-
PDF
- Split View
-
Views
-
Cite
Cite
Filipa Lynce, Ana Barac, Ming T. Tan, Federico M. Asch, Karen L. Smith, Chau Dang, Claudine Isaacs, Sandra M. Swain, SAFE‐HEaRt: Rationale and Design of a Pilot Study Investigating Cardiac Safety of HER2 Targeted Therapy in Patients with HER2‐Positive Breast Cancer and Reduced Left Ventricular Function, The Oncologist, Volume 22, Issue 5, May 2017, Pages 518–525, https://doi.org/10.1634/theoncologist.2016-0412
Close - Share Icon Share
Abstract
Human epidermal growth receptor 2 (HER2) targeted therapies have survival benefit in adjuvant and metastatic HER2 positive breast cancer but are associated with cardiac dysfunction. Current U.S. Food and Drug Administration recommendations limit the use of HER2 targeted agents to patients with normal left ventricular (LV) systolic function.
The objective of the SAFE‐HEaRt study is to evaluate the cardiac safety of HER2 targeted therapy in patients with HER2 positive breast cancer and mildly reduced left ventricular ejection fraction (LVEF) with optimized cardiac therapy. Thirty patients with histologically confirmed HER2 positive breast cancer (stage I–IV) and reduced LVEF (40% to 49%) who plan to receive HER2 targeted therapy for ≥3 months will be enrolled. Prior to initiation on study, optimization of heart function with beta‐blockers and angiotensin converting enzyme inhibitors will be initiated. Patients will be followed by serial echocardiograms and cardiac visits during and 6 months after completion of HER2 targeted therapy. Myocardial strain and blood biomarkers, including cardiac troponin I and high‐sensitivity cardiac troponin T, will be examined at baseline and during the study.
LV dysfunction in patients with breast cancer poses cardiac and oncological challenges and limits the use of HER2 targeted therapies and its oncological benefits. Strategies to prevent cardiac dysfunction associated with HER2 targeted therapy have been limited to patients with normal LVEF, thus excluding patients who may receive the highest benefit from those strategies. SAFE‐HEaRt is the first prospective pilot study of HER2 targeted therapies in patients with reduced LV function while on optimized cardiac treatment that can provide the basis for clinical practice changes.
Human epidermal growth receptor 2 (HER2) targeted therapies have survival benefit in adjuvant and metastatic HER2 positive breast cancer but are associated with cardiac dysfunction. To our knowledge, SAFE‐HEaRt is the first clinical trial that prospectively tests the hypothesis that HER2 targeted therapies may be safely administered in patients with mildly reduced cardiac function in the setting of ongoing cardiac treatment and monitoring. The results of this study will provide cardiac safety data and inform consideration of clinical practice changes in patients with HER2 positive breast cancer and reduced cardiac function, as well as provide information regarding cardiovascular monitoring and treatment in this population.
Introduction
Human epidermal growth factor receptor 2 (HER2) is overexpressed in approximately 25% of breast cancers [1] and in the era preceding HER2 targeted therapies was a marker of poor prognosis [2]. The development of trastuzumab, a monoclonal antibody against the HER2 receptor, resulted in dramatic improvements in survival in both adjuvant and metastatic HER2 positive breast cancer , but its use has been limited by cardiac toxicity. A retrospective analysis of the initial trials of trastuzumab for metastatic breast cancer identified unexpected cardiac dysfunction in 3%–27% of patients with the highest incidence of cardiac toxicity in those who received concomitant anthracyclines. Among such patients, 19% developed class III or IV New York and Heart Association symptoms [7]. As a result, when trastuzumab was evaluated as an adjuvant therapy, most trials avoided coadministration of trastuzumab with anthracyclines and limited previously received cumulative anthracyclines doses. In addition, adjuvant trastuzumab trials employed stringent cardiovascular eligibility criteria, cardiac monitoring schema with frequent assessments of left ventricular ejection fraction (LVEF), and algorithms for holding trastuzumab in the setting of cardiac toxicity as well as early trial‐stopping rules . Although difficult to generalize due to the different definitions of cardiac endpoints used, the observed rates of severe trastuzumab‐associated cardiac toxicity, including symptomatic heart failure and cardiac death, in the adjuvant trastuzumab trials were low (0%–4.1%) and early stopping rules were not reached [9], thus leading to widespread adoption of trastuzumab‐containing regimens in oncology clinical practice for patients with early HER2‐positive breast cancer.
