
The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle
Introduction
The clinical definition of infertility is a lack of pregnancy after 12 consecutive months of unprotected sexual intercourse. The incidence of infertility is 10–15% globally, and this has risen in recent years (1). Approximately 50% of infertility cases involve male factors, among which 20% are cases of pure “male factor” infertility, and approximately 30% are cases of combined female and male factor infertility (2). Currently, evaluation and diagnosis of male infertility mainly rely on traditional semen analysis, including the volume, concentration, vitality and morphology of the semen (3). However, approximately 15% of men with a normal semen analysis are diagnosed as infertile (4). This suggests that semen analysis alone can only provide limited information for the assessment of male fertility, and it does not fully reflect the fertilization potential of the sperm. With the development of assisted reproductive technology (ART), traditional semen analysis has failed to meet the needs of reproductive clinical practice. We need better clinical indicators to determine the cause of male infertility and its relationships with reproductive outcomes. The sperm DNA fragmentation index (DFI) reflects the integrity of and the damage to the DNA, the genetic material of the sperm, thereby detecting potential sperm damage. It is considered a crucial indicator in evaluating semen quality. Sperm DNA fragmentation (SDF) has important impacts on fertilization, embryonic development and paternal genetic information transmission during both spontaneous and ART pregnancies (5,6). Studies (4,7) have shown that SDF assessment is a very valuable tool in assessing male infertility, but its clinical significance in predicting the prognostic outcomes of ART is unknown (8).
DNA damage in sperm and its impact on pregnancy outcomes following ART is inconclusive in the literature. A study by Li et al. suggested that sperm DNA damage reduced the pregnancy rate following IVF, but does not affect the pregnancy rate in cycles using ICSI (9). However, Collins et al. suggested that the assessment of sperm DNA damage is not sufficient to provide conclusive clinical evidence regarding male infertility (10). The Practice Committee of the American Society for Reproductive Medicine states that the current data do not support an adverse effect of sperm DNA damage on pregnancy outcomes following ART (11). A recent meta-analysis by Simon et al. showed that sperm DNA damage is associated with reduced pregnancy rates in IVF/ICSI (12). Conversely, the results of a meta-analysis by Zini et al. suggested that sperm DNA damage has no effect on outcomes of ART pregnancies, though there was evidence that sperm DNA damage causes an increased risk of early abortion in IVF or ICSI pregnancies (13). A recent meta-analysis suggested that for patients with recurrent miscarriage, an assessment of sperm DNA damage prior to ART should be strongly recommended to reduce the risk of early abortion (14).
One reason for the inconsistent literature is the variety of methods used to detect SDF in different studies. Main SDF detection methods include the sperm chromatin structure assay (SCSA), the sperm chromatin diffusion method (SCD), the comet assay (CA) and terminal deoxyuridine nick end labeling (TUNEL). It is inevitable that the different mechanisms underlying these methods will result in discrepancies. Even with the same detection method, the use of varying thresholds and inconsistencies in laboratory practice can also lead to studies drawing differing conclusions. Another possible cause for the discrepancies is the small sample sizes of most previous analyses, which could result in error.
In this study, we used SCSA, which is widely used clinically, to determine sperm DFI. Three thresholds of DFI (low, medium and high) were defined to analyze the predictive value of DFI for pregnancy outcomes from 2,622 ART (IUI, IVF, and ICSI) treatment cycles performed in our medical center. The effect of sperm DFI on oocyte fertilization and embryo development in IVF/ICSI was also evaluated. Furthermore, the relationship between SDF and lifestyle factors such as age, body mass index (BMI), smoking and alcohol consumption, as well as semen analysis parameters, were analyzed to investigate the value of SDF in assessing male fertility and the impact of its associated factors on pregnancy outcomes following ART.
