Abstract
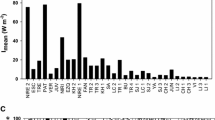
We described phytoplankton productivity in a floodplain wetland of the Lower Paraná River, Argentina. Four samplings encompassing periods of high and low water levels were conducted in a highly colored shallow lake. Photosynthesis-irradiance (P-E) curves and the areal photosynthetic rate (PA) were estimated following the 14C assimilation technique. Likewise, physical and chemical variables and phytoplankton composition, density, and chlorophyll a concentration were measured. Phytoplankton assemblages shifted from cyanobacteria blooms in summer to cryptophycean dominance in winter, and co-dominance of cryptophytes and chlorophytes in autumn. Assimilation number Pmax ranged from 3– 7.8 μg C (μg Chl a h)−1 and peaked in early summer (low water level) when water color was highest, suggesting that phytoplankton productivity was not depressed by the high content of colored humic acids. Photosynthetic efficiency (α) ranged from 0.021–1 μg C (μg Chl a h)−1 μmol photons−1 m2 s and reached its maximum value during winter even when the assemblage, dominated by cryptophyceans, did not achieve light saturation. In early and late summer and in autumn, optimal irradiance (Eopt) ranged from 544–1,397 μmol photons m−2 s−1. The highest PA (207 mg C m−2 h−1) was registered in late summer (high water level) when the lowest mean irradiance (Emean) was observed (341 μmol photons m−2 s−1). The lowest PA (28 mg C m−2 h−1) occurred in winter when Emean was maximum (1,432 μmol photons m−2 s−1). Our results indicate that productivity was similar to those recorded for other latitudes and appeared not limited by the humic content of the water because phytoplankton was dominated by algae well-adapted to low light conditions.
Similar content being viewed by others
Literature Cited
APHA. 1992. Standard Methods for the Examination of Water and Wastewater, twentieth edition. APHA, Washington, DC, USA.
Arvola, L., P. Kankaala, T. Tulonen, and A. Ojala. 1996. Effects of phosphorus and allochthonous humic matter enrichment on the metabolic processes and community structure of plankton in a boreal lake (Lake Pääjärvi). Canadian Journal of Fisheries and Aquatic Sciences 53: 1646–62.
Buma, A. G. J., A. A. M. Noordeloos, and J. Larsen. 1993. Strategies and kinetics of photoacclimation in three Antarctic nanophytoflagellates. Journal of Phycology 29: 407–17.
Carvalho, P., L. M. Bini, S. M. Thomaz, L. Gonçalves de Oliveira, B. Robertson, W. L. Gomes Tavechio, and A. J. Darwisch. 2001. Comparative limnology of South American floodplain lakes and lagoons. Acta Scientiarum 23: 265–73.
Chauton, M. S., G. H. Tilstone, C. Legrand, and G. Johnsen. 2004. Changes in pigmentation, bio-optical characteristics and photophysiology, during phytoflagellate succession in mesocosms. Journal of Plankton Research 26: 315–24.
Chichizola, S. E. 1993. Las comunidades vegetales de la Reserva Natural Estricta Otamendi y sus relaciones con el ambiente. Parodiana 8: 227–63.
Côté, B. and T. Platt. 1983. Day to day variations in the springsummer photosynthetic parameters of coastal marine phytoplankton. Limnology and Oceanography 28: 320–44.
Cuthbert, I. D. and P. del Giorgio. 1992. Toward a standard method of measuring color in freshwater. Limnology and Oceanography 37: 1319–26.
de Tezanos Pinto, P., L. Allende, and I. O’Farrell. 2007. Influence of free-floating plants on the structure of a natural phytoplankton assemblage: an experimental approach. Journal of Plankton Research 28: 47–56.
Eilers, P. H. C. and J. C. H. Peeters. 1988. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling 42: 199–215.
Eloranta, P. 1999. Humus and water physics. p. 61–74. In J. Keskitalo and P. Eloranta (eds.) Limnology of Humic Waters. Backhuys Publishers, Leiden, The Netherlands.
Falkowski, P. G. 1981. Light-shade adaptations and assimilation numbers. Journal of Plankton Research 3: 203–16.
Helbling, E. W., V. E. Villafañe, and O. Holm-Hansen. 1994. Effects of ultraviolet radiation on antarctic marine phytoplankton photosynthesis with particular attention to the influence of mixing. p. 207–27. In S. Weiler and P. Penhale (eds.) Ultraviolet Radiation in Antarctica: Measurements and Biological Effects. American Geophysical Union, Washington, DC, USA.
Henley, W. J. 1993. Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. Journal of Phycology 29: 729–39.
Henry, R. 1993. Primary production by phytoplankton and its controlling factors in Jurumirim reservoir (São Paulo, Brazil). Revista Brasileira de Biologia 53: 489–99.
Henry, R., M. A. Nunes, P. M. Mitsuka, N. Lima, and S. M. C. De e Casanova. 1998. Variação espacial e temporal da produtividade primária pelo fitoplâncton na represa de Jurumirim (Rio Paranapanema, Sp). Revista Brasileira de Biologia 58: 571–90.
Hill, W. 1996. Effects of light. p. 121–48. In R. Stevenson, M. Bothwell, and R. Lowe (eds.) Algal Ecology: Freshwater Benthic Ecosystems. Academic Press, London, UK.
Holopainen, A. L., R. Niinioja, and A. Rämö. 2003. Seasonal succession, vertical distribution and long term variation of phytoplankton communities in two shallow forest lakes in eastern Finland. Hydrobiologia 506-09: 237–45.
Huszar, L. V. and C. S. Reynolds. 1997. Phytoplankton periodicity and sequences of dominance in an Amazonian flood-plain lake (Lago Batata, Pará, Brazil): responses to gradual environmental change. Hydrobiologia 346: 169–81.
Izaguirre, I. and I. O’Farrell. 1999. Temporal fluctuation of phytoplanktonic chlorophyll a and primary production in a turbid floodplain lake of the River Parana (Argentina). Gayana Botanica 56: 39–51.
Izaguirre, I., I. O’Farrell, F. Unrein, R. Sinistro, M. Dos Santos Afonso, and G. Tell. 2004. Algal assemblages and limnological features across a wetland, from a shallow lake to the relictual oxbow lakes (Lower Paraná River, South America). Hydrobiologia 511: 25–36.
Izaguirre, I., R. Sinistro, I. O’Farrell, F. Unrein, and G. Tell. 2001. Algal assemblages in anoxic relictual oxbow lakes from the Lower Paraná Floodplain. Nova Hedwigia 123: 95–106.
Jackson, T. A. and R. E. Hecky. 1980. Depression of primary productivity by humic matter in lake and reservoir waters of the boreal forest zone. Canadian Journal of Fisheries and Aquatic Sciences 37: 2300–17.
Jansson, M., P. Blomqvist, A. Jonsson, and A. K. Bergstöm. 1996. Nutrient limitation of bacterioplankton, autotrophic and mixotrophic phytoplankton, and heterotrophic nanoflagellates in Lake Örträsket. Limnology and Oceanography 41: 1552–59.
Junk, W., P. Bayley, and R. Sparks. 1989. The flood pulse concept in river-floodplain systems. In D. P. Dodge (ed.) Proceedings of the International Large River Symposium. Canadian Journal of Fisheries and Aquatic Sciences 106: 110–27.
Kirk, J. T. O. 1994. Light and Photosynthesis in Aquatic Ecosystems, second edition. Cambridge University Press, Cambridge, UK.
Macedo, M. F., P. Duarte, P. Mendes, and J. G. Ferreira. 2001. Annual variation of environmental variables, phytoplankton species composition and photosynthetic parameters in a coastal lagoon. Journal of Plankton Research 23: 719–32.
Marker, A. F. H., C. A. Crowther, and R. J. M. Gunn. 1980. Methanol and acetone as solvents for estimating chlorophyll a and phaeopigments by spectophotometry. Archive für Hydrobiologie 14: 52–69.
Neiff, J. J., M. H. Iriondo, and R. Carignan. 1994. Large tropical South American wetland: an overview. p. 156–65. In G. L. Link and R. L. Naiman (eds.) Proceedings of the International Workshop on The Ecology and Management of Aquaticterrestrial ecotones. University of Washington, Seattle, WA, USA.
O’Farrell, I., R. Sinistro, I. Izaguirre, and F. Unrein. 2003. Do steady state assemblages occur in shallow lentic environments from wetlands? Hydrobiologia 502: 197–209.
Pålsson, C. and W. Granéli. 2004. Nutrient limitation of autrotrophic and mixotrophic phytoplankton in a temperate and tropical humic lake gradient. Journal of Plankton Research 26: 1005–14.
Présing, M., S. Herodek, T. Preston, and L. Vörös. 2001. Nitrogen uptake and the importance of internal nitrogen loading in Lake Balaton. Freshwater Biology 46: 125–39.
Reynolds, C. 2006. Ecology of Phytoplankton. Cambridge University Press, Cambridge, UK.
Robinson, G., S. E. Gurney, and L. G. Goldsborough. 1997. The primary productivity of benthic and planktonic algae in a prairie wetland under controlled water-level regimes. Wetlands 17: 182–94.
Sakshaug, E., A. Bricaud, Y. Dandonneau, P. G. Falkowski, D. A. Kiefer, L. Legendre, A. Morel, J. Parslow, and M. Takahashi. 1997. Parameters of photosynthesis: theory and interpretation of results. Journal of Plankton Research 19: 1637–70.
Steeman-Nielsen, E. 1952. The use of radioactive carbon (14C) for measuring organic production in the sea. Journal du Conseil pour l’Exploration de la Mer. 18: 117–40.
Stumm, W. and J. J. Morgan. 1996. Aquatic Chemistry. Chemical Equilibria and Rates in Natural Waters. John Wiley & Sons, Inc., New York, NY, USA.
Train, S. and L. C. Rodrigues. 1998. Temporal fluctuations of the phytoplankton community of the Baia River, in the upper Paraná River floodplain, Mato Grosso do Sul, Brazil. Hydrobiologia 361: 125–34.
Tulonen, T., P. Kankaala, A. Ojala, and L. Arvola. 1994. Factors controlling production of phytoplankton and bacteria under ice in a humic, boreal lake. Journal of Plankton Research 16: 1411–32.
Utermöhl, M. 1958. Zur Vervollkommung der quantitativen Phytoplankton Methodik. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Venrick, E. L. 1978. How many cells to count? p. 167–80. In A. Sournia (ed.) Phytoplankton Manual. UNESCO, Paris, France.
Welcomme, R. L. 1985. River fisheries. United Nations FAO Fisheries Technical Paper 262, Rome.
Williamson, C. E., D. P. Morris, M. L. Pace, and O. G. Olson. 1999. Dissolved organic carbon and nutrients as regulators of lake ecosystems: resurrection of a more integrated paradigm. Limnology and Oceanography 44: 795–803.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rodríguez, P., Pizarro, H. Phytoplankton productivity in a highly colored shallow lake of a South American floodplain. Wetlands 27, 1153–1160 (2007). https://doi.org/10.1672/0277-5212(2007)27[1153:PPIAHC]2.0.CO;2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1672/0277-5212(2007)27[1153:PPIAHC]2.0.CO;2