Trastuzumab‐associated cardiac toxicity often occurs early during the course of treatment (median time to presentation 7.8 months) and is most commonly manifested by an asymptomatic decrease in LVEF [10]. In contrast to anthracyclines‐associated cardiac toxicity, trastuzumab‐associated cardiac toxicity is not dose‐dependent and is reversible in the majority of patients within 6 months of discontinuing trastuzumab therapy . Results from long‐term follow‐up of cardiac function in the National Surgical Adjuvant Breast and Bowel Project B‐31 trial revealed a 7‐year cumulative incidence of protocol‐defined cardiac events (CEs) of 4.0% of patients who received trastuzumab in comparison to 1.3% of patients who did not, resulting in an absolute difference in cardiac events of only 2.7%, thus providing evidence of long term favorable benefit‐to‐risk ratio of trastuzumab for early HER2‐positive breast cancer [13]. Real‐world studies in community settings have validated the survival benefit of adjuvant trastuzumab, but report significantly higher incidence of CEs, particularly among elderly patients and those with cardiovascular (CV) risk factors ], thus highlighting the importance of cardiac surveillance [18].
Based on the U.S. Food and Drug Administration (FDA)‐approved trastuzumab package insert, patients should have LVEF evaluation prior to initiation of therapy to confirm normal baseline left ventricular (LV) systolic function and then at regular intervals during treatment. It is recommended that trastuzumab be held or stopped if significant decreases in LVEF (LVEF ≥16% from pretreatment values or LVEF ≤50% and ≥10% absolute decrease from baseline) occur or persist, respectively [21]. Similar recommendations are in place for two other currently approved HER2‐targeted therapies for breast cancer, pertuzumab [22] and ado‐trastuzumab emtansine [23] (T‐DM1). Several recently completed clinical investigations have explored neurohormonal blockade, using beta‐blockers and/or renin‐angiotensin system antagonists, to reduce treatment‐related LV dysfunction and associated interruptions in HER2 therapy. Only patients with normal LVEF were enrolled in the recent prospective trials, thus excluding patients at higher risk of developing cardiac toxicity and who may potentially have the greatest benefit from cardiac intervention.
Another approach to prevent LVEF decline in patients receiving HER2 targeted therapies is the use of early markers of cardiac injury as predictors of cardiac dysfunction. Myocardial strain is a new echocardiographic measure of cardiac contractility that offers the potential to detect subtle signs of cardiac dysfunction [28], and in patients treated with trastuzumab, early (within 3 months of treatment initiation) decrease in myocardial strain has been shown to predict subsequent decline in LVEF [29]. Cardiac troponins, structural proteins unique to the heart, have also been identified in patients with cancer undergoing high‐dose chemotherapy where they predicted subsequent development of cardiac dysfunction [30] and in patients treated with doxorubicin, taxanes, and trastuzumab, early increases in troponin provided additive information about the risk of cardiotoxicity [31]. There are limited data on the values of strain and serum biomarkers in patients with cardiac dysfunction undergoing HER2 targeted therapies.