Methods
Study design
From September 2016 to September 2018, couples suffering from infertility were examined and evaluated for ART treatment at the Reproductive Center of the First Affiliated Hospital of Zhengzhou University. The inclusion criteria for the study were females less than 42 years of age with a basal level of follicle stimulating hormone (FSH) that was <12 mIU/mL and males with a sperm concentration >0.5×106/mL. Couples diagnosed with unexplained infertility were referred to IUI; the IVF mainly consisted of couples with female factor infertility and the criteria for ICSI was a total sperm count of <800,000 after gradient centrifugation. One couple who met the inclusion criteria was excluded due to hereditary or metabolic disease. A total of 2,262 treatment cycles were included in the study, including 1,185 cases of artificial insemination by husband intrauterine insemination (AIH-IUI), 1,221 cases of IVF, and 216 cases of ICSI. Based on the results from the sperm DFI assessment and previous reports (7,15), the cases were divided into the following three groups: low DFI (DFI ≤15%), medium DFI (15%< DFI <30%) and high DFI (DFI ≥30%). The rates of pregnancy, early abortion, IVF/ICSI fertilization and good quality embryos were compared among the 3 groups. Additionally, the relationship between DFI and age, body mass index, smoking, alcohol consumption and routine semen parameters were analyzed. The DFI and its associated factors in evaluating male fertility were discussed. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Semen analysis
All tests were conducted according to World Health Organization (WHO) laboratory manual for the examination and processing for human semen (Fifth Edition) (3). The male remained abstinent for 3–7 days before collecting the semen into a sterile container by masturbation. Once the semen liquefaction was complete, 10 µL of the sample was taken and counted in a Makler chamber (Sefi medical instruments), and sperm concentration and viability were recorded. All tests were completed within 1 hour after sperm collection; sperm morphology assessment (SMA) was performed using H-E staining analysis following Kruger’s strict criteria.
Detection of sperm DFI by SCSA
All of the male fertility laboratory evaluations were completed prior to ART treatment. Only freshly liquefied semen produced after 3–7 days of abstinence were used in the DFI tests. An appropriate amount of semen was diluted in TNE buffer (0.01M Tris-HCL, 0.15M NaCl, 1 mM EDTA) to adjust the semen density to (0.5–1.0)×106/mL. Acridine orange (AO; PH6.0) solution was added for staining, and then the sperm DFI was calculated with fluorescence signals detected with a flow cytometer (BD FACS Canto II). The single-stranded DNA fragments combined with acridine orange emitted red fluorescence, while the intact double-strand DNA with intact DNA combined with acridine orange emitted green fluorescence. At least 5,000 sperm were counted with flow cytometry. The proportion of the sperm with red fluorescence of the total number of sperms was calculated, namely DFI. The detailed protocol was based on the previous description (16).
ART treatment plan
IUI is usually performed on couples with unexplained infertility (17), which refers to a diagnosis made in couples in whom all the standard investigations such as tests of ovulation, tubal patency and semen analysis are normal. The procedures were performed as follows. For IUI, ovulation was induced using clomiphene citrate (Pergotime; Serono Nordic, Copenhagen, Denmark). Once 1–2 follicles reached 17 mm in diameter, 10,000 IU of human chorionic gonadotropin (hCG) was injected. Thirty-six hours after the injection, fresh semen was collected and subjected to density gradient centrifugation (DGC) in sperm-grade 40% and 80% (Vitrolife, Sweden) solutions. One milliliter of treated sperm suspension was injected into the uterine cavity. After 16 days, serum β-hCG was collected to detect pregnancy. Positive test results (>50 mIU/mL) were followed by an ultrasound examination after 7 weeks to confirm clinical pregnancy. For IVF and ICSI, ultralong treatment regimens were used for ovulation induction. On the second or third day of menstruation, 3.75 mg of gonadotropin-releasing hormone-a (GnRH-a, triptorelin acetate) was injected intramuscularly to downregulate the pituitary secretion of gonadotropin (Gn), followed by an ultrasound examination and blood tests for FSH, LH, and E2 after 28–35 days. If the Gn level reached the low threshold, the ovulation induction program with Gn was initiated. The initial injected regimen contained recombinant human follicle stimulating hormone (r-FSH, Gonal-F, Serono, Swiss), and the amount of Gn was adjusted according to the follicular development, which was monitored by vaginal ultrasound and blood tests of the reproductive hormone levels. When at least 3 dominant follicles reached an average diameter of at least 17 mm, as monitored by ultrasound, Gn usage was stopped, and 10,000 IU of r-hCG (Serono, Swiss) was injected intramuscularly. At 35–37 h after injection, eggs were collected with the guidance of vaginal ultrasound.
Oocyte fertilization and embryo culture and transfer
For IVF fertilization, pretreatment consisted of fresh semen being subjected to DCG in sperm-grade 40% and 80% solutions, followed by use of the sperm swim-up technique to reach a final sperm density of 1×106/mL through adjustment. The solution was then cultured with oocytes for fertilization. In ICSI fertilization, after DCG of the sperm from fresh semen, viable sperm with good morphology were collected under the microscope and directly injected into the egg cytoplasm for fertilization. The pronuclei of the oocytes were examined 16–18 h after injection to assess the success of fertilization. The rate of normal fertilization (NF) is calculated by dividing the number of double pronuclear embryos (2PN) by the number of MII eggs (NF rate = number of 2PN embryos/number of MII eggs). Fertilized embryos were cultured to day 3 (D3), and embryonic development was monitored. Good quality embryos on D3 or blastocysts on day 5 (D5) were selected and transferred, and progesterone support was provided to the patient. The high-quality embryo rate was defined as the number of embryos/2PN cleavage with grade I or II Peter score from fertilization to the third day (D3). The good quality embryos rate is defined as the proportion of good quality embryos with grade I or II at the 8-cell stage [using Peter scoring system (18)] by the total number of 2PN embryos.
Evaluation of pregnancy outcome
Serum β-hCG levels were measured 14 days after embryo transfer, and biochemical pregnancy was confirmed by hCG levels greater than 50 mIU/mL. Clinical pregnancy was confirmed by intrauterine pregnancy with a normal fetal heart rate seen on ultrasound 7 weeks after embryo transfer. Loss of the fetus within 12 weeks of pregnancy was classified as an early abortion.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software. Data from each group are expressed as the mean ± standard deviation (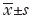 ), and the mean values of each group were compared using ANOVA. Rates of clinical pregnancy and early abortion were compared using the χ2 test. Correlation analysis was performed using bivariate Pearson analysis, while categorical variables (smoking and alcohol consumption) were analyzed using a Spearman correlation. Values of P<0.05 were considered statistically significant.
), and the mean values of each group were compared using ANOVA. Rates of clinical pregnancy and early abortion were compared using the χ2 test. Correlation analysis was performed using bivariate Pearson analysis, while categorical variables (smoking and alcohol consumption) were analyzed using a Spearman correlation. Values of P<0.05 were considered statistically significant.
Results
Relationship between sperm DFI and pregnancy outcomes following IUI
By analyzing 1,185 IUI cycles, we examined the association between groups of sperm DFI values and pregnancy outcomes; the results are shown in Table 1. There were no differences in general health (age, BMI, and basal FSH levels) between patients with high, medium or low DFI (P>0.05), but semen parameters such as sperm concentration and progressive motility differed significantly between the three groups (P<0.01). With regard to pregnancy outcomes, the clinical pregnancy rates among the high, medium, and low sperm DFI groups were 12.5% (11/88), 14.3% (48/360), and 13.4% (102/761), respectively. The χ2 test confirmed that these rates did not differ between the three groups (P=0.88). However, the early abortion rates were 27.3% (3/11), 14.6% (7/48) and 4.9% (5/102), respectively, and these rates differed significantly between the groups (P=0.02). Pairwise comparisons of the early abortion rates showed statistically significant differences between the high and low DFI groups (P=0.03) and between the medium and low DFI groups (P=0.04).
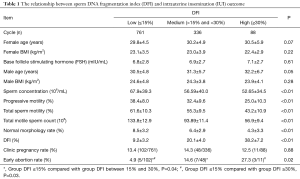
Full table
Relationships between sperm DFI and pregnancy outcomes and embryo quality following IVF/ICSI
Analysis of 1,211 IVF cycles and 216 ISCI cycles showed that the rates of clinical pregnancy and early abortion did not differ significantly among the three DFI groups. The rates of fertilization and good quality embryos also did not differ significantly among the DFI groups (P<0.05), as shown in Table 2.
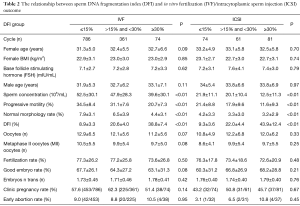
Full table
Correlation between sperm DFI and semen parameters and general lifestyle factors
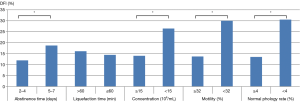
Correlation analysis showed that sperm DFI was negatively associated with sperm concentration, progressive motility and normal morphology (correlation coefficients r were −0.307, –0.552 and −0.620, respectively; all P<0.01). Sperm DFI was positively correlated with age, abstinence time, smoking and alcohol consumption (r values were 0.159, 0.133, 0.109 and 0.064, respectively; all P<0.01). There was no correlation between sperm DFI and male BMI, sperm liquefaction time or semen volume (P>0.05) (Table 3). We also looked for associations between the sperm DFI values and semen analysis parameters. From the semen analysis results displayed in Figure 1, sperm DFI values were notably lower among men with normal semen parameters compared to those with abnormal semen parameters.
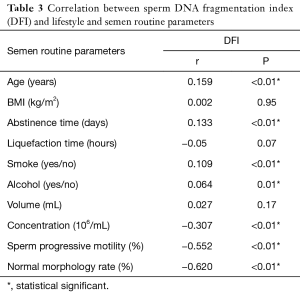
Full table
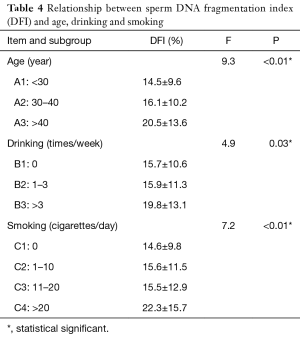
Age, alcohol drinking and smoking were also analyzed in subgroups. As shown in Table 4, among the age groups, there was a significant difference in DFI values between the three groups A1, A2, and A3 (P<0.01), and there was also a significant difference in DFI values between any two of the three groups (P<0.01). Among the drinking groups, there were significant differences in DFI between the three groups (P=0.03), B1 and B3 (P=0.03) and B2 and B3 (P=0.04). There were no significant differences between B1 and B2. There was no statistically significant difference between C1, C2, and C3 among the smoking groups, while there was a statistically significant difference in DFI between C4 and the other three groups (P<0.01).
Full table
Discussion
SDF occurs during spermatogenesis and maturation whereby broken DNA fragments are produced in sperm cells as a result of damaged chromosomes and impaired DNA integrity (5). The sperm DFI is used to assess the DNA damage and directly reflects the degree of sperm DNA destruction. Human sperm DNA carries the paternal genetic information, and its integrity is required to correctly transmit genetic materials to the offspring. Damage to sperm chromatin can directly affect the sperm’s normal functions (19). Currently, the following three major factors are known to cause sperm DNA damage: abnormal sperm chromatin assembly, aberrant apoptosis of sperm cells, and excessive oxidative stress (20). During sperm maturation, histones are gradually replaced by the smaller arginine- and cysteine-rich protamine (HP), a process that reduces the ability of sperm DNA to repair itself in response to changes in the internal and external environments. Furthermore, the misfolding of DNA supercoiling structures in the chromosome due to twisting tensions generated by the double-stranded DNA helix can also lead to aberrant DNA repair, causing SDF or abnormalities in the chromatin structures (21). Inflammation in the external genital tracts and varicocele can also increase the risks of SDF by inducing reactive oxygen species in the sperm. Poor lifestyle habits including smoking, alcohol consumption, environmental radiation and pollution can all lead to increased SDF, as shown in another study (22). Consistent with previous reports, we found that sperm DFI rises significantly with age, and unhealthy lifestyle habits such as smoking and alcohol consumption can lead to an elevated risk of DFI.
Many investigators believe that SDF has a negative impact on embryo quality and pregnancy outcomes following IVF/ICSI (23,24). Studies by Zheng et al. found that high sperm DFI is not only related to reduced fertilization rate and poor embryo quality in IVF, but it is also associated with decreased rates of pregnancy (25). However, recent studies (26,27) suggest otherwise, showing that sperm DFI has no correlation with fertilization rates, and high DFIs do not affect the development of embryos or pregnancy outcomes following IVF/ICSI. However, the study by Niu et al. (28) indicated that high DFIs affect embryo quality (rates of good quality embryos and blastocyst formation) but have no impact on oocyte fertilization rates or pregnancy outcomes following IVF. In this study, we found that although both the fertilization rates and the rates of good quality embryos decreased as the sperm DFI increased, there was no significant difference in either rate between the three groups (P>0.05). These results indicate that sperm DFI is not a predictor of the fertilization rate or embryo development, which is consistent with the conclusion of Sun et al. (27). The fertilization rate and embryo development may be impacted by pre-treating the semen before IVF fertilization. In our study, the semen was centrifuged in a DGC, followed by 15 minutes of swim-up treatment at 36 °C; the optimized sperm in the upper layer were selected for oocyte fertilization. Pre-treating the semen optimizes the semen solution for further screening of good-quality sperm to ensure successful fertilization. Yet at the same time, it may somewhat minimize the effect of high DFIs on fertilization and subsequent embryo development. Xue et al. (29) reported that different methods utilized in semen pre-treatment may greatly influence the sperm DFI, and sperm DFI is significantly reduced after DGC. Studies by Bungum et al. (30) and Niu et al. (28) also confirmed that sperm DFI is not associated with pregnancy outcomes in IVF using preoptimized sperm by DGC, suggesting that appropriate semen pre-treatment removes most high DFI sperms to eliminate the adverse effects of high DFI on oocyte fertilization, embryo development, and the final clinical pregnancy outcome. In our study, the semen used in ICSI also required optimization before the procedure, and a single sperm with good viability and morphology was selected from the optimized sperm solution and injected into the cytoplasm of the oocyte. This study demonstrates that sperm DFI is positively associated with sperm motility and morphology, as sperm with better morphological activity have a low incidence of DNA fragmentation. Therefore, arbitrary selection of sperm for ICSI could potentially lower the possibility of using a sperm with high DFI for fertilization, since it may weaken the binding ability to the zona pellucida of the oocyte. This could also be a major reason why our study saw no effect of sperm DFIs on embryonic development or pregnancy outcomes following ISCI. Additionally, both animal (31,32) and human studies (33) have confirmed that oocytes play a role in repairing sperm DNA damage to some extent. Thus, DNA repair by oocytes could be another reason why DFI does not influence embryo development or pregnancy outcomes following IVF/ICSI (34).
Although researchers have controversial opinions on the relationship between DFI and IVF/ICSI pregnancy outcomes, the negative effect of high sperm DFI on outcomes of natural pregnancies or IUI pregnancies has been broadly recognized. It was shown that sperm DFIs is significantly elevated in couples with unexplained infertility (35). Rates of pregnancy and early abortions are significantly higher among those with high DFI compared to those with low DFI (36). Nonetheless, in this study, we did not find any differences in the pregnancy rates following IUI among the three DFI groups, which may be attributed to the optimization treatment of the semen using GDC potentially eliminating any differences in the sperm DFI. However, the rates of early abortion in the high, medium and low DFI groups were 27.3%, 14.6% and 4.9%, respectively, and these differences were statistically significant. The early abortion rates in the high and medium DFI groups were significantly higher than that in the low DFI group, with P values of 0.03 and 0.04, respectively. This suggests that although DCG-optimization of sperm may minimize the potential effects of high sperm DFI on pregnancy rates following IUI, it may increase the risk of early abortion, which is in agreement with the previous findings by Duran et al. (37).
