High intensity focused ultrasound (HIFU) applied to hepato-bilio-pancreatic and the digestive system—current state of the art and future perspectives
Introduction
High intensity focused ultrasound (HIFU) is a medical technology which uses acoustic lenses or curved piezoelectric transducers to focus beams of ultrasounds, on a target located deep in the body. This translates into the ability to deliver energy in the human body transcutaneously, in a totally non-invasive manner. The transfer and concentration of this mechanical vibrational energy to the distant target point occurs with minimal impact on the pathway followed, providing a good transonic pairing between the source and the target (1). Highly conductive means of acoustic energy (e.g., water) will allow waves to pass through without generating echoes (transonic = hypo echogenic) while low-conductive or non-conductive media (e.g., bones and air) generate hyper echogenic images, and block the transmission of US energy. US in HIFU are generally used at relatively low frequency (0.8-1.6 MHz) but when the beam is focused, typically in an olive shape (its length is superior to its width according to the axis of the transducer), the therapy acoustic power (W) can be high enough to induce tissue damage. Destruction of the target can be obtained either by localized thermic effect (beyond the range of hyperthermia) which generates coagulation necrosis or, at higher acoustic intensities, by the phenomenon of cavitation. Inertial cavitation, i.e., the generation of gas microbubbles within the fluids due to the impact of HIFU, is a chaotic and unpredictable mechanical effect, in which oscillating gas bubbles accumulate more and more heat due to the mechanical friction with the US waves, and can implode with consequent tissue damage (2). Tissue ablation can be defined by the thermal dose which depends on the actual heating temperature and by application time. The volume of the target depends on the design of the acoustic lens and on the ultrasound parameters. It can range from 1 mm × 1.5 mm to 10 mm × 16 mm in size (3). This fascinating technology has a long history, however, it is only during the last decade that the HIFU have been increasingly used to treat a variety of diseases, especially in eastern countries. There is an increasing interest around the potential application of HIFU energy, in various clinical applications, and this interest is confirmed by a growing number of players (corporate companies and start-ups) which are currently manufacturing HIFU-based systems. However, despite its great appeal, the clinical use of HIFU remains quite limited. One of the reasons for the timid uptake of HIFU technologies could be seen in the multiple challenges to handle in the clinical setting, including cost/effectiveness and logistic considerations and the relatively tiny treatment/complications cut-off.
Currently, FDA-approved clinical applications are limited to bone metastases and uterine fibroids treatment (Focused Ultrasound Foundation: http://www.fusfoundation.org). Outside the United States, HIFU is being explored in several conditions such as cancer of the prostate (4), the breast (5), the pancreas (6), the liver (7) and also in non-oncologic applications, e.g., the management of back pain, neuromodulation for essential tremor, or Parkinson’s disease (8). Preliminary trials are also being conducted to use HIFU to treat hypertension by selective renal denervation (9).
Image-guidance is crucial to plan the treatment strategy and also to follow-up the results of HIFU treatment (10-12) and the different devices used, including US and/or MRI-guidance.
In 2012, we created a scientific foundation, IHU-Strasbourg, to develop the concept of minimally invasive hybrid image-guided therapies for the digestive system (13). Our aim is to create a joint venture between the three main interventional disciplines (minimally invasive surgery, interventional radiology and interventional endoscopy) and to create the “hybrid physician” with cross abilities in order to optimize patient outcomes (14). In this context, HIFU immediately appeared as one of the potential weapons which deserved attention to improve the treatment of digestive cancers, particularly for those in which HIFU remains only conceptual, like gastrointestinal tumors.
We aimed to review the current state of the art of HIFU therapy for the digestive system, and provide some perspectives on potential improvements and some preliminary experimental results on the endoluminal use of miniature HIFU systems.
Materials and methods
Until November 2014, a systematic search of the literature was performed interrogating PubMed and Embase search engines. The following keywords were used in various combinations: high intensity focused ultrasound (HIFU); HIFU and esophagus, stomach, liver, pancreas, gallbladder, colon, rectum, and cancer. A prefilled excel database was used to enter the records according to a defined exclusion criteria algorithm. Exclusion criteria applied hierarchically were: (I) not relevant to HIFU technology; (II) not relevant to the digestive system; (III) not including human subjects; (IV) not in English; (V) review articles. Abstracts were manually screened by LS and MD separately, and subsequently matched for accuracy. Pertinent full-text articles were retrieved and analyzed, and data were extracted on the database. The flow chart of article selection is described following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (15) (Figure 1).

Results
Study selection and level of evidence
The initial database literature search yielded 2,007 records, including duplicates and non-pertinent articles. After manual screening of the abstracts, 1,596 records were excluded as non-pertinent to our search. The remaining 411 articles were further assessed for eligibility. After removal of duplicate records (n=47), 364 articles were assessed according to selection criteria. At that stage, n=37 records were not pertinent to HIFU technology; n=46 were not pertinent to the digestive system; n=100 referred to experimental non-human trials, n=31 were not in English and n=83 reviews or editorials were excluded from data analysis. A total of 67 articles were identified and were included for data extraction. From this pool, 46 were included for quantitative analysis and the remaining for qualitative analysis (Figure 1).