The SAFE‐HEaRt study tests the hypothesis that HER2 targeted therapies may be safely used in patients with mildly decreased LVEF with appropriate cardiac monitoring and treatment. The rationale for our pilot study is based on the substantial oncologic benefit of HER2 targeted therapies and the retrospective data demonstrating resolution or improvement of most trastuzumab‐induced cardiotoxicity with appropriate cardiac management . This is the first prospective evaluation of HER2 targeted therapy in patients with HER2 positive breast cancer and decreased cardiac function, a group of patients who at present have limited treatment options and who are at risk for adverse outcomes. The results of our study will inform clinical practice about the safety of HER2 treatment in patients with reduced cardiac function and provide new data about predictors of high risk that may allow consideration of alternative oncological treatments and/or different cardiac prevention strategies for the prevention of progressive LV dysfunction and heart failure.
SAFE‐HEaRt Design
SAFE‐HEaRt is a pilot study evaluating the cardiac safety of HER2 targeted therapy of the physician's choice (trastuzumab, pertuzumab, or T‐DM1) in 30 patients with HER2 positive invasive breast cancer and mildly decreased LV function (LVEF 40%–49%) while on concomitant cardiac treatment with beta‐blockers and angiotensin converting enzyme (ACE) inhibitors. An Investigational New Drug Application was obtained for all three agents (trastuzumab, pertuzumab and T‐DM1) and an Institutional Review Board has approved the study. The trial is being conducted at three academic centers in the U.S. with the recent addition of the Memorial Sloan Kettering Cancer Center. Enrollment began in October 2013 and is projected to end in December 2017.
Endpoints and Definitions
The primary objective of the study is to evaluate the cardiac safety of HER2 targeted therapy in patients with HER2 positive breast cancer and reduced LVEF when given concomitantly with cardiac treatment. The primary endpoint is the proportion of patients who complete their planned HER2 targeted oncologic therapy without the development of a CE or asymptomatic worsening of cardiac function. For this study, a specific definition of CEs and asymptomatic worsening of cardiac function was applied. Cardiac events are defined as the presence of symptoms and signs attributable to heart failure (HF) (increasing shortness of breath, orthopnea, paroxysmal nocturnal dyspnea, bilateral ankle swelling) confirmed by a cardiologist; cardiac arrhythmia requiring pharmacological or electrical treatment; myocardial infarction and/or sudden cardiac death; or death due to myocardial infarct, arrhythmia, or HF. Asymptomatic worsening of cardiac function is defined as a decline in LVEF ≥10% points from baseline and/or ejection fraction ≤35% corroborated by a confirmatory echocardiogram in 2–4 weeks in the absence of symptoms/signs suggestive of HF. Planned oncologic therapy is defined in the adjuvant setting as completion of 1 year total of HER2 targeted therapy and in the metastatic setting as 1 year of treatment or cessation of treating regimen due to progressive disease or noncardiac toxicity or noncardiac death. Secondary objectives include cardiac injury biomarkers (high‐sensitivity troponin and myocardial strain) as early predictors of CEs and asymptomatic further worsening of cardiac function, time to development of events, absolute changes in LVEF, and delays in HER2 targeted therapies attributed to cardiac causes.
Eligibility Criteria
Patients are eligible for the study if they have a confirmed diagnosis of stage I–IV HER2 positive breast cancer, defined by immunohistochemical staining for HER2 protein of 3+ intensity and/or amplification of the HER2 gene on fluorescence in situ hybridization ≥2.0 on breast specimen or biopsy of a metastatic site. In addition, they must have LVEF <50% and ≥40% documented in cardiac imaging done within 30 days prior to enrollment. Decreased LV systolic function may be an existing condition prior to the initiation of cancer treatment or have developed during cancer treatment including HER2 therapy. Patients are candidates for study participation if they are currently receiving or plan to receive trastuzumab, trastuzumab/pertuzumab, or TDM1 in the (neo) adjuvant or metastatic setting and are expected to receive at least 3 months of HER2 targeted therapy from the time of study enrollment. Patients with current signs or symptoms of HF, active coronary ischemia, or a history of recent hospitalization(s) due to documented HF in the preceding 12 months are not eligible. Patients who received prior anthracyclines are eligible for the study greater than 50 days after the last anthracycline administration (Table 1).
