of molecular mutation has got a certain effect (2). In addition to EGFR, ALK etc. the estrogen receptor is another promising targeting gene (7).
Recently, it has been suggested that estrogen plays an important role in the occurrence, development, and metastasis of lung cancer. In lung cancer, similar to breast cancer, aromatase is a crucial enzyme in estrogen synthesis (7), and enzymatic activity in lung cancer cell lines and normal lung tissue was exhibited (8). Although tobacco smoking remains a significant risk factor for adenocarcinoma, approximately 20% of women with lung cancer are never smokers (9). This phenomenon maybe suggests that estrogen is associated with lung cancer in the other hand.
In view of the fact that there is an association between ER overexpression and human lung cancer, most studies reported so far are limited in their sample size and discrete outcomes. We therefore carried out a meta-analysis of data from published studies to quantitatively review the effect of ER overexpression in tumor tissue on survival in patients with NSCLC.
Methods
Search strategy and study selection
The selected publications were identified by using up-to-date electronic databases, including PubMed, Web of Science, China National Knowledge Infrastructure (CNKI). An upper date limit of August 31, 2015 was applied; we used no lower date limit. Searches included the terms “estrogen receptor”, “ER”, “ERβ” and “prognosis”. We also reviewed the Cochrane Library for relevant articles. The references reported in the identified studies were also used to complete the search.
Studies eligible for inclusion in this meta-analysis met the following criteria: (I) measure estrogen receptor β (ERβ) expression in the primary lung cancer tissue with immunohistochemistry (IHC) or enzyme linked immunosorbent assay (ELISA)/reverse transcription-polymerase chain reaction (RT-PCR); (II) provide information on survival (studies investigating response rates only were excluded); (III) studies that reported a hazard ratio (HR) and confidence interval (CI) or could be calculated from the sufficient data; and (IV) when the same author reported results obtained from the same patient population in more than one publication, only the most recent report, or the most complete one, was included in the analysis. Two reviewers (P Zhan and L Ma) independently determined study eligibility. Disagreements were resolved by consensus.
Data extraction and quality assessment
The final articles included were assessed independently by two reviewers (P Zhan and L Ma). Data retrieved from the reports included first author, publication year, patient source, histology, disease stage, test method, cut-off value, and HR with 95% CI (Table 1).

Full table
If data from any of the above categories were not reported in the primary study, items were treated as “not applicable”. We did not contact the author of the primary study to request the information.
Statistical methods
For the quantitative aggregation of the survival results, hazard ratios (HR) and their 95% CIs were combined to give the effective value. When these statistical variables were not given explicitly in an article, they were calculated from available numerical data using methods reported by Parmar et al. (10).
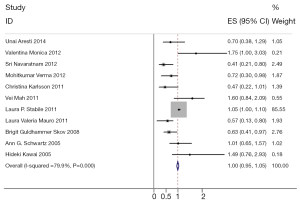
Heterogeneity of the individual HRs was calculated with Chi-squared tests according to Peto’s method (11). Meanwhile, heterogeneity test with I2 statistic and Q statistic was performed. All the studies included were categorized by patient race, histology, disease stage. Individual meta-analysis was conducted in each subgroup. If HRs were found to have fine homogeneity, a fixed effect model was used for secondary analysis; if not, a random effect model was used. In this meta-analysis, DerSimonian-Laird random effects analysis (12) was used to estimate the effect of estrogen receptor overexpression on survival. By convention, an observed HR >1 implies worse survival for the group with estrogen receptor overexpression. The impact of estrogen receptor on survival was considered to be statistically significant if the 95% CI did not overlap with 1. Horizontal lines represent 95% CIs. Each box represents the HR point estimate, and its area is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with CI represented by its width. The unbroken vertical line is set at the null value (HR =1.0).
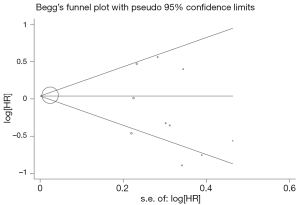
Evidence of publication bias was sought using the methods of Egger et al. (13) and of Begg et al. (14). Moreover, contour-enhanced funnel plot (15) was performed to aid in interpreting the funnel plot. If studies appear to be missing in areas of low statistical significance, then it is possible that the asymmetry is due to publication bias. If studies appear to be missing in areas of high statistical significance, then publication bias is a less likely cause of the funnel asymmetry. Intercept significance was determined by the t-test suggested by Egger (P<0.05 was considered representative of statistically significant publication bias). All calculations were performed using STATA version 11.0 (Stata Corporation, College Station, TX, USA).
Results
Study selection and characteristics
Eleven studies (16-26) published between 2005 and 2014 were eligible for this systematic review with meta-analysis. All reported the prognostic value of ERβ status for survival in NSCLC patients. The total number of patients included was 1,650 ranging from 58 to 377 patients per study (median 218). The major characteristics of the eleven eligible publications are reported in Table 1.
These publications followed several different patient cohorts. The NSCLC studies considered either all lung cancer subtypes (n=4) and adenocarcinomas (n=1). All eleven studies used IHC or RT-PCR/ELISA to evaluate ERβ expression in NSCLC.
Meta-analysis
The results of the meta-analysis are reported in Figure 1. Overall, the combined HR for all eligible studies evaluated ERβ expression in NSCLC was 1.000 (95% CI: 0.954–1.047), indicating that ERβ overexpression was an indicator of negative results for NSCLC patients. Meanwhile, no significant heterogeneity was detected among these studies (I2=79.9%, P=0.000) (Figure 1).
Publication bias
Begg’s funnel plot and Egger’s test were performed to assess the publication bias in the literature. All five eligible studies investigating NSCLC patients yielded a Begg’s test score of P=0.274 and an Egger’s test score of P=0.183, meanwhile according to the contour-enhanced funnel plot (Figure 2), the absence of publication bias was found in all eleven studies. These results suggest that there is no publication bias.
Discussion
The development of lung cancer is a complex process, which is regulated by many kinds of cytokines, chemokines, tumor inflammatory microenvironment, vascular normalization, gene pathway and so on. Some studies indicate that ER is playing an important role in this process. In the recent years, with the continuous development of estrogen receptor researches, the understanding of the mechanism of estrogen receptors in cancer is deepening, but the main mechanism is not clear in lung cancer. They exert their functions through two different specific pathways, genomic and non-genomic (27,28), with the same biological effects: proliferation, growth, apoptosis, differentiation and angiogenesis.
The non-genomic pathway is characterized by the rapid initiation of signaling molecules such as MAPK (29), Akt, etc., and the related signal molecules are phosphorylated, which play an important role in the process. Genomic pathway is mainly mediated by classical pathway (ERE) and AP-1 (30), which is composed of the non-classical pathway mediated by transcription factors (27). All in all, a large number of studies on the mechanism of estrogen receptor action still need to be proved.
Stabile’s research proposed that in an in vivo lung tumor xenograft model, the drug combination of gefitinib (an EGFR receptor tyrosine kinase inhibitor) and fulvestrant (an ER antagonist) treatment decreased tumor volume (31). In addition, the clinical trials of estrogen receptor antagonists in the treatment of lung cancer are also in the exploration (32-35). This result above all suggested that ERβ could play a more important role in biological behavior of NSCLC (36). In contrast, in the present meta-analysis, we have combined eleven published studies including 3,300 patients with NSCLC to yield summary statistics that indicate that ERβ overexpression has a significant correlation with negative result of survival in NSCLC patients.
Although we performed a meta-analysis of the overall survival and ERβ expression, there were several limitations that should be considered. First, different countries’ studies were included in this analysis, which may cause language bias. Second, the risks calculated in our meta-analysis may be an overestimate due to publication bias. Some eligible studies were excluded from this meta-analysis because they lacked sufficient data on survival; negative or small-sample studies may be less likely to be published. Third, the method used to extrapolate the HR was different, which was extracted from the data included directly or which was calculated from the survival curves. Fourth, it could be explained by the fact that differences in antibodies used for staining, different criteria for defining stain positivity, and different patient demographics such as tumor stage, histological subtype, and adjuvant treatment. Nevertheless, no publication bias was detected using Begg’s test, indicating that the statistics obtained approximate the actual results. All that analyses suggest that our results were statistically reliable.
At first, the ER is deeply researched in breast cancer. Selective estrogen receptor modulators (SERMs) are ER ligands that in some tissues (i.e., bone and cardiovascular system) act like estrogens but block estrogen action in others (37). Tamoxifen and raloxifene have emerged effect on breast cancer treatments. Some studies have examined how estrogen signaling can influence NSCLC growth and how interactions with growth factors signaling pathways promote lung cancer. Further, new findings are presented to show that selective targeting of these complexes, intersecting pathways can effectively inhibit the growth of human NSCLC. Sequential or combined use of ER antagonists (fulvestrant or tamoxifen) and EGFR TKIs can block the growth of NSCLC tumor (38). These results also confirmed that estrogen receptors are associated with lung cancer.
In the recent years, scholars have been concerned about the status of estrogen receptor in lung cancer, and they are trying to understand the relationship between the occurrence, development and prognosis of lung cancer. They would like to find a new way for the treatment of lung cancer. But there are a lot of differences between domestic and foreign literature reports, and even contradictory results. So far, the intrinsic link between ER and lung cancer, and the exact mechanism are not clear yet. In the future, large prospective studies are needed to confirm the clinical utility of ERβ as an independent prognostic marker.