No articles discussed the use of HIFU technology to treat hollow organ pathologies (esophagus, colon, rectum, and gallbladder).
Forty-four studies reported on the clinical use of HIFU (7,10-12,16-33) to treat liver lesions (34-55), while 19 series were found on HIFU treatment for pancreatic cancers (6,56-73) and 4 studies included patients suffering from both liver and pancreatic cancers (74-77), reporting on a total of 1,682 and 823 cases for the liver and pancreas, respectively. However, the real number of patients who benefited from HIFU treatment is much larger, since a recent review article from Zhou reported on over 3,000 cases of advanced pancreatic cancer treated with HIFU alone or in combination with chemotherapy (CHT) or radiotherapy (RT) (78). On the other hand, a recent and authoritative systematic review of the literature on the use of HIFU in advanced pancreatic cancer by Dr. Wu, a very active researcher in the field, reported data on 561 patients (79). Several trials have been published in languages other than English and were excluded from our analysis.
Demographic and clinical data are summarized in Table 1.
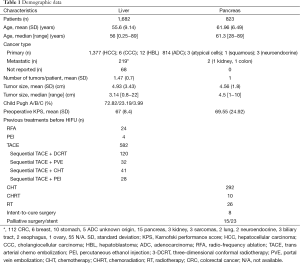
Full table
While the majority of pancreatic cancer patients undergoing HIFU therapy were in the advanced stages (37.5% stage III and 61% stage IV), in selected case HIFU was used for less advanced cases of hepatocellular carcinoma (HCC), e.g., as a “bridging therapy” in cirrhotic patients listed in an orthotopic liver transplantation (OLT) waiting list (37,44,51).
There is only one small-sized prospective randomized clinical trial comparing HIFU combined with trans arterial chemo embolization (TACE) vs. TACE alone (30). The largest published series on HIFU treatment for liver cancer included 151 unresectable cases which were prospectively compared to 30 comparable patients receiving only supportive palliative care (21). The largest series of HIFU treatment for pancreatic tumors reported on 224 cases (6).
Technical considerations
The vast majority of reported cases were treated using the US-guided HIFU delivery devices JC Model Chongqing HIFU Technology Co, Ltd., Chongqing, China, and the FEB-BY02 HIFU system (Yuande Biomedical Engineering Limited Corporation, Beijing, China), which differ essentially in ergonomics of HIFU energy delivery to the patient. Both can deliver up to 300 W of acoustic power (which corresponds to a focal peak intensity of about 20 KW/cm2). No clinical MRI or CT-guided procedures were reported. One study reported the use of pre-HIFU CT to determine the optimal depth of treatment ensuring safe ablation (71).
In case of liver treatment, the HIFU ablation procedure is more frequently performed under general or epidural anesthesia, while in pancreatic procedures, it is often performed without anesthesia but only under analgesia and/or sedation. HIFU total sonication time is largely variable. It may take up to 30 minutes and mainly depends on the size and location of the tumors. Total HIFU session duration (from first to last sonication) may range from 30-40 minutes to several hours (33). Several HIFU sessions, with a few interval days, might be required to treat large lesions, especially in advanced pancreatic cancers.
To optimize acoustic windowing, pre-HIFU surgical rib removal (approximately two weeks before liver lesions treatment) has been reported in 95 cases (21,29,34,54). Planned iatrogenic right hydrothorax with intra-pleural infusion of warm saline solution was reported in 272 cases, to enhance HIFU coupling in cases of liver dome tumor (26,35,36,38,41,45,52,55,75). Other studies reported on the use of artificial pleural infusion or artificial ascites in selected cases, without detailing the number of patients (42-44,49,51).
Intragastric water filling and colon irrigation have also been described to optimize acoustic coupling and consequently reduce the risks of burn injuries to air-filled viscera that might interpose between the HIFU transducer and the target (41). The placement of degassed water-filled balloons on the application site, with slight pressure to displace bowel and clear gas, is an additional mean to enhance the coupling and reduce the risk of injuries to innocent organs.
HIFU setting parameters, i.e., therapeutic frequency (MhZ), therapy power (W), focal peak intensity (W/cm2), were highly variable depending on the study, even when considering populations of homogenous patients. One study (66) reported a preliminary dosimetric analysis in 136 patients presenting advanced pancreatic cancer, which suggested a minimal dose intensity of 11 KJ/cm3 and a minimal therapy power of 260 W.
Outcomes
Tumor ablation rates as assessed by post-procedure imaging (US and/or CT and/or MRI) are reported in Tables 2 and 3. The variability of ablation rates is very wide and protocols and patient characteristics are also very inhomogeneous. No relationship could be established, based on those published data, between sonication parameters, tumor size and ablation rates or the occurrence of complications. Mean follow-up was 26.16±18.8 months (reported in 711 patients with liver lesions) and 25.07±19.09 months (reported for 264 patients with pancreas tumors).
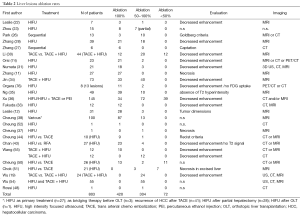
Full table
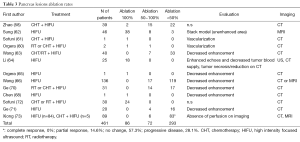
Full table
Real-time increase of US reflection intensity during tumor ablation was predictive of a >30% tumor ablation ratio (70).