Key inclusion and exclusion criteria for the SAFE‐HEaRt study
| Inclusion criteria | – Histologically or cytologically confirmed stage I–IV HER2 positive breast cancer, defined by IHC staining for HER2 protein of 3+ intensity and/or amplification of the HER2 gene on FISH ≥2.0 on breast specimen or biopsy of a metastatic site |
| – LVEF <50% and ≥40% documented in cardiac imaging done within 30 days prior to enrollment | |
| – Expected to receive at least 3 months of HER2 targeted therapy from the time of study enrollment | |
| Exclusion criteria | – Current signs or symptoms of HF, active coronary ischemia or a history of recent hospitalization(s) due to documented HF in the preceding 12 months are not eligible |
| – Patients who received prior anthracyclines are eligible for the study greater than 50 days after the last anthracycline administration |
| Inclusion criteria | – Histologically or cytologically confirmed stage I–IV HER2 positive breast cancer, defined by IHC staining for HER2 protein of 3+ intensity and/or amplification of the HER2 gene on FISH ≥2.0 on breast specimen or biopsy of a metastatic site |
| – LVEF <50% and ≥40% documented in cardiac imaging done within 30 days prior to enrollment | |
| – Expected to receive at least 3 months of HER2 targeted therapy from the time of study enrollment | |
| Exclusion criteria | – Current signs or symptoms of HF, active coronary ischemia or a history of recent hospitalization(s) due to documented HF in the preceding 12 months are not eligible |
| – Patients who received prior anthracyclines are eligible for the study greater than 50 days after the last anthracycline administration |
Abbreviations: FISH, fluorescence in situ hybridization; HER2, human epidermal growth receptor 2; HF, heart failure; IHC, immunohistochemistry; LVEF, left ventricular ejection fraction.
Key inclusion and exclusion criteria for the SAFE‐HEaRt study
| Inclusion criteria | – Histologically or cytologically confirmed stage I–IV HER2 positive breast cancer, defined by IHC staining for HER2 protein of 3+ intensity and/or amplification of the HER2 gene on FISH ≥2.0 on breast specimen or biopsy of a metastatic site |
| – LVEF <50% and ≥40% documented in cardiac imaging done within 30 days prior to enrollment | |
| – Expected to receive at least 3 months of HER2 targeted therapy from the time of study enrollment | |
| Exclusion criteria | – Current signs or symptoms of HF, active coronary ischemia or a history of recent hospitalization(s) due to documented HF in the preceding 12 months are not eligible |
| – Patients who received prior anthracyclines are eligible for the study greater than 50 days after the last anthracycline administration |
| Inclusion criteria | – Histologically or cytologically confirmed stage I–IV HER2 positive breast cancer, defined by IHC staining for HER2 protein of 3+ intensity and/or amplification of the HER2 gene on FISH ≥2.0 on breast specimen or biopsy of a metastatic site |
| – LVEF <50% and ≥40% documented in cardiac imaging done within 30 days prior to enrollment | |
| – Expected to receive at least 3 months of HER2 targeted therapy from the time of study enrollment | |
| Exclusion criteria | – Current signs or symptoms of HF, active coronary ischemia or a history of recent hospitalization(s) due to documented HF in the preceding 12 months are not eligible |
| – Patients who received prior anthracyclines are eligible for the study greater than 50 days after the last anthracycline administration |
Abbreviations: FISH, fluorescence in situ hybridization; HER2, human epidermal growth receptor 2; HF, heart failure; IHC, immunohistochemistry; LVEF, left ventricular ejection fraction.