Correlation analysis between sperm DFI and semen parameters showed that sperm DFIs is negatively associated with sperm density and viability but positively associated with sperm morphology. The results presented here are consistent with most recent reports (38,39), implying an equivalent correlation between SDF and semen analysis in assessing sperm quality, i.e., DFI can also be used as a standard measure in the assessment of male fertility. In terms of sperm DFI and age, BMI, and lifestyle factors, we discovered that sperm DFI increases with age and is also closely associated with smoking and alcohol drinking. From another perspective, these results can also suggest that sperm DFI is more sensitive than regular semen analysis in detecting latent DNA damage in the sperm. We also demonstrated that sperm DFI is positively correlated with time of abstinence, which confirms the results by Agarwal et al. (40). Thus, to avoid adverse effects of potential high sperm DFIs on pregnancy outcomes, a recommendation should be made to shorten the male abstinence time to an appropriate period that does not affect the quality of semen while monitoring ovulation or providing bedroom guidance during IUI treatment
In this study, the DFI Groups are heterogeneous in terms of number of patients. The number of cases in the high DFI group was small, the small sample size may lead to research bias, especially in the comparison of pregnancy and early abortion rates. Additionally, as a retrospective study, there is a lack of some male laboratory indicators, such as white blood cell counts, and there is no matching of the differences in male clinical examinations, which is also one of the limitations of this study. In future studies, the sample size should be expanded, and the male and female subjects’ various interference indicators should be matched and grouped strictly, to allow more accurate conclusions.
Conclusions
Sperm DFI is negatively correlated with sperm density, viability and normal sperm morphology, though it is positively correlated with age, abstinence time and unhealthy lifestyle habits (smoking and alcohol drinking). High sperm DFI does not affect the clinical pregnancy rate following IUI, but it may increase the risk of early abortion. Pregnancy outcomes following IVF and ICSI are not related to sperm DFI, and elevated sperm DFI does not impact oocyte fertilization or embryo development. As an increasingly common technology in clinical testing for reproduction, sperm DFI has proven to be very valuable in male fertility evaluation, but its significance as a predictor of pregnancy outcomes following ART requires further investigation.
Acknowledgments
The authors would like to thank our colleagues of the center for reproductive medicine, their help is most grateful. We also like to thank the patients who participated in this study.
Funding: This work was supported by the Key Science and Technology Foundation of Henan Province (182102310397 to H Yang) and Youth Innovation Project of the First Affiliated Hospital of Zhengzhou University (to H Yang), and the National Natural Science Foundation of China (81771534 to G Li).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The ID of ethics approval is Keyan-2018-lw-044.
References
- Saleh RA, Agarwal A, Nelson DR, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril 2002;78:313-8. [Crossref] [PubMed]
- Nallella KP, Sharma RK, Aziz N, et al. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril 2006;85:629-34. [Crossref] [PubMed]
- World Health Organization. WHO laboratory manual for the examination and processing for human Semen. 5th ed. Switzerland: WHO Press; 2010:223-5.
- Agarwal A, Allamaneni SS. Sperm DNA damage assessment: a test whose time has come. Fertil Steril 2005;84:850-3. [Crossref] [PubMed]
- Barratt CL, Aitken RJ, Bjorndahl L, et al. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications-a position report. Hum Reprod 2010;25:824-38. [Crossref] [PubMed]
- Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons J Androl 2009;30:219-29. [Crossref] [PubMed]
- Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 2011;13:69-75. [Crossref] [PubMed]
- Agarwal A, Cho CL, Majzoub A, et al. The Society for Translational Medicine: clinical practice guidelines for sperm DNA fragmentation testing in male infertility. Transl Androl Urol 2017;6:S720-33. [Crossref] [PubMed]
- Li Z, Wang L, Cai J, et al. Correlation of sperm DNA damage with IVF and ICSI outcomes: a systematic review and meta-analysis. J Assist Reprod Genet 2006;23:367-76. [Crossref] [PubMed]
- Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril 2008;89:823-31. [Crossref] [PubMed]
- The Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril 2013;99:673-7. [Crossref] [PubMed]
- Simon L, Zini A, Dyachenko A, et al. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl 2017;19:80-90. [PubMed]
- Zini A, Boman JM, Belzile E, et al. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod 2008;23:2663-8. [Crossref] [PubMed]
- Zhao J, Zhang Q, Wang Y, et al. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization /intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril 2014;102:998-1005.e8. [Crossref] [PubMed]
- Venkatesh S, Singh A, Shamsi MB, et al. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci 2011;18:1005-13. [Crossref] [PubMed]
- Evenson DP. Sperm chromatin structure assay (SCSA®). Methods Mol Biol 2013;927:147-64. [Crossref] [PubMed]
- Ray A, Shah A, Gudi A, et al. Unexplained infertility: an update and review of practice. Reprod Biomed Online 2012;24:591-602. [Crossref] [PubMed]
- Brinsden PR. A textbook of in vitro fertilization and assisted reproduction. New York: The Parthenon Publishing Group Inc, 1999:1-564.
- Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015;30:120-7. [Crossref] [PubMed]
- Gomes M, Goncalves A, Rocha E, et al. Effect of in vitro exposure to lead chloride on semen quality and sperm DNA fragmentation. Zygote 2015;23:384-93. [Crossref] [PubMed]
- Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 2010;93:1027-36. [Crossref] [PubMed]
- Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online 2014;28:684-703. [Crossref] [PubMed]
- Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 2004;81:1289-95. [Crossref] [PubMed]
- Bounartzi T, Dafopoulos K, Anifandis G, et al. Pregnancy prediction by free sperm DNA and sperm DNA fragmentation in semen specimens of IVF/ICSI-ET patients. Hum Fertil (Camb) 2016;19:56-62. [Crossref] [PubMed]
- Zheng WW, Song G, Wang QL, et al. Sperm DNA damage has a negative effect on early embryonic development following in vitro fertilization. Asian J Androl 2018;20:75-9. [Crossref] [PubMed]
- Lin MH, Kuo-Kuang Lee R, Li SH, et al. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril 2008;90:352-9. [Crossref] [PubMed]
- Sun TC, Zhang Y, Li HT, et al. Sperm DNA fragmentation index, as measured by sperm chromatin dispersion, might not predict assisted reproductive outcome. Taiwan J Obstet Gynecol 2018;57:493-8. [Crossref] [PubMed]
- Niu ZH, Shi HJ, Zhang HQ, et al. Sperm chromatin structure assay results after swim-up are related only to embryo quality but not to fertilization and pregnancy rates following IVF. Asian J Androl 2011;13:862-6. [Crossref] [PubMed]
- Xue X, Wang WS, Shi JZ, et al. Efficacy of swim-up versus density gradient cen-trifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet 2014;31:1161-6. [Crossref] [PubMed]
- Bungum M, Spanò M, Humaidan P, et al. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Hum Reprod 2008;23:4-10. [Crossref] [PubMed]
- Tesarík J, Kopecný V, Plachot M, et al. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J Reprod Fertil 1986;78:463-70. [Crossref] [PubMed]
- Santos R, Palos-Ladeiro M, Besnard A, et al. Relationship between DNA damage in sperm after ex vivo exposure and abnormal embryo development in the progeny of the three-spined stickleback. Reprod Toxicol 2013;36:6-11. [Crossref] [PubMed]
- Ahmadi A, Ng SC. Developmental capacity of damaged spermatozoa. Hum Reprod 1999;14:2279-85. [Crossref] [PubMed]
- Meseguer M, Santiso R, Garrido N, et al. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril 2011;95:124-8. [Crossref] [PubMed]
- Oleszczuk K, Augustinsson L, Bayat N, et al. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013;1:357-60. [Crossref] [PubMed]
- Vandekerckhove FW, De Croo I, Gerris J, et al. Sperm chromatin dispersion test before sperm preparation is predictive of clinical pregnancy in cases of unexplained infertility treated with intrauterine insemination and induction with clomiphene citrate. Front Med (Lausanne) 2016;3:63. [Crossref] [PubMed]
- Duran EH, Morshedi M, Taylor S, et al. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002;17:3122-8. [Crossref] [PubMed]
- Winkle T, Rosenbusch B, Gagsteiger F, et al. The correlation between male age, sperm quality and sperm DNA fragmentation in 320 men attending a fertility center. J Assist Reprod Genet 2009;26:41-6. [Crossref] [PubMed]
- Smit M, Romijn JC, Wildhagen MF, et al. Sperm chromatin structure is associated with the quality of spermatogenesis in infertile patients. Fertil Steril 2010;94:1748-52. [Crossref] [PubMed]
- Agarwal A, Gupta S, Du Plessis S, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology 2016;94:102-10. [Crossref] [PubMed]