For pancreatic cancer, there was only a small sized (n=12) study in which HIFU alone was compared with concurrent CHT and HIFU (59), demonstrating a clear survival advantage in the combined group. In remaining studies, HIFU was used as adjuvant treatment to RT or systemic CHT. In advanced pancreatic cancer, HIFU could provide significant relief of cancer pain (56-58,62-64) and significant improvement of the Karnofski performance score (KPS) (21,64).
In liver lesions, HIFU was also mainly used as a co-adjuvant therapy in sequential protocols. However, in few studies, some prospective comparisons have been performed between HIFU and TACE or radio frequency (RF) ablations (Table 4). When HIFU was compared as sole strategy vs. radio-frequency ablation (RFA) alone, in selected cases presenting with recurrent HCC (43), there were comparable results with no significant differences in terms of survival, and a tendency towards a better tolerance profile with HIFU.
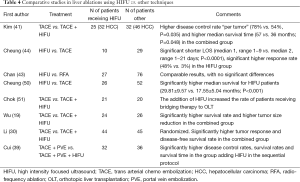
Full table
When compared to TACE (44,50), a significantly higher tumor response and higher survival along with decreased length of hospital stay was reported in the HIFU group.
Studies reporting HIFU as a co-adjuvant of TACE, including the only randomized trial (30), suggested, almost univocally, that this combination achieves better disease control as compared to TACE alone (Table 4).
Complications
Post-HIFU complications or side-effects were reported in 31/48 and 16/23 trials, describing liver and pancreatic oncologic cases, respectively. The most frequent complications were skin burns at the application sites and osteonecrosis of ribs or vertebra along the US pathway (Tables 5,6). Post-HIFU pain was not assessed systematically and was reported in 17 studies (384 patients undergoing liver HIFU procedures) (7,12,19-22,24,26,28,29,32,39,41,45,49,54,75) and 6 studies (62 patients receiving HIFU for pancreatic malignancies) (56,59,62,70,71,75). In only a few of those trials, a semi-quantitative evaluation tool was used to report pain level, based on the analgesic requirements (mild = no analgesic; moderate = non-steroidal anti-inflammatory drugs; severe = required morphine) (7,19,20,24,39). Post-HIFU pain was generally described as transient and mild, with less than 10% of patients requiring narcotics.
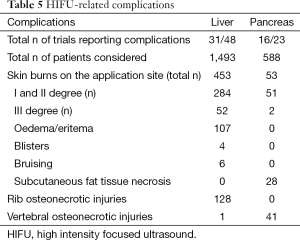
Full table
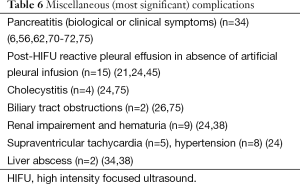
Full table
Similarly, post-HIFU fever, as part of the “post-ablation syndrome”, was not systematically reported and was described in approximately 10% of post-hepatic and 15% of post-pancreatic treatments (as mild or transient fever). The occurrence of some rare miscellaneous complications is detailed in Tables 5,6.
Discussion
Recent fascinating discoveries are revealing new insights in the mechanisms of action of HIFU which go beyond the mere local generation of hyperthermia for tumor ablation (80).
In fact, synergistic and distinct thermal and non-thermal effects have been recognized, with blurred boundaries. Among non-thermal effects, the transfer of mechanical energy seems to be able to induce cascade phenomena that could profoundly affect the host response.
A most intriguing phenomenon is the modulation of immune-response: the disintegration of neoplastic masses induced by vibration seems to enhance the anti-tumor immune-surveillance by amplification of cancer antigens (81,82). Zhou et al. demonstrated a significant decrease of immunosuppressive cytokines after HIFU, which means a shift towards improved anti-cancer immune response (23).
For those reasons and for the intrinsically non-invasive delivery modality, HIFU could play a major role in the management of neoplasia of the digestive system, although epidemiology and anatomical factors impose organ-specific considerations.
For pancreatic cancer, as we could verify through our systematic review, the frequent diagnostic delay with the ineluctable poor prognosis associated with advanced stages, relegates HIFU to a mere role of co-adjuvant therapy, the best effects of which are probably linked to pain management and quality of life for patients (83). In fact, advanced pancreatic cancer often presents with severe pain which is commonly managed with morphine administration and/or celiac plexus alcoholization (84). HIFU can achieve a spectacular decrease of cancer-related pain and could complement or even replace opioids and plexus neurolysis. The mechanical effect of HIFU seems to induce neuromodulation and pain relief through a reversible block of nerve activity (85). Different considerations have to be made for HIFU in liver lesions, in which the hepatobiliary multidisciplinary team (surgeons, oncologists, interventional radiologists), faces multiple layers of complexity: primary vs. metastatic, location, size, preserved vs. impaired liver function, criteria for OLT etc. Data published so far suggest some advantages of HIFU treatment of liver lesions under some precise conditions. For example, in presence of ascites or coagulopathy, HIFU could be the only possible option to keep a patient in an OLT list or to treat HCC recurrences, as the other more invasive locoregional ablative therapies [cryoblation, percutaneous ethanol injection (PEI), TACE, and RFA] are contraindicated. A recent meta-analysis demonstrated that combined HIFU and TACE treatment for HCC confers higher survival when compared to TACE (86) alone. The only randomized trial comparing TACE vs. TACE + HIFU, showed higher disease control and increased disease-free survival (30). This enhanced effect seems to be due to the increased action of HIFU on the tissue retaining the ethiodized oil (lipiodol) used for TACE.