Screening and Pre‐Enrollment Procedures
Protocol‐specific screening procedures will be performed to exclude coronary ischemia or other treatable causes of cardiomyopathy. Stress testing and coronary artery imaging will be performed for this purpose at the discretion of cardiology study investigator(s). Standard cardiac troponin I (cTnI) will be collected as a screening procedure for each patient. If the results of the standard cTnI assay are >1 ng/mL, appropriate clinical work‐up will be initiated. The patient will be allowed to participate in the study only after the exclusion of ongoing cardiac ischemia or injury. The LVEF used to meet eligibility criteria for each patient will be confirmed by review of the echocardiogram at the MedStar Health Research Institute Echocardiography Core Laboratory (Core Lab). If the images of the clinical echocardiogram used to determine eligibility are not available, or if LVEF was obtained using a different cardiac imaging technique (cardiac magnetic resonance (MR) or multigated acquisition scan), the patient will undergo a new study echocardiogram during the screening period to confirm LVEF is within the appropriate range for study participation.
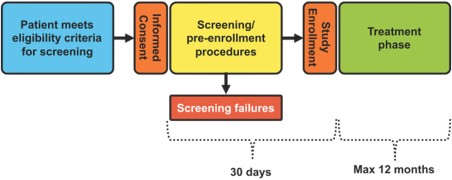
After completion of all screening procedures, ineligible patients will be considered screen failures. The following instances will be considered a screen failure: Core Lab does not confirm LVEF reported in initial cardiac imaging (LVEF <50% and ≥40%) and/or a treatable and reversible HF cause and/or cardiac ischemia is identified. Patients able to continue in the study, that is, those who do not meet any screen failure criteria, will enroll and move to the treatment phase (Fig. 1). Patients will be followed for up to 12 months on study.
Study phases. Protocol‐specific screening procedures will be performed to exclude coronary ischemia or other treatable causes of cardiomyopathy. The left ventricular ejection fraction used to meet eligibility criteria for each patient will be confirmed by review of the echocardiogram at the MedStar Health Research Institute Echocardiography Core Laboratory. After completion of all screening procedures, eligible patients will proceed with treatment and ineligible patients will be considered screen failures.
Echocardiograms and Other Evaluations
All transthoracic echocardiograms will be performed at clinical echocardiography laboratories following an acquisition protocol developed for the SAFE‐HEaRt study that includes comprehensive 2D, 3D, and strain imaging. Echocardiograms will be performed at baseline and after starting HER2 targeted therapy every 6 weeks for 2 assessments and then every 3 months during the study. Additional echocardiograms may be performed at the discretion of study investigators. All echocardiograms will be analyzed by an independent investigator at the Core Lab, blinded to any clinical data and following standard procedures as recommended by the American Society of Echocardiography .
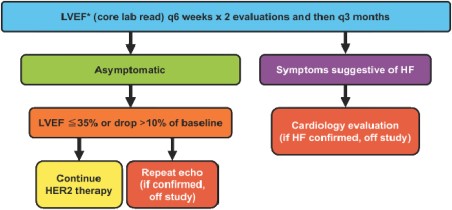
During the study, patients will be followed by an investigator cardiologist for evaluation of symptoms and signs of HF and for initiation and titration of cardiac medications (beta‐blockers and ACE inhibitors). After receiving the first dose of HER2 targeted therapy on study, patients will come for cardiology assessments every 6 weeks for two visits and then every 12 weeks throughout study participation. The treating physicians will have access to the LVEF assessed by the Core Lab that will be used for clinical decision making. A schema will be followed to determine when HER2 targeted therapy should be held, rechallenged, or stopped (Fig. 2). If a patient has an asymptomatic absolute decline in LVEF of ≥10% points from baseline or to ≤35%, HER2 targeted therapy will be temporarily withheld. At any time that HER2 targeted therapy is withheld, the patient will undergo follow‐up cardiology assessment to evaluate for the presence of HF signs and symptoms. A confirmatory study echocardiogram will be performed within 2–4 weeks. If the repeated study echocardiogram confirms the change in LVEF that meets holding criteria, the patient will come off the study. This will be named asymptomatic worsening of cardiac function and considered a primary endpoint. However, if the repeated echocardiogram shows improvement in LVEF and the holding criteria are no longer met, HER2 targeted therapy will be resumed and the patient will undergo regular cