

Indian Journal of Science and Technology
DOI: 10.17485/IJST/v14i36.1612
Year: 2021, Volume: 14, Issue: 36, Pages: 2842-2849
Original Article
Nargiz Kamal Bayramova1, Nargiz Fakhraddin Sultanova2,Durna Rafail Aliyeva2, Irada Mammad Huseynova3*
1Junior researcher, Institute of Molecular Biology and Biotechnologies, Azerbaijan National Academy of Sciences
2Associate professor, PhD in biology, Institute of Molecular Biology and Biotechnologies,
Azerbaijan National Academy of Science
3Professor. Director, Academician, Doctor of biological sciences, Institute of Molecular Biology and Biotechnologies, Azerbaijan National Academy of Sciences
*Correspondign Author
Email: [email protected]
Received Date:30 August 2021, Accepted Date:04 October 2021, Published Date:03 November 2021
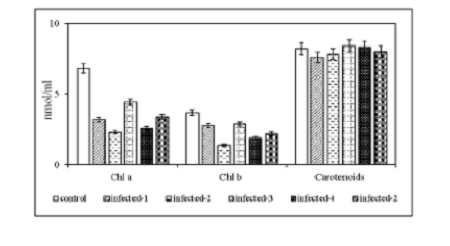
Objective: Grapevine (Vitis vinifera L.) is one of the major crops widely cultivated for the wine industry, as well as for the production of fresh and dried fruit in Azerbaijan. Grapevine leafroll disease (GLD) affects the vines throughout the world and is considered the most economically destructive among all virus and virus-like diseases. Vineyard surveys were conducted to determine the virus infection in the major grapevine growing region of Azerbaijan during 2019-2020. Methods/Statistical analysis: Forty-six samples were collected from grapevine fields and screened by rapid one-step assay AgriStrip and double-antibody sandwich Enzyme-linked immunosorbent assay (DASELISA). In our study, the levels of malondialdehyde (MDA), hydrogen peroxide (H2O2), pigments, relative water content (RWC), alterations in the activities of peroxidase enzymes (the activities of ascorbate peroxidase (APO), benzidine peroxidase (BPO) and guaiacol peroxidase (GPO) were investigated. Findings: The results revealed that tested samples were infected with Grapevine leafrollassociated virus 3 (GLRaV-3), however, no sample was found infected with other Grapevine leafroll-associated viruses. Changes in the levels of MDA, H2O2, pigments, RWC, alterations in the activities of peroxidase enzymes were studied. Significant reduction in green pigments like chlorophylls (a, b and total) and a gradual reduction in carotenoids were observed in all infected species. Obtained results showed that the level of RWC and the amount of hydrogen peroxide had increased in all infected leaves. The activities of APO, BPO and GPO were observed to increase in virus-infected leaves compared to the healthy control. Based on the results, it can be concluded that the effect of GLRaV-3 was more destructive in the N2 sample. The antioxidant defense system works more effectively in the N5 sample and this plant is more resistant to viral infection. Novelty: These results indicate that GLRaV-3 was the only most prevalent endemic viral pathogen of grapevine in the dominantly warmhumid continental climate of our country. All variable physiological parameters are assessed as the plant’s response to the pathogen.
Keywords: activity; chlorophyll; hydrogen peroxide; malondialdehyde; peroxidase; virus disease
© 2021 Bayramova et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Published By Indian Society for Education and Environment (iSee)
Subscribe now for latest articles and news.