As technology has advanced over the last decade, so have the treatment options for patients with type 1 diabetes (T1D). The goal of these technological advances is improved regulation of glucose levels to more closely resemble glucose metabolism in patients without diabetes. Perhaps even more importantly, new technologies seek to improve quality of life by decreasing the burden of disease for patients and those involved in their care.1 Recent advances have allowed for some patients to achieve tighter glycaemic control and reduced frequency of hypoglycaemia, a combination that allows for the potential reduction of both long term and short term complications.2,3 However, with gains in both the quality and number of current technologies come challenges for providers to stay up to date and prescribe the best system to fit each patient’s specific needs and lifestyle. Providers must be aware of, and understand, all currently available treatment options in order to assist patients in making informed decisions on which technologies they should utilise. The following review summarises advances in glucose meters, continuous glucose monitors (CGMs), insulin pumps, automated insulin delivery systems, Bluetooth insulin pens, alternate glucagon administration and patient-facing diabetes software. Refer to Table 1 and Table 2 for specific details of currently available CGMs and insulin pumps.
Glucose meters
The first blood glucose meter was invented in 1971 and was rightfully celebrated as a marked improvement over urine glucose monitoring. Within a few years, self-monitoring of blood glucose (SMBG) became a standard of care. Contemporary blood glucose meters are small, provide results in 5 seconds and use just 3–5 µL of blood. In addition, many meters now have the capability to connect wirelessly via radiofrequency or Bluetooth to insulin pumps or to smartphone applications.4 When a glucose meter communicates a blood glucose value to an insulin pump, the steps required to administer insulin or calibrate a connected CGM are minimised as the user does not have to manually enter the value. In addition, Bluetooth devices create the possibility of interoperable and manufacturer agnostic devices. Apps are available that can automatically receive blood glucose values, identify trends, and share data with the provider between clinic visits. These apps can also track food intake, exercise and other data.
Continuous glucose monitors
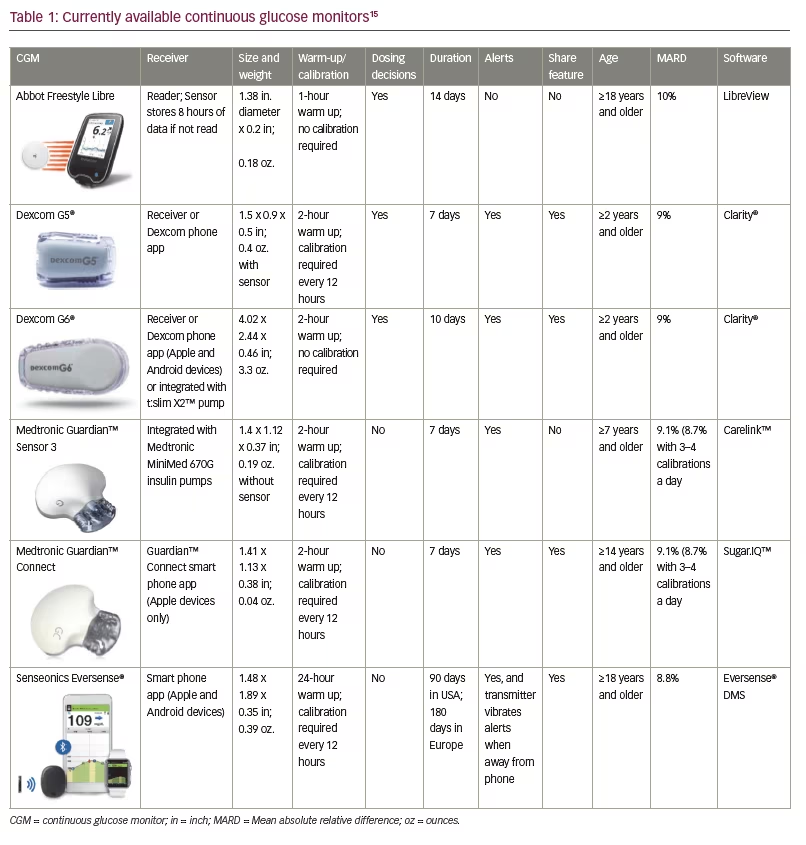
CGMs, first released in 1999, allow patients and their healthcare providers to obtain a more complete understanding of glycaemic variability by examining glucose levels every 5–15 minutes throughout the day. As such, CGMs allow patients and providers to assess trends, patterns and percentages of time spent in or out of glycaemic targets. Patients can account for rapidly changing blood glucose levels when determining corrections for hyper or hypoglycaemia. CGMs provide the benefits of improved control, decreased time spent in hyper or hypoglycaemia, and optimally, decreased risk of complications secondary to T1D.5,6 CGMs have been associated with the most substantial improvement in glycated haemoglobin (HbA1c) in patients who had a higher baseline HbA1c and in those with the most frequent sensor use.5,6 In a 2011 meta-analysis, overall mean HbA1c decreased by 0.3% in patients using a CGM compared to SMBG. Results also showed that for each 1-day-a-week increase in CGM usage, the effect on HbA1c increased by 0.15%.7 Each of these systems requires a sensor that is inserted subcutaneously and measures interstitial fluid glucose values; data are then relayed to a transmitter.8 CGM accuracy is often evaluated using the mean absolute relative difference (MARD), the difference between the CGM reading and the blood glucose level measured by a reference system.9 Current CGMs are able to achieve a MARD under 10%.
Medtronic
Medtronic (Dublin, Ireland) released the first CGM, the Sof-sensor™, in 1999. While revolutionary for home glucose monitoring, users noted discomfort with insertion and wear, and accuracy was not optimal with a MARD of 19.7%.9 Medtronic next released the Enlite™ sensor in 2011. This CGM added predictive alerts to notify users of impending hyper- or hypoglycaemic events.10 The CGM data are available on the integrated Medtronic Paradigm®, 530G, and 630G insulin pumps. A finger-stick calibration is required every 12 hours, and the CGM communicates wirelessly with Ascensia Diabetes Care’s (Basel, Switzerland) Contour™ Next Link glucometer. The CGM data can be uploaded to data management systems such as Glooko, Tidepool, Diasend® or Carelink™.11 Results for the Enlite have been mixed as it provides an improved but still suboptimal MARD of 17% and persistent issues with inaccuracy measuring glucose in the hypoglycaemic range.9,12
The Guardian™ 3 CGM was released in 2017 and is integrated with the Medtronic 670G insulin pump as a component of the first hybrid closed-loop insulin delivery and glucose monitoring system. Calibrations are required every 12 hours and allow for a MARD of 9.1–10.6% depending on the site location. Improved accuracy with a MARD of 8.7–9.6% can be achieved with 3–4 calibrations daily.13,14 The Contour Next Link 2.4 glucometer communicates wirelessly with the system and can also utilise a USB connection to upload data from the pump to the Carelink data management software. Medtronic CGMs, as of yet, are not approved by the United States Food and Drug Administration (FDA) for insulin dosing decisions but continue to require confirmation with a glucometer. This CGM is approved for 7 days of use.15
The Guardian™ Connect CGM was released in 2018 and is Medtronic’s first standalone consumer CGM and can be used by patients on multiple daily injections or other insulin pumps. This system utilises the Guardian 3 sensor and provides predictive alerts up to 60 minutes in advance of potential hypoglycaemic or hyperglycaemic events. CGM data and alerts are viewed on a smart device via Medtronic’s Guardian Connect app and are automatically uploaded to Carelink. Users can share their data and alerts with others via this app as well. Those who use this system will have access to Medtronic’s Sugar.IQ™ assistant on their smart device, which uses artificial intelligence software powered by IBM Watson to make recommendations for changes to the patient’s regimen or behavior.15
Dexcom
Dexcom (San Diego, California, USA) began developing its CGM in 1999 and released the Dexcom STS CGM system in 2006 and the Dexcom SEVEN® CGM in 2009. The Dexcom SEVEN CGM had a MARD of 16.5% and was able to be worn for up to 7 days.9 The Dexcom G4®, with an improved MARD of 12.6%, was released in 2012.9,16 The G4 CGM required a finger-stick calibration every 12 hours and had pre-set hypoglycaemia alarms and other customisable alerts. Blood glucose values were transmitted to a separate handheld receiver that was kept within 20 feet of the transmitter. This system later became compatible with the Dexcom Share® 2 app which could then be used to share the data with up to five other people via the Dexcom Follow® app. Data could be uploaded with multiple data management systems such as Tidepool, Glooko, Diasend, and Clarity®.11
The Dexcom G5® Mobile system was released in 2015 and was approved for patients aged ≥2 years.17 With a MARD of 9%, the G5 was the first CGM approved for non-adjunctive use, requiring finger-sticks for calibration but allowing for CGM data to be used in making insulin dosing decisions.18 The G5 transmitter can be paired with a rechargeable G5 receiver or with the Dexcom G5 App on compatible smart devices. Data can be shared with others via the Dexcom app or uploaded to data management systems.15
The Dexcom G6® received FDA approval in March 2018 and is factory calibrated, no longer requiring finger-stick calibrations. While shedding the requirement for calibration, the G6 has a MARD of 9%.19 As with the G5, users are able to make treatment decisions using CGM data without having to confirm their blood glucose level using a glucose meter. The G6 has the capability to function as a standalone CGM or to be integrated with compatible insulin pumps as companies plan for future advances. The sensor can now be worn for up to 10 days and there is no interference with acetaminophen as with earlier sensors.20 Data can be shared or uploaded using the Dexcom G6 and Clarity apps.21 It is approved for patients ≥2 years of age.15
Dexcom and Verily (South San Francisco, California, USA) are anticipated to launch the G7 CGM in 2020. This CGM is planned to be thinner, have a disposable sensor and transmitter, and to be more affordable. It will continue to communicate directly with smart phones and will likely have extended wear of 14 days.22
Abbott
Abbott’s (Chicago, Illinois, USA) FreeStyle Libre was approved in 2016 and is a water-resistant, disposable CGM that is factory calibrated and provides a MARD of 10%.17 While the Libre was initially approved for 10 days of use, the duration was recently extended to 14 days of use and now requires only 1 hour to warm up.23 The Libre is referred to as a ‘flash glucose monitoring system’ as the data is not displayed continuously but is stored and obtained by scanning the device with the reader which also contains a glucose meter. As such, there are no real time alarms or alerts.24 It is important to note, however, that this device is typically more affordable than other CGMs and is available in most major pharmacies with a prescription. The device has a rechargeable battery and can be uploaded with the Tidepool data management software, the Abbott Libre software, or with the LibreView app on compatible devices.15 This device is approved in patients ≥18 years old in the US and ≥4 years old in Europe.
The Libre 2 CGM is currently available in Germany and is planned to be available in other parts of Europe in the near future. Bluetooth capability and blood glucose alarms have been integrated while maintaining the original model’s small size and affordability.25
Senseonics
Senseonics’ (Germantown, Maryland, USA) Eversense® CGM is an implantable CGM that was recently approved in 2018 for up to 90 days of use in the United States (180 days in Europe). The sensor is inserted under the skin by procedure in a medical office and therefore can be performed at each quarterly appointment. Insertion and removal require both local anaesthesia and an incision into the skin which may cause apprehension for patients. Calibrations are required every 12 hours and provide a MARD of 8.8%.20 The transmitter contains a rechargeable battery and is worn over the skin but can be disconnected without disturbing the underlying sensor. Blood glucose readings are viewed on a smartphone app, and the data is updated every 5 minutes. The app contains predictive alerts and the transmitter itself vibrates to alert the user of hyper- or hypoglycaemia.26 While Senseonics is seeking FDA approval for adjunctive use for this CGM, the use of a meter is currently required to make treatment decisions. This device utilises Bluetooth technology making it interoperable as well.
Insulin injections
In 2007 and 2014, Eli Lilly (Indianapolis, Indiana, USA) and Novo Nordisk (Bagsværd, Denmark), respectively, released insulin pens with a memory function that logs the date, time, and amount of insulin given for several previous doses. These insulin pens work in tandem with pen caps that log the data. The Timesulin® insulin pen cap was released in 2011, fits all insulin pens and shows users of the last insulin injection administered. Gocap was released more recently and fits on most insulin pens, connecting via Bluetooth to a smartphone app and allowing users to track the date, time, and amount of insulin doses. The Gocap app can also be used to log diet and exercise, and remind patients of missed or abnormal insulin doses. Patients can share data from the app with family and healthcare providers as needed, which is beneficial when confirming appropriate administration and adjusting insulin regimens.27 The InPen is another smart pen device that uses insulin cartridges in a reusable pen and links to a smartphone app that provides a bolus advisor and insulin on-board calculations. Dexcom CGM links to the InPen app to provide trends and blood glucose averages as well.20 Barriers to compliance with insulin administration including inappropriate dosing and remembering to administer injections can be overcome with these devices.28
Inhaled insulin
Afrezza® (Westlake Village, California, USA), an ultra-rapid-acting inhaled insulin, is comprised of Technosphere insulin powder (TI) and was FDA approved in 2014. Afrezza is currently the only inhaled insulin on the market.20 Upon inhalation, Afrezza’s TI particles are dissolved in the alveoli and insulin is systemically absorbed. Afrezza peaks in 53 minutes, has a shorter duration of action than subcutaneous insulin and has demonstrated fewer episodes of post prandial hyper- or hypoglycaemia. Afrezza is available in 4-, 8-, or 12-unit fixed doses which can pose dosing challenges for some patients. Afrezza is used for meal time boluses and subcutaneous basal insulin is still required. Afrezza is contraindicated for patients with asthma or chronic lung disease as it has been associated with bronchospasm. Periodic monitoring of pulmonary function is required for Afrezza users.29 Data regarding Afrezza utilisation in paediatric patients is limited, though paediatric safety studies are underway. Afrezza is currently only approved for adults.
Insulin pumps
The first insulin pump, the Biostator, was invented in 1974 but was so large it was only used during diabetic ketoacidosis (DKA) and hospitalisations.30 The first commercial insulin pump was released by MiniMed™ (Medtronic) in the 1980s. While the last 4 decades have seen remarkable improvements in these devices, there is no doubt that the next 10 years will be characterised by a near total transformation of the interaction patients and doctors have with insulin pumps and CGMs. Studies have shown that insulin pumps can provide many benefits including improved HbA1c, decreased variability of blood glucose levels, and decreased episodes of hypoglycaemia without increasing the rate of DKA regardless of baseline HbA1c.31
Medtronic
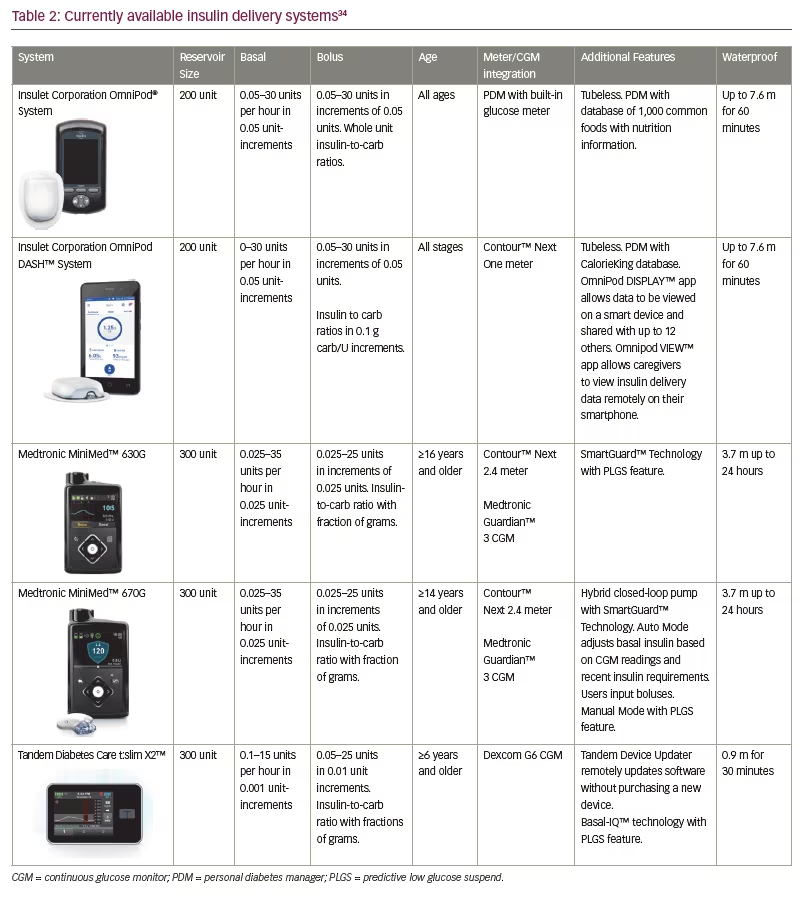
MiniMed was acquired by Medtronic in 2001 and continued developing new technologies including a wirelessly connected insulin pump and glucose meter in 2003, and Carelink management software in 2004. The Paradigm Real-Time Revel™ system was approved by the FDA in 2010, and functioned as a stand-alone insulin pump or could be integrated with the Medtronic CGM. CGM data was viewable directly on the insulin pump screen.32 The MiniMed 530G system was released in 2013, and could also function as a stand-alone insulin pump or be integrated with the newer Enlite CGM. Upgrades to this system included a predictive alert that notified the patient of rapidly changing, high, or low blood glucose levels. It also introduced the threshold suspend function, which used SmartGuard™ technology to suspend basal insulin for up to 2 hours if the patient did not respond to a low glucose alert.11 This feature was shown to reduce night-time hypoglycaemia without increasing HbA1c.33 Medtronic has discontinued production of these legacy insulin pumps as newer devices have been released.
In 2016, Medtronic released the MiniMed 630G system. The 630G has similar functionality to the previous insulin pumps including the insulin cartridge size, basal and bolus increments and threshold suspend feature, but has a colour screen. Remote boluses can be performed using the connected Contour Next Link 2.4 glucometer, and the threshold suspend feature can be used when the pump is connected to the Guardian III CGM.34
Most recently, Medtronic released the 670G, the first hybrid closed-loop system which uses the Guardian 3 CGM and SmartGuard technology. The 670G can be utilised as a sensor-augmented pump in ‘manual mode’ that includes a predictive low glucose suspend (PLGS) feature, in which basal insulin can be suspended when hypoglycaemia is predicted but before reaching the low glucose threshold. It can also be used in ‘auto mode’ where the system adjusts insulin delivery every 5 minutes to treat to a target glucose of 120 mg/dL. The algorithm uses CGM values and a sensitivity factor that is readjusted daily based on the previous day’s total daily insulin and the target blood glucose level of 120 mg/dL.35 Patients can use a temporary glucose target of 150 mg/dL during events where hypoglycaemia is possible such as during exercise. Users must still enter a meter glucose reading and bolus for corrections and carbohydrates, but this system has been shown in an in-home study to increase time in range and decrease variability and post prandial excursions. In this study, adults and adolescents demonstrated an improvement in HbA1c from 7.3% to 6.8% and 7.7% to 7.1%, respectively.36 Time spent with sensor glucose between 71–180 mg/dL improved from 68.8% to 73.8% in adults and from 60.4% to 67.2% in adolescents. There were no episodes of DKA or severe hypoglycaemia in this study.36 Thousands of hours of real-world data have confirmed the findings in the pivotal study. The system will revert to manual mode if the CGM is not calibrated when requested or if maximum or minimum basal rates are utilised for longer than a set amount of time.36 The 670G has been approved by the FDA for patients 7 years of age and older.34 Trials for the next generation Medtronic automated insulin delivery system will begin in 2019.
Animas
The Animas (West Chester, Pennsylvania, USA) OneTouch Ping® insulin pump received FDA approval in 2008 and connected to the OneTouch meter via radio frequency communication. Blood glucose values were sent directly from the meter to the pump, and the meter could be used remotely to programme insulin delivery from the pump.37 The Animas Vibe® insulin pump was FDA approved for adults in 2014 and for children aged ≥2 years in 2016. This system was the first to be integrated with Dexcom and the first integrated system to be approved in children this young. Users could view their CGM data directly on their pump and could customise alerts.38 Animas partnered with the Juvenile Diabetes Research Foundation (JDRF) in 2010 to work towards developing a hybrid closed-loop system, and released data in 2014 and 2016 with promising results using a Hypoglycemia-Hyperglycemia Minimizer system in conjunction with an Animas OneTouch Ping insulin pump and a Dexcom G4 CGM.39 In October 2017, however, Animas announced it would no longer make insulin pumps.
Tandem
Tandem Diabetes Care (San Diego, California, USA) developed the t:slim insulin pump with significant end-user input and released the product in 2012. Surveys and focus groups were performed to determine patient priorities leading to the development of a touch-screen device with a rechargeable battery that lasts up to 7 days. The home screen displays important information including the remaining amount of insulin, insulin on board, battery life and quick access to bolus and pump functions.40 The t:slim was connected to the Dexcom G4 CGM. It used Tandem’s t:connect® application, Glooko, Tidepool and Diasend data management systems. In 2015, the t:flex® insulin pump was released with a larger insulin cartridge holding 480 units and was marketed towards patients requiring more than 100 units a day.11 Both the G4 and the t:flex have since been discontinued.
The t:slim X2™ insulin pump was released in 2016. In addition to being thinner than its predecessor, the X2 is the first insulin pump capable of updating its software at home via personal computer. The t:slim X2 is approved for patients ≥6 years.34 After the completion of the PROLOG study in 2018, the t:slim X2 integrated with Dexcom G6 CGM, and using Basal IQ™ technology, was FDA approved with a PLGS feature. This algorithm uses CGM readings to discontinue basal insulin when hypoglycaemia is predicted and resumes insulin delivery when blood glucose levels improve. Results of the PROLOG study demonstrated a decrease in median time spent with a blood glucose less than 70 mg/dL from 3.6% to 2.6% without an increase in hyperglycaemia. Therefore time in range from 70–180 mg/dL increased slightly from 64% to 65%.41 T:slim X2 users who also wear the Dexcom G6 are not required to perform finger-sticks for calibrations or insulin delivery.42 T:slim X2 was recently FDA approved as the first interoperable insulin pump allowing it to be used with a variety of CGMs, blood glucose meters and software platforms, allowing greater customisation of care and providing more opportunities for developing future closed loop systems.43
Insulet
The OmniPod® insulin pump was first released by Insulet (Acton, Massachusetts, USA) in 2011 with a smaller second-generation pod released in 2013. The OmniPod is tubeless, can be worn for up to 72 hours and is controlled by a personal diabetes manager (PDM) that also functions as a glucose meter. The PDM contains nutrition information for more than 1,000 common foods and can store pre-set carb values. The OmniPod connects to multiple data management applications including Glooko, Diasend and Tidepool.11 In a 2016 study by Layne et al., subjects had an average decrease in HbA1c of 0.6% independent of previous treatment after utilising the OmniPod for 3 months.44 Total daily dose of insulin also decreased by 16.4% which has been attributed to the tubeless system allowing more consistent and continuous insulin delivery.45
In June 2018, the FDA approved The Omnipod DASH™ Insulin Management System which includes a Bluetooth enabled pod and an updated touch screen PDM. This system can be connected to the Contour Next One glucose meter and enables data to be viewed remotely on a separate smart phone through the PDM. CGM data cannot currently be sent directly to the PDM, but it can be viewed on the patient’s phone app side-by-side with the PDM data.45 A randomised two-arm open crossover study was performed in 2012 comparing the original OmniPod insulin pump to standard insulin pumps in regards to patient satisfaction; while patients were pleased overall with both types of therapy, subjects did note the advantages of the OmniPod including an automated cannula insertion and continuous insulin infusion without the need to disconnect.46
Insulet is currently developing the OmniPod Horizon™ hybrid closed loop system which will be integrated with the Dexcom G6 CGM and controlled by an embedded algorithm. Smart devices will be used to monitor blood glucose levels, insulin delivery, and administer prandial boluses.47
Many people living with T1D have created their own do-it-yourself fully automated insulin delivery devices. Tidepool’s Loop is one example in which a CGM, compatible insulin pump and smart device are integrated with hardware to adjust basal insulin rates based on a programmed algorithm. Tidepool recently partnered with OmniPod to allow the device to communicate directly with the Loop app which is currently under production with support from the JDRF and the Helmsley Charitable Trust.47 As this type of closed-loop device becomes more common, endocrinologists should be aware and prepared to support these patients.48
Each of these companies, along with new entrants such as TypeZero Technologies, Beta Bionics, Bigfoot Biomedical, Cellnovo, Diabeloop and Eli Lilly partnered with DEKA are developing advanced automated insulin delivery projects, and their respective clinical trials can be reviewed on ClinicalTrials.gov. This site is easy to navigate as users can find related trials by searching “artificial pancreas.” Searches can be narrowed by location, company and recruitment status. Examples include NCT03040414 (Fuzzy Logic Automated Insulin Regulation), NCT03840278 (The Bihormonal iLet Bionic Pancreas Feasibility Study), and NCT02871089 (Closed Loop From Onset in Type 1 Diabetes [CLOuD].
Stable glucagon formulation
Glucagon is necessary for emergency treatment of severe hypoglycaemia and is currently only available as a powder that must be mixed with sterile water immediately prior to injection. The reconstituted solution is stable for only 24 hours. This medication is expensive, difficult to mix and administer for those who have not been trained, and is not able to be reused or stored once reconstituted. Both Eli Lilly and Novo Nordisk have glucagon emergency kits of this nature. In a recent study by Yale et al. in which a hypoglycaemic episode was simulated, only 13% of caregivers and 0% of acquaintances were able to successfully deliver a full dose of glucagon, while three participants actually delivered insulin instead.49 Newer formulations of glucagon including stable glucagon and intranasal glucagon are discussed below and will provide a solution to many of these issues and are critical needs in advancement of bihormonal pump systems.
Dasiglucagon is a more stable analogue of human glucagon being developed by Zealand Pharma (Copenhagen, Denmark). Phase II trials have been completed and demonstrated similar pharmacokinetics to current glucagon formulations with similar side effects of dose-dependent nausea and vomiting.50 Xeris (Chicago, Illinois, USA) has developed a stable liquid glucagon that is a native human glucagon dissolved in a carrier solution, dimethyl sulfoxide. Phase II trials have also been completed for this product that has similar pharmacokinetics with a slightly slower absorption time.51 Both companies plan to develop multi-use pens for home-use and infusion formulations that could be utilised in a bihormonal pump.52
Intranasal glucagon, which is pending FDA approval, will be a pivotal change in hypoglycaemic management. Studies have shown this formulation allows for more accurate and more rapid administration by untrained caregivers during severe and emergent hypoglycaemic episodes.53 Intranasal glucagon has slightly slower, but similar blood glucose raising effects at lower blood glucagon levels. Side effects of nausea and vomiting occur at similar rates to intramuscular glucagon.53 More than 90% of caregivers and acquaintances in the previously mentioned study were able to successfully deliver a full dose of intranasal glucagon.49 Eli Lilly is currently the only company with this product in phase III clinical trials.52
Applications and algorithms
Multiple data management software systems are mentioned earlier in this review and are available for use by providers and patients. These systems enable users to download and consolidate data from glucometers, insulin pumps, and CGMs into standardised reports that help elicit patterns and trends. Data management software systems are especially beneficial when utilised between clinic visits.54 The most commonly employed systems are reviewed here.
Diasend is compatible with over 100 devices, and data can be uploaded online from home, and shared with providers. Diasend was acquired by Glooko in 2016. Glooko has a standard uploader, and also utilises Bluetooth technology to upload data to applications available on Android and Apple devices. Glooko/Diasend links with fitness trackers, and users can also record data related to fitness and diet. Tidepool is a non-profit organisation and therefore free of charge to providers and patients. This web-based system is compatible with multiple devices.55 Similar data management systems are specifically designed to be compatible with their respective devices such as Tandem’s t:connect, Dexcom’s Clarity, and Medtronic’s Carelink.
Numerous smart-device applications are available to aid users with multiple aspects of diabetes care including logging blood glucose levels, insulin doses, diet and fitness. Many of these apps include a database to facilitate looking up carbohydrate amounts in common foods, and some even contain an insulin dose calculator based on the patient’s regimen, accounting for insulin on board.56 More recent advancements, such as Medtronic’s Sugar.IQ™, include artificial intelligence systems that are able to recognise and point out important trends to patients.20 Other applications are currently being developed such as Advice4U which uses the DreaMed Advisor Pro algorithm to determine and recommend insulin pump dose adjustments.
Decision-making for continuous glucose monitors and insulin pump wear
An individualised approach is vital in determining which type of technology will be most beneficial for a specific patient. Considerations should include patient motivation, cost, insurance coverage, and features that would be most helpful for that patient and family. For example, those with hypoglycaemia unawareness may do best with a CGM with audible alerts or an insulin pump with the ability to suspend insulin delivery. Parents of paediatric patients often prefer devices that can share data to smart devices. Certified Diabetes Educators (CDE) can play a crucial role in informing patients of the benefits and realistic expectations of these devices. CDEs have access to resources including the Diabetes Advanced Network Access which serves as a central tool to provide updates and education on available technologies.
Conclusion
The goal of these devices and systems is to advance the care and management of patients’ diabetes and help improve quality of life by easing the burden of a disease that has required such intensive focus on daily care.57 Patient motivation continues to be required, however, to achieve optimal results from new technologies. Education for healthcare provider teams is also necessary to encourage the standardised methodologies for reviewing data and making device adjustments.17 More than ever before, patients are being empowered with the tools to maximise self-care and collaboration with their healthcare providers. While there remains much work to do, the last decade of technological advancements has brought us to the threshold of fully closed-loop systems and reduced burden of disease.







