Cystic lymphangioma of the pancreas: a hard diagnostic challenge between pancreatic cystic lesions—review of recent literature
Introduction
The widespread use of abdominal ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) has resulted in an increased identification of asymptomatic pancreatic lesions defined “incidentalomas” (1). In fact Bauer (2) had reported an increasing frequency in the detection and evaluation of pancreatic cystic lesions (PCLs) over the last three decades. The term PCLs denotes a histologically heterogeneous collection of neoplasms showing a wide spectrum of diagnoses, ranging from completely benign to potentially malignant, carcinoma in situ, frankly invasive and malignant. The management of patients with PCLs can be challenging and varies considerably among the different subtypes of PCLs. Also the treatment is extremely various; it consists from resection to simple observation and more or less tight follow up. It is of extreme importance to identify suspicious features indicating potential or certain malignancy in order to select the appropriate treatment. However, the conventional radiology has been generally inadequate for the evaluation (3), and pancreatic cystic lymphangioma have been classically diagnosed on histopathological examination following surgical excision. Lymphangioma of the pancreas is extremely rare accounting for less than 1% of these tumours (4). It may clinically mimic pancreatic carcinoma and should be considered as a differential diagnosis in any patient with PCL. We present a rare case of a pancreatic lymphangioma in an adult, diagnosed only after histopathological examination and a review of the recent international literature.
Case presentation
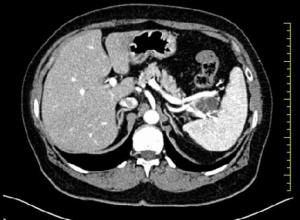
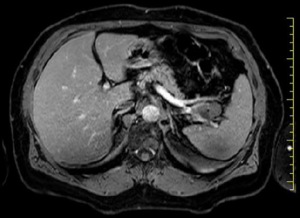
A 67-year-old woman with no history of systemic disease was referred to our hospital due to an incidental US finding of an intra-abdominal cyst. At the admission, the patient was asymptomatic, without abdominal fullness, tenderness or jaundice. The patient had no history of heavy alcohol use. There was no personal or family history of pancreatic or hepatobiliary disease. Laboratory findings were all within normal limits. Tumor markers were negative: CEA, CA19-9 and pancreatic enzymes were all normal. The CT (Figure 1) scan revealed a 32 mm × 22 mm multiloculate solid cystic mass with thin septa within the lesion in the tail of pancreas, adjacent to splenic vein and splenic artery. This mass did not show signs of infiltration or compression to adjacent structures. No soft tissue component and no calcification were found. Based on CT findings, pancreatic serous cystadenoma was suspected. MRI study further confirmed a pancreatic mass with fluid content and thin septa with mild enhancement (Figure 2). Therefore, due to the impossibility to achieve a definitive diagnosis and to cannot exclude with certainty a malignant origin: we have decided to submit the patient to a laparotomy surgery through a left subcostal approach extended to the right side. Intra-operatively it was confirmed the presence of a cystic mass which was located in the pancreas tail extending to the splenic hilum. To perform an en-bloc resection of the mass we have been obligated to execute the spleen mobilization. It was impossible to execute a spleen preserving technique because splenic vessels were severely adherent to the lesion. Therefore, we have performed a distal splenopancreatectomy with a vascular GIA mechanic stapler 45 mm. The definitive histological examination has revealed a cystic lymphangioma of pancreatic origin that compressed splenic hilum vessels. Her postoperative course was uneventful. Nowadays, two years after the operation, the patient is disease free without signs of recurrence at the US and CT follow-up.
Materials and methods
In light of this rare clinical case and its diagnostic difficulty, we have decided to perform a review of the literature to evaluate the role of imaging and the radiological characteristics of pancreatic lymphangioma in order to reach the correct pre-operative diagnosis. The review tries to identify the features described in literature of the pancreatic lymphangioma. We have performed a PubMed research of the world literature between January 1st 2000, to November 31st 2017 (last 17 years), using the keyword [Lymphangioma pancreas], [diagnosis], [CT lymphangioma] and [MRI lymphangioma]. We have found 158 articles, of which about 100 were case reports. All papers in English and Italian reporting the radiological characteristics were included. We have excluded also articles concerning children, the cases in which the radiological aspects were not reported, and the hemolymphangioma of the pancreas. In case of multiple publications on the same group of patients, only the most recent and complete paper was retained. All types of study design were included. There was no restriction on the patient number. The following data were analysed: year, sex, age (year), position in pancreas, size (cm), presence of septa, imaging modalities positive for septa, presence of solid part, loculation and wall thickness.
Results
Based on our search criteria, we have identified 31 pancreatic lymphangioma in literature reporting their imaging characteristics (Table 1). Patients were 9 males and 22 females with a median age of 43.22 and a standard deviation (SD) of 15.06. The anatomical location was: head in 10 patients; body in 8; tail in 12; body/tail in 1. The medium size in centimetres (cm) of the lesion was 9.53 cm with a SD 5.55 cm. In 21 patients it was described the presence of septa, which can be thin or thick. CT imaging was positive for septa in 16 patients, MRI-imaging was positive in 10, and both CT and MRI were positive in 3. CT was negative to identify the septa in 10 patients, MRI in 3; not reported images for septa in 2. The lesion appeared multiloculated cysts in 23 patients, uniloculate in 6, not reported in 2. The wall thickness was thin in 9 cases, thick in 2 and not reported in 20.
In only one case (5) there is a solid part, this could be explain because patient was affected of acute recurrent pancreatitis. As shown in the Table 1, imaging alone is not specific and not able to exclude cystadenomas or other cystic neoplasm. In 4 cases the pancreatic lymphangioma has been diagnosed by EUS-FNA and the authors decided to keep following the patients with image studies.

Full table
In our review 26 patients have undergone different surgical interventions: 11 patients were treated with a wedge resection of the cyst [5 of them was a video laparoscopic (VLS) wedge resection]; 7 patients underwent distal-pancreatectomy associated with splenectomy (1 of them was VLS distal-spleno-pancreatectomy) and 1 was a distal-pancreatectomy with spleen preserving technique; 5 surgeons have performed a Pancreaticduodenectomy and 2 performed Whipple procedure. No recurrence was observed in all the cases treated surgically.
Discussion
Lymphangiomas are benign, slow-growing cystic lesions most commonly affecting the paediatric population and less frequently the adults with a female predominance. The majority are found in the neck (75%) and axilla (20%), with many other sites reported in literature including the pleura, pericardium, groin, bones, liver, spleen, pancreas, colon, omentum, and genital organs. Less than 1% of lymphangiomas are found as distal pancreatic, peripancreatic, or retroperitoneal masses. The first reported case of a pancreatic lymphangioma was published in 1913 by Koch (35). Since then, to our knowledge 82 cases have been reported in the literature (6). Some patients usually present with abdominal pain and nausea (7-9,36); Erguney (10) and Schneider (11) described patients with a palpable abdominal mass. In some cases this pathology was found following an acute abdomen pain/syndrome (12-14). Wang (37) described a case with acute abdominal pain associated to intrahepatic and extrahepatic duct dilatation. However, in most cases lymphangiomas are symptomless and discovered as an incidental finding (6,15,16) as in our case. Based on the imaging findings the correct differential diagnosis of a pancreatic cystic lymphangioma should exclude: pseudocyst, cystadenoma, other congenital cysts, and cystic ductal carcinoma. US reveals a complex cystic mass with internal septa or internal echoes with calcifications seen rarely. CT shows a well-circumscribed, thin-walled, low-density, and homogenous cystic mass that maybe unilocular or multilocular with thin-enhancing endocystic septae (38). These features are similar to cystadenomas that occur far more frequently. On MRI, the lesion appears hypodense on T1- sequence and hyperintense on T2. MRI is more useful to exclude communication between the cystic lesion and pancreatic duct when compared to CT (39).
On the other hand, only six cases in the literature (15,17-20,40) reported a preoperative diagnosis with endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). For the diagnosis of pancreatic cystic lymphangioma the role of the EUS-FNA is less defined with respect to rare cystic diseases, but has been evolving over the last decade. The important diagnostic role of EUS-FNA is due to the acquisition of cytological and biochemical markers of cystic fluid. In our case, although CT and MRI images indicated diagnosis of serous cystadenoma, we could not exclude a potential malignant lesion (such as mucosal cystadenoma) and due to the small dimension of the lesion and its tight relationship with the splenic hilum vessels, we decided to do not perform the EUS-FNA. For this reason, we decided to remove the whole mass. An en-bloc resection is the treatment of choice.
Obviously, an incomplete excision may results in recurrence (36). According to Gerry et al. (41) the observation is recommended for typical benign lesions (serous cystadenoma), and on the other hand, upfront resection is recommended for likely malignant lesions such as intraductal papillary mucinous neoplasm (IPMN), mucinous cystadenoma, solid pseudopapillary tumor, and cystic pancreatic neuroendocrine tumors). In general, resection should be considered whenever the risk of malignancy is higher than the risk of the operation. No malignant transformation of pancreatic cystic lymphangiomas has been reported in the literature.
Conclusions
Although pancreatic lymphangioma is rare, we believe that it should be considered in the differential diagnosis of cystic-solid tumors of the pancreas, especially in women, particularly when there is no sufficient evidence for diagnosing cystadenoma, cystadenocarcinoma or some other relatively common disease of the pancreas. Although traditional radiology is not yet able to give a specific diagnosis, it can help to identify and suspect this possibility of diagnosis. Some authors advocate the usefulness of EUS-FNA in the preoperative work-up of suspected lymphangiomas. The endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) can have a potential role to reach the correct diagnosis, but it should be better validate, in fact only in six cases it was applied till today.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Okun SD, Lewin DN. Non-neoplastic pancreatic lesions that may mimic malignancy. Semin Diagn Pathol 2016;33:31-42. [Crossref] [PubMed]
- Bauer F. Pancreatic cystic lesions: diagnostic, management and indications for operation. Chirurgia (Bucur) 2017;112:97-109. [Crossref] [PubMed]
- Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR Am J Roentgenol 2007;189:648-56. [Crossref] [PubMed]
- Leung TK, Lee CM, Shen LK, et al. Differential diagnosis of cystic lymphangioma of the pancreas based on imaging features. J Formos Med Assoc 2006;105:512-7. [Crossref] [PubMed]
- Tadic M, Cabrijan Z, Stoos-Veic T, et al. Lymphangioma as a rare cause of acute recurrent pancreatitis. Endoscopy 2014;46 Suppl 1 UCTN:E598-9.
- Fujii M, Saito H, Yoshioka M, et al. Rare case of Pancreatic Cystic Lymphamgioma. Intern Med 2018;57:813-7. [Crossref] [PubMed]
- Colovic RB, Grubor NM, Micev MT, et al. Cystic lymphangioma of the pancreas. World J Gastroenterol 2008;14:6873-5. [Crossref] [PubMed]
- Fahimi H, Faridi M, Gholamin S, et al. Cystic lymphangioma of the pancreas: diagnostic and therapeutic challenges. JOP 2010;11:617-9. [PubMed]
- Dalla Bona E, Beltrame V., Blandamura S, et al. Huge cystic lymphangioma of the pancreas mimicking pancreatic cystic neoplasm. Case Rep Med 2012;2012. [Crossref] [PubMed]
- Erguney S, Teksoz S, Erdamar S, et al. Extended pancreaticoduodenectomy for a huge cystic cavernous lymphangioma: a case report. JOP 2012;13:289-91. [PubMed]
- Schneider G, Seidel R, Altmeyer K, et al. Lymphangioma of the pancreas and the duodenal wall: MR imaging findings. Eur Radiol 2001;11:2232-5. [Crossref] [PubMed]
- Ghatak S, Ray S, Sanyal S, et al. An unusual cause of acute abdomen in adults: giant cystic lymphangioma of the pancreatic head. A clinical case and literature review. JOP 2011;12:266-70. [PubMed]
- Kawaguchi K, Motoi F, Ohtsuka H, et al. Cystic Lymphangioma of the Pancreas with Spontaneous Rupture: Report of a Case. Case Rep Gastroenterol 2011;5:288-94. [Crossref] [PubMed]
- Goh BK, Tan YM, Yap WM, et al. Hemorrhage into a pancreatic lymphangioma after blunt trauma mimicking a post-traumatic pancreatic pseudocyst. J Trauma 2006;61:992-4. [Crossref] [PubMed]
- Coe AW, Evans J, Conway J. Pancreas cystic lymphangioma diagnosed with EUSFNA. JOP 2012;13:282-4. [PubMed]
- Santes O, Chan C. Cystic lymphangioma of the pancreas: a rare entity. J Gastrointest Surg 2016;20:2100-1. [Crossref] [PubMed]
- Bhatia V, Rastogi A, Saluja S, et al. Cystic pancreatic lymphangioma. The first report of a preoperative pathological diagnosis by endoscopic ultrasound-guide cyst aspiration. JOP 2011;12:473-6. [PubMed]
- Hussain I, Ang TL. Cystic pancreatic lymphangioma diagnosed with endoscopic ultrasound-guided fine needle aspiration. Endosc Ultrasound 2017;6:136-9. [Crossref] [PubMed]
- Black T, Guy CD, Burbridge RA. Retroperitoneal cystic lymphangioma diagnosed by endoscopic ultrasound-guided fine needle aspiration. Clin Endosc 2013;46:595-7. [Crossref] [PubMed]
- Carvalho D, Costa M, Russo P, et al. Cystic pancreatic lymphangioma: diagnostic role of endoscopic ultrasound. GE Port J Gastroenterol 2016;23:254-8. [Crossref] [PubMed]
- Koenig TR, Loyer EM, Whitman GJ, et al. Cystic lymphangioma of the pancreas. AJR Am J Roentgenol 2001;177:1090. [Crossref] [PubMed]
- Igarashi A, Maruo Y, Ito T, et al. Huge Cystic Lymphangioma of the pancreas: report of a case. Surg Today 2001;31:743-6. [Crossref] [PubMed]
- Chung JC, Kim HC, Chu CW, et al. Huge cystic lymphangioma of the pancreas. Can J Surg 2009;52:E303-5. [PubMed]
- Yüceyar S, Kapan M, Ozben V, et al. Pancreatic cystic lymphangioma: Report of a case. Turk J Gastroenterol 2009;20:228-30. [Crossref] [PubMed]
- Kim HH, Park EK, Seoung JS, et al. Cystic lymphangioma of the pancreas mimicking pancreatic pseudocyst. J Korean Surg Soc 2011;80:S55-8. [Crossref] [PubMed]
- Sohn BK, Cho CH, Chae HD. Cystic lymphangioma of the pancreas. J Korean Surg Soc 2011;81:141-5. [Crossref] [PubMed]
- Margiotta M, Marrano N, Monari F, et al. Approccio combinato laparoscopico e minilaparotomico in un voluminoso linfangioma cistico pancreatico. Giornale di Chirurgia 2010;31:75-9. [PubMed]
- Gureş N, Gurluler E, Alim A, et al. Cystic pancreatic lymphangioma. Rare Tumors 2012;4. [Crossref] [PubMed]
- Mousavi SR, Moradi A, Sobhiyeh MR, et al. A patient with cystic lymphangioma in pancreas. Gastroenterol Hepatol Bed Bench 2013;6:159-64. [PubMed]
- DI Marco M, Grassi E, Vecchiarelli S, et al. Retroperitoneal lymphangioma: a report of 2 cases and a review of the literature regarding the differential diagnoses of retroperitoneal cystic masses. Oncol Lett 2016;11:3161-6. [Crossref] [PubMed]
- Bihari C, Rastogi A, Rajesh S, et al. Cystic lymphangioma of pancreas. Indian J Surg Oncol 2016;7:106-9. [Crossref] [PubMed]
- Ishibashi Y, Tsujimoto H, Kouzu K, et al. Laparoscopic resection of a huge retroperitoneal cystic lymphangioma after successful reduction of tumor size with a double balloon catheter. Int J Surg Case Rep 2015;11:8-10. [Crossref] [PubMed]
- Sato T, Matsuo Y, Shiga K, et al. Laparoscopic resection of retroperitoneal lymphangioma around the pancreas: a case report and review of the literature. J Med Case Rep 2015;9:279. [Crossref] [PubMed]
- Talaiezadeh A, Ranjbari N, Bakhtiari M. Pancreatic lymphangioma as a rare pancreatic mass: a case report. Iran J Cancer Prev 2016;9. [PubMed]
- Koch K. Beiträge zur Pathologie der Bauchspeicheldrüse. Virchows Arch. path Anat 1913;214:180-206.
- Mansour NM, Salyers WJ Jr. Recurrence of a Pancreatic Cystic Lymphangioma After Diagnosis and Complete Drainage by Endoscopic Ultrasound with FineNeedle Aspiration. JOP 2013;14:280-2. [PubMed]
- Wang Y, Tang SS, Ma Y. Cystic lymphangioma of the pancreas with congenital intrahepatic duct dilatation and choledochal cyst. J Clin Ultrasound 2011;39:104-7. [Crossref] [PubMed]
- Macin G, Hekimoglu K, Uner H, et al. Pancreaticcystic lymphangioma: diagnostic approach with MDCT and MR imaging. JBR-BTR 2014;97:97-9. [PubMed]
- Afzal S, Masroor I, Shafq G. Pancreatic lymphangioma. J Coll Physicians Surg Pak 2014;24:60-1. [PubMed]
- Dries AM, McDermott J. Diagnosis of cystic lymphangioma of the pancreas with endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol 2008;103:1049-50. [Crossref] [PubMed]
- Gerry JM, Poultsides GA. Surgical Management of pancreatic Cyst: a shifting paradigm toward selective resection. Dig Dis Sci 2017;62:1816-26. [Crossref] [PubMed]