Acknowledgements
Funding: This study was supported by the Natural Science Foundation of Jiangsu Province (No. BK20140736), Clinical Science and Technology Project of Jiangsu Province (No. BL2013026) and the National Natural Science Foundation of China (No. 81302032, No. 81401903, No. 81572937, and No. 81572273).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Zhou C. Lung cancer molecular epidemiology in China: recent trends. Transl Lung Cancer Res 2014;3:270-9. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Weinberg OK, Marquez-Garban DC, Fishbein MC, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res 2005;65:11287-91. [Crossref] [PubMed]
- Mah V, Seligson DB, Li A, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res 2007;67:10484-90. [Crossref] [PubMed]
- Radzikowska E, Głaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol 2002;13:1087-93. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335-71. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Peters JL, Sutton AJ, Jones DR, et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008;61:991-6. [Crossref] [PubMed]
- Aresti U, Carrera S, Iruarrizaga E, et al. Estrogen receptor 1 gene expression and its combination with estrogen receptor 2 or aromatase expression predicts survival in non-small cell lung cancer. PLoS One 2014;9:e109659. [Crossref] [PubMed]
- Monica V, Longo M, Felice B, et al. Role of hormone receptor expression in patients with advanced-stage lung cancer treated with chemotherapy. Clin Lung Cancer 2012;13:416-23. [Crossref] [PubMed]
- Navaratnam S, Skliris G, Qing G, et al. Differential role of estrogen receptor beta in early versus metastatic non-small cell lung cancer. Horm Cancer 2012;3:93-100. [Crossref] [PubMed]
- Verma MK, Miki Y, Abe K, et al. Co-expression of estrogen receptor beta and aromatase in Japanese lung cancer patients: gender-dependent clinical outcome. Life Sci 2012;91:800-8. [Crossref] [PubMed]
- Karlsson C, Helenius G, Fernandes O, et al. Oestrogen receptor beta in NSCLC - prevalence, proliferative influence, prognostic impact and smoking. APMIS 2012;120:451-8. [Crossref] [PubMed]
- Mah V, Marquez D, Alavi M, et al. Expression levels of estrogen receptor beta in conjunction with aromatase predict survival in non-small cell lung cancer. Lung Cancer 2011;74:318-25. [Crossref] [PubMed]
- Stabile LP, Dacic S, Land SR, et al. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res 2011;17:154-64. [Crossref] [PubMed]
- Mauro LV, Dalurzo M, Carlini MJ, et al. Estrogen receptor beta and epidermal growth factor receptor as early-stage prognostic biomarkers of non-small cell lung cancer. Oncol Rep 2010;24:1331-8. [PubMed]
- Skov BG, Fischer BM, Pappot H. Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer 2008;59:88-94. [Crossref] [PubMed]
- Schwartz AG, Prysak GM, Murphy V, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res 2005;11:7280-7. [Crossref] [PubMed]
- Kawai H, Ishii A, Washiya K, et al. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res 2005;11:5084-9. [Crossref] [PubMed]
- Safe S, Kim K. Nonclassical genomic ER/Sp and ER/AP-1 signaling pathways. J Mol Endocrinol 2008;41:263-75. [Crossref] [PubMed]
- Arpino G, Wiechmann L, Osborne CK, et al. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev 2008;29:217-33. [Crossref] [PubMed]
- Duan R, Xie W, Burghardt RC, et al. Estrogen receptor-mediated activation of the serum response element in MCF-7 cells through MAPK-dependent phosphorylation of Elk-1. J Biol Chem 2001;276:11590-8. [Crossref] [PubMed]
- Paech K, Webb P, Kuiper GG, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997;277:1508-10. [Crossref] [PubMed]
- Stabile LP, Lyker JS, Gubish CT, et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res 2005;65:1459-70. [Crossref] [PubMed]
- Márquez-Garbán DC, Chen HW, Goodglick L, et al. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann N Y Acad Sci 2009;1155:194-205. [Crossref] [PubMed]
- Perez EA, Gandara DR, Edelman MJ, et al. Phase I trial of high-dose tamoxifen in combination with cisplatin in patients with lung cancer and other advanced malignancies. Cancer Invest 2003;21:1-6. [Crossref] [PubMed]
- Yang CH, Cheng AL, Yeh KH, et al. High dose tamoxifen plus cisplatin and etoposide in the treatment of patients with advanced, inoperable nonsmall cell lung carcinoma. Cancer 1999;86:415-20. [Crossref] [PubMed]
- Lara PN Jr, Gandara DR, Longmate J, et al. Activity of high-dose toremifene plus cisplatin in platinum-treated non-small-cell lung cancer: a phase II California Cancer Consortium Trial. Cancer Chemother Pharmacol 2001;48:22-8. [Crossref] [PubMed]
- Luo Z, Wu R, Jiang YF, et al. Overexpression of estrogen receptor beta is a prognostic marker in non-small cell lung cancer: a meta-analysis. Int J Clin Exp Med 2015;8:8686-97. [PubMed]
- Lewis JS, Jordan VC. Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance. Mutat Res 2005;591:247-63. [Crossref] [PubMed]
- Pietras RJ, Márquez DC, Chen HW, et al. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids 2005;70:372-81. [Crossref] [PubMed]