Two main techniques are used as a protective means during liver HIFU ablations: (I) pre-HIFU surgical rib removal (approximately two weeks before) to obtain a favorable acoustic therapeutic windowing to the liver (21,29,34,54); and (II) intra-pleural infusion of warm saline solution. Although effective, those methods drastically reduce the appeal of HIFU in terms of minimal invasiveness.
A fundamental development lies in the ability to perform HIFU treatment without the need to stop breathing. This would allow to increase the number of patients that could be treated under conscious sedation, instead of using general anesthesia, and might reduce the length of procedures, in either pulsed or continuous HIFU applications. Breathing movements generate a very large cranio-caudal displacement of the liver, up to several cm, even during quiet breathing as can be seen in Figure 2. The ability to track organ displacement and constantly focus on the same target requires some technological developments which are currently underway. An interesting solution has been proposed by Auboiroux et al. who placed an MRI-compatible camera to track respiratory movements and synchronize HIFU delivery (87). We propose different approaches.

One of our main fields of expertise is the concept of augmented reality (AR) applied to the digestive system. AR is an image-guided surgical navigation system in which computer-based patient-specific images (virtual clone of the patient) are overlapped (registered) with real-life images. This allows to visualize some anatomical structures such as vessels (88,89) by transparency. The virtual clone of the patient is obtained through 3D reconstruction of preoperative CT or MRI images and computed with a specific software to obtain organ segmentation. In addition to allowing to visualize resection planes and plan the procedure, the VR-RENDER® software, developed at the Research Institute against Cancer of the Digestive System (IRCAD), can also calculate resection and future remnant volumes. It has been applied to minimally invasive liver resections (90,91), in video-assisted minimally invasive parathyroidectomies (92,93), and in duodeno-cephalo-pancreatectomy (94-96). A targeted therapy, surgical or ablative, can be simulated on the virtual model to plan the most adapted strategy. Intraoperatively, the 3D model may be superimposed with real-time patient images (Figure 2). The main problems with registration of AR in digestive surgery and interventional radiology include organ deformation or displacement by surgical manipulation, needle insertion, transducer application, and during breathing motion. To overcome these problems we have developed software which is able to predict organ motion (97) and organ deformation (98), based on biomechanical properties (99) (Figure 2).
Those works on “flexible” AR might be transferrable to predict organ motion and allow a constant targeting using a robotized arm and a visual servoing tracking system, and constantly adjust the direction of the HIFU transducer.
Perspectives: conceptual application
Inspired by the transrectal HIFU probes for prostate cancer ablations (e.g., Ablatherm, EDAP, France), we aimed to use an endoscopic mounted miniature HIFU transducer in direct contact with the gastrointestinal mucosa. We formulated the hypothesis that HIFU could replace RF ablation of premalignant lesions (e.g., Barrett’s esophagus) and potentially treat gastric or colon malignancies. The potential advantage of HIFU over RF in this specific application, could be in the possibility to prevent US energy spread to adjacent structures by injecting a bolus of air (which would block the diffusion of the HIFU) in the submucosal space. This technique could mimic the oncologic performance of an endoscopic submucosal dissection (100) with the advantage of being easier to perform. A flexible surgical endoscopy robotized platform equipped with an integrated ultrasound probe and HIFU applicator, has been already described (101). Recently, a similar endoscopic HIFU system has been successfully tested in the animal model to achieve transluminal (transgastric) ablation of liver or pancreatic tumors (102). Our aim is to treat lesions directly originating from the GI tract and we have developed a miniature piezoceramic transducer mounted on the tip of an endoscope. The device can deliver acoustic intensities from 14 to 30 W/cm2 Based on preliminary studies, the device was set to deliver 600 joules (30 W × 20 sec) for stomach sonications and 350 J for colon sonications to create effective destruction down to the submucosa layer. Tests were performed in porcine models, under Animal Care Committee protocol approved by the French Ministry of Superior Education and Research (acronym FURTHER, Focused UltRasound THERapies, protocol number: 38.2014.01.062).
A bolus of air was injected in the submucosal space, to create a protective interface and a long-lasting lifting of the mucosa (Figure 3). The effects of the sonications were assessed by confocal endomicroscopy (Cellvizio®, MaunaKea Technologies, France) (Figure 4). The system could achieve effective ablation of the mucosa/submucosa without creating full-thickness lesions and burns to adjacent organs. Confocal endomicroscopy could provide some optical signature of efficacy (disappearance of enterocyte borders signs of coagulation necrosis). However, the limited depth of penetration of the laser, could only provide information on the mucosa. Histology also presented mucosa/submucosa architecture distortion and coagulation necrosis. Further studies are underway to refine the technique and establish optimal doses and effects profiles.


Conclusions
Digestive system clinical applications of HIFU are limited to pancreatic and liver cancer. It is a safe and well tolerated therapeutic modality. The exact place in the algorithm for the management of HCC remains to be defined. However, HIFU seems to add clear survival advantages over TACE alone and similar results when compared to RFA. Current evidence is insufficient and only very limited comparative prospective studies have been performed. The role in pancreatic cancer seems to be mostly palliative, with consistent effects when it comes to cancer-related pain relief. Further research is warranted to improve targeting accuracy and efficacy monitoring. Additional work is required to transfer this technology to appealing treatments such as endoscopic HIFU-based therapies.
Acknowledgements
The authors are grateful to Christopher Burel for his assistance in proofreading the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 2007;23:89-104. [Crossref] [PubMed]
- Zhou Y, Gao XW. Variations of bubble cavitation and temperature elevation during lesion formation by high-intensity focused ultrasound. J Acoust Soc Am 2013;134:1683-94. [Crossref] [PubMed]
- Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol 2011;2:8-27. [Crossref] [PubMed]
- Crouzet S, Chapelon JY, Rouviere O, et al. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients. Eur Urol 2014;65:907-14. [Crossref] [PubMed]
- Cavallo Marincola B, Pediconi F, et al. High-intensity focused ultrasound in breast pathology: non-invasive treatment of benign and malignant lesions. Expert Rev Med Devices 2015;12:191-9. [Crossref] [PubMed]
- Wang K, Zhu H, Meng Z, et al. Safety evaluation of high-intensity focused ultrasound in patients with pancreatic cancer. Onkologie 2013;36:88-92. [Crossref] [PubMed]
- Kennedy JE, Wu F, ter Haar GR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004;42:931-5. [Crossref] [PubMed]
- Dobrakowski PP, Machowska-Majchrzak AK, Labuz-Roszak B, et al. MR-guided focused ultrasound: a new generation treatment of Parkinson’s disease, essential tremor and neuropathic pain. Interv Neuroradiol 2014;20:275-82. [Crossref] [PubMed]
- Wang Q, Guo R, Rong S, et al. Noninvasive renal sympathetic denervation by extracorporeal high-intensity focused ultrasound in a pre-clinical canine model. J Am Coll Cardiol 2013;61:2185-92. [Crossref] [PubMed]
- Fukuda H, Numata K, Nozaki A, et al. Usefulness of US-CT 3D dual imaging for the planning and monitoring of hepatocellular carcinoma treatment using HIFU. Eur J Radiol 2011;80:e306-10. [Crossref] [PubMed]
- Zhang Y, Zhao J, Guo D, et al. Evaluation of short-term response of high intensity focused ultrasound ablation for primary hepatic carcinoma: utility of contrast-enhanced MRI and diffusion-weighted imaging. Eur J Radiol 2011;79:347-52. [Crossref] [PubMed]
- Leslie T, Ritchie R, Illing R, et al. High-intensity focused ultrasound treatment of liver tumours: post-treatment MRI correlates well with intra-operative estimates of treatment volume. Br J Radiol 2012;85:1363-70. [Crossref] [PubMed]
- Marescaux J, Diana M. Next step in minimally invasive surgery: hybrid image-guided surgery. J Pediatr Surg 2015;50:30-6. [Crossref] [PubMed]
- Marescaux J, Diana M. Inventing the future of surgery. World J Surg 2015;39:615-22. [Crossref] [PubMed]
- Moher D, Altman DG, Liberati A, et al. PRISMA statement. Epidemiology 2011;22:128; author reply. [Crossref] [PubMed]
- Li CX, Xu GL, Jiang ZY, et al. Analysis of clinical effect of high-intensity focused ultrasound on liver cancer. World J Gastroenterol 2004;10:2201-4. [PubMed]
- Wu F, Wang ZB, Jin CB, et al. Circulating tumor cells in patients with solid malignancy treated by high-intensity focused ultrasound. Ultrasound Med Biol 2004;30:511-7. [Crossref] [PubMed]
- Illing RO, Kennedy JE, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer 2005;93:890-5. [Crossref] [PubMed]
- Wu F, Wang ZB, Chen WZ, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005;235:659-67. [Crossref] [PubMed]
- Li JJ, Xu GL, Gu MF, et al. Complications of high intensity focused ultrasound in patients with recurrent and metastatic abdominal tumors. World J Gastroenterol 2007;13:2747-51. [Crossref] [PubMed]
- Li YY, Sha WH, Zhou YJ, et al. Short and long term efficacy of high intensity focused ultrasound therapy for advanced hepatocellular carcinoma. J Gastroenterol Hepatol 2007;22:2148-54. [Crossref] [PubMed]
- Leslie TA, Kennedy JE, Illing RO, et al. High-intensity focused ultrasound ablation of liver tumours: can radiological assessment predict the histological response? Br J Radiol 2008;81:564-71. [Crossref] [PubMed]
- Zhou Q, Zhu XQ, Zhang J, et al. Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol 2008;34:81-7. [Crossref] [PubMed]
- Li JJ, Gu MF, Luo GY, et al. Complications of high intensity focused ultrasound for patients with hepatocellular carcinoma. Technol Cancer Res Treat 2009;8:217-24. [Crossref] [PubMed]
- Noterdaeme O, Leslie TA, Kennedy JE, et al. The use of time to maximum enhancement to indicate areas of ablation following the treatment of liver tumours with high-intensity focused ultrasound. Br J Radiol 2009;82:412-20. [Crossref] [PubMed]
- Park MY, Jung SE, Cho SH, et al. Preliminary experience using high intensity focused ultrasound for treating liver metastasis from colon and stomach cancer. Int J Hyperthermia 2009;25:180-8. [Crossref] [PubMed]
- Zhang L, Fan WJ, Huang JH, et al. Comprehensive sequential interventional therapy for hepatocellular carcinoma. Chin Med J (Engl) 2009;122:2292-8. [PubMed]
- Zhang L, Zhu H, Jin C, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol 2009;19:437-45. [Crossref] [PubMed]
- Zhu H, Zhou K, Zhang L, et al. High intensity focused ultrasound (HIFU) therapy for local treatment of hepatocellular carcinoma: role of partial rib resection. Eur J Radiol 2009;72:160-6. [Crossref] [PubMed]
- Li C, Zhang W, Zhang R, et al. Therapeutic effects and prognostic factors in high-intensity focused ultrasound combined with chemoembolisation for larger hepatocellular carcinoma. Eur J Cancer 2010;46:2513-21. [Crossref] [PubMed]
- Numata K, Fukuda H, Ohto M, et al. Evaluation of the therapeutic efficacy of high-intensity focused ultrasound ablation of hepatocellular carcinoma by three-dimensional sonography with a perflubutane-based contrast agent. Eur J Radiol 2010;75:e67-75. [Crossref] [PubMed]
- Wang Y, Wang W, Wang Y, et al. Ultrasound-guided high-intensity focused ultrasound treatment for needle-track seeding of hepatocellular carcinoma: preliminary results. Int J Hyperthermia 2010;26:441-7. [Crossref] [PubMed]
- Fukuda H, Ito R, Ohto M, et al. Treatment of small hepatocellular carcinomas with US-guided high-intensity focused ultrasound. Ultrasound Med Biol 2011;37:1222-9. [Crossref] [PubMed]
- Jin C, Zhu H, Wang Z, et al. High-intensity focused ultrasound combined with transarterial chemoembolization for unresectable hepatocellular carcinoma: long-term follow-up and clinical analysis. Eur J Radiol 2011;80:662-9. [Crossref] [PubMed]
- Ng KK, Poon RT, Chan SC, et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg 2011;253:981-7. [Crossref] [PubMed]
- Xu G, Luo G, He L, et al. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol 2011;37:1993-9. [Crossref] [PubMed]
- Cheung TT, Chok KS, Lo RC, et al. High-intensity focused ultrasound ablation as a bridging therapy for hepatocellular carcinoma patients awaiting liver transplantation. Hepatobiliary Pancreat Dis Int 2012;11:542-4. [Crossref] [PubMed]
- Cheung TT, Chu FS, Jenkins CR, et al. Tolerance of high-intensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg 2012;36:2420-7. [Crossref] [PubMed]
- Cui L, Liu XX, Jiang Y, et al. Comparative study on transcatheter arterial chemoembolization, portal vein embolization and high intensity focused ultrasound sequential therapy for patients. Asian Pac J Cancer Prev 2012;13:6257-61. [Crossref] [PubMed]
- Fukuda H, Numata K, Nozaki A, et al. Findings of multidetector row computed tomography of HCCs treated by HIFU ablation. Eur J Radiol 2012;81:e239-43. [Crossref] [PubMed]
- Kim J, Chung DJ, Jung SE, et al. Therapeutic effect of high-intensity focused ultrasound combined with transarterial chemoembolisation for hepatocellular carcinoma <5 cm: comparison with transarterial chemoembolisation monotherapy--preliminary observations. Br J Radiol 2012;85:e940-6. [Crossref] [PubMed]
- Ni S, Liu L, Shu Y. Sequential transcatheter arterial chemoembolization, three-dimensional conformal radiotherapy, and high-intensity focused ultrasound treatment for unresectable hepatocellular carcinoma patients. J Biomed Res 2012;26:260-7. [Crossref] [PubMed]
- Chan AC, Cheung TT, Fan ST, et al. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg 2013;257:686-92. [Crossref] [PubMed]
- Cheung TT, Fan ST, Chan SC, et al. High-intensity focused ultrasound ablation: an effective bridging therapy for hepatocellular carcinoma patients. World J Gastroenterol 2013;19:3083-9. [Crossref] [PubMed]
- Cheung TT, Fan ST, Chu FS, et al. Survival analysis of high-intensity focused ultrasound ablation in patients with small hepatocellular carcinoma. HPB (Oxford) 2013;15:567-73. [Crossref] [PubMed]
- Fukuda H, Numata K, Nozaki A, et al. High-intensity focused ultrasound ablation assisted using color Doppler imaging for the treatment of hepatocellular carcinomas. Abdominal Imaging 2013;38:1263-8. [Crossref] [PubMed]
- Ma WH, Ho WY, Lai AS, et al. Characteristic uptake pattern of bone scintigraphy in patients with hepatocellular carcinoma following treatment with high-intensity focused ultrasound. Nucl Med Mol Imaging 2013;47:273-7. [Crossref] [PubMed]
- Rossi M, Raspanti C, Mazza E, et al. High-intensity focused ultrasound provides palliation for liver metastasis causing gastric outlet obstruction: case report. J Ther Ultrasound 2013;1:9. [Crossref] [PubMed]
- Zhu J, Zhu H, Mei Z, et al. High-intensity focused ultrasound ablation for treatment of hepatocellular carcinoma and hypersplenism: preliminary study. J Ultrasound Med 2013;32:1855-62. [Crossref] [PubMed]
- Cheung TT, Poon RT, Jenkins CR, et al. Survival analysis of high-intensity focused ultrasound therapy vs. transarterial chemoembolization for unresectable hepatocellular carcinomas. Liver Int 2014;34:e136-43. [Crossref] [PubMed]
- Chok KS, Cheung TT, Lo RC, et al. Pilot study of high-intensity focused ultrasound ablation as a bridging therapy for hepatocellular carcinoma patients wait-listed for liver transplantation. Liver Transpl 2014;20:912-21. [Crossref] [PubMed]
- Cheung TT, Poon RT, Yau T, et al. High-intensity focused ultrasound as a treatment for colorectal liver metastasis in difficult position. Int J Colorectal Dis 2012;27:987-8. [Crossref] [PubMed]
- Wu F, Chen WZ, Bai J, et al. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol 2001;27:1099-106. [Crossref] [PubMed]
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol 2004;11:1061-9. [Crossref] [PubMed]
- Wang S, Yang C, Zhang J, et al. First experience of high-intensity focused ultrasound combined with transcatheter arterial embolization as local control for hepatoblastoma. Hepatology 2014;59:170-7. [Crossref] [PubMed]
- Wang X, Sun J. High-intensity focused ultrasound in patients with late-stage pancreatic carcinoma. Chin Med J (Engl) 2002;115:1332-5. [PubMed]
- Wu F, Wang ZB, Zhu H, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology 2005;236:1034-40. [Crossref] [PubMed]
- Zhao H, Yang G, Wang D, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs 2010;21:447-52. [Crossref] [PubMed]
- Lee JY, Choi BI, Ryu JK, et al. Concurrent chemotherapy and pulsed high-intensity focused ultrasound therapy for the treatment of unresectable pancreatic cancer: initial experiences. Korean J Radiol 2011;12:176-86. [Crossref] [PubMed]
- Orgera G, Krokidis M, Monfardini L, et al. High intensity focused ultrasound ablation of pancreatic neuroendocrine tumours: report of two cases. Cardiovasc Intervent Radiol 2011;34:419-23. [Crossref] [PubMed]
- Sofuni A, Moriyasu F, Sano T, et al. The current potential of high-intensity focused ultrasound for pancreatic carcinoma. J Hepatobiliary Pancreat Sci 2011;18:295-303. [Crossref] [PubMed]
- Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas 2011;40:1080-6. [Crossref] [PubMed]
- Wang K, Chen Z, Meng Z, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia 2011;27:101-7. [Crossref] [PubMed]
- Li PZ, Zhu SH, He W, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hepatobiliary Pancreat Dis Int 2012;11:655-60. [Crossref] [PubMed]
- Orgera G, Krokidis M, Monfardini L, et al. Ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation in pancreatic metastasis from renal cell carcinoma. Cardiovasc Intervent Radiol 2012;35:1258-61. [Crossref] [PubMed]
- Wang K, Chen L, Meng Z, et al. High intensity focused ultrasound treatment for patients with advanced pancreatic cancer: a preliminary dosimetric analysis. Int J Hyperthermia 2012;28:645-52. [Crossref] [PubMed]
- Yuan Y, Shen H, Hu XY, et al. Multidisciplinary treatment with chemotherapy, targeted drug, and high-intensity focused ultrasound in advanced pancreatic carcinoma. Med Oncol 2012;29:957-61. [Crossref] [PubMed]
- Chen Q, Zhu X, Chen Q, et al. Unresectable giant pancreatic neuroendocrine tumor effectively treated by high-intensity focused ultrasound: a case report and review of the literature. Pancreatology 2013;13:634-8. [Crossref] [PubMed]
- Gao HF, Wang K, Meng ZQ, et al. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepato-gastroenterology 2013;60:1906-10. [PubMed]
- Ge HY, Miao LY, Wang JR, et al. Correlation between ultrasound reflection intensity and tumor ablation ratio of late-stage pancreatic carcinoma in HIFU therapy: dynamic observation on ultrasound reflection intensity. ScientificWorldJournal 2013;2013:852874.
- Ge HY, Miao LY, Xiong LL, et al. High-intensity focused ultrasound treatment of late-stage pancreatic body carcinoma: optimal tumor depth for safe ablation. Ultrasound Med Biol 2014;40:947-55. [Crossref] [PubMed]
- Sofuni A, Moriyasu F, Sano T, et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J Gastroenterol 2014;20:9570-7. [PubMed]
- Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP 2009;10:123-9. [PubMed]
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol 2010;195:W245-52. [Crossref] [PubMed]
- Jung SE, Cho SH, Jang JH, et al. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdominal Imaging 2011;36:185-95. [Crossref] [PubMed]
- Orgera G, Monfardini L, Della Vigna P, et al. High-intensity focused ultrasound (HIFU) in patients with solid malignancies: evaluation of feasibility, local tumour response and clinical results. La Radiologia medica 2011;116:734-48. [Crossref] [PubMed]
- Seo YY. Whole-Body Bone Scan Findings after High-Intensity Focused Ultrasound (HIFU) Treatment. Nucl Med Mol Imaging 2011;45:268-75. [Crossref] [PubMed]
- Zhou Y. High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pract 2014;2014:205325.
- Wu F. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol 2014;20:16480-8. [Crossref] [PubMed]
- Haen SP, Pereira PL, Salih HR, et al. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol 2011;2011:160250.
- Unga J, Hashida M. Ultrasound induced cancer immunotherapy. Adv Drug Deliv Rev 2014;72:144-53. [Crossref] [PubMed]
- Liu HL, Hsieh HY, Lu LA, et al. Low-pressure pulsed focused ultrasound with microbubbles promotes an anticancer immunological response. J Transl Med 2012;10:221. [Crossref] [PubMed]
- Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. J Gastrointest Oncol 2011;2:175-84. [PubMed]
- Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol 2007;102:430-8. [Crossref] [PubMed]
- Wrenn SP, Dicker SM, Small EF, et al. Bursting bubbles and bilayers. Theranostics 2012;2:1140-59. [Crossref] [PubMed]
- Liao M, Huang J, Zhang T, et al. Transarterial chemoembolization in combination with local therapies for hepatocellular carcinoma: a meta-analysis. PloS One 2013;8:e68453. [Crossref] [PubMed]
- Auboiroux V, Petrusca L, Viallon M, et al. Respiratory-gated MRgHIFU in upper abdomen using an MR-compatible in-bore digital camera. Biomed Res Int 2014;2014:421726.
- Nicolau S, Soler L, Mutter D, et al. Augmented reality in laparoscopic surgical oncology. Surg Oncol 2011;20:189-201. [Crossref] [PubMed]
- D’Agostino J, Diana M, Soler L, et al. 3D Virtual Neck Exploration Prior to Parathyroidectomy. N Engl J Med 2012;367:1072-3. [Crossref] [PubMed]
- Mutter D, Soler L, Marescaux J. Recent advances in liver imaging. Expert Rev Gastroenterol Hepatol 2010;4:613-21. [Crossref] [PubMed]
- Pessaux P, Diana M, Soler L, et al. Towards cybernetic surgery: robotic and augmented reality-assisted liver segmentectomy. Langenbecks Arch Surg 2015;400:381-5. [Crossref] [PubMed]
- D’Agostino J, Diana M, Vix M, et al. Three-dimensional metabolic and radiologic gathered evaluation using VR-RENDER fusion: a novel tool to enhance accuracy in the localization of parathyroid adenomas. World J Surg 2013;37:1618-25. [Crossref] [PubMed]
- D’Agostino J, Wall J, Soler L, et al. Virtual neck exploration for parathyroid adenomas: a first step toward minimally invasive image-guided surgery. JAMA Surg2013;148:232-8; discussion 8. [Crossref] [PubMed]
- Marzano E, Piardi T, Soler L, et al. Augmented reality-guided artery-first pancreatico-duodenectomy. J Gastrointest Surg 2013;17:1980-3. [Crossref] [PubMed]
- Diana M, Pessaux P, Marescaux J. New technologies for single-site robotic surgery in hepato-biliary-pancreatic surgery. J Hepatobiliary Pancreat Sci 2014;21:34-42. [Crossref] [PubMed]
- Pessaux P, Diana M, Soler L, et al. Robotic duodenopancreatectomy assisted with augmented reality and real-time fluorescence guidance. Surg Endosc 2014;28:2493-8. [Crossref] [PubMed]
- Hostettler A, Nicolau SA, Remond Y, et al. A real-time predictive simulation of abdominal viscera positions during quiet free breathing. Prog Biophys Mol Biol 2010;103:169-84. [Crossref] [PubMed]
- Haouchine N, Dequidt J, Berger MO, et al. Deformation-based augmented reality for hepatic surgery. Stud Health Technol Inform 2013;184:182-8. [PubMed]
- Umale S, Chatelin S, Bourdet N, et al. Experimental in vitro mechanical characterization of porcine Glisson’s capsule and hepatic veins. J Biomech 2011;44:1678-83. [Crossref] [PubMed]
- Diana M, Chung H, Liu KH, et al. Endoluminal surgical triangulation: overcoming challenges of colonic endoscopic submucosal dissections using a novel flexible endoscopic surgical platform: feasibility study in a porcine model. Surg Endosc 2013;27:4130-5. [Crossref] [PubMed]
- Konishi K, Hashizume M. Future perspective of gastrointestinal ‘intelligent’ endoscopy. Nihon Rinsho 2010;68:1279-84. [PubMed]
- Li T, Khokhlova T, Maloney E, et al. Endoscopic high-intensity focused US: technical aspects and studies in an in vivo porcine model (with video). Gastrointest Endosc 2015;81:1243-50. [Crossref] [PubMed]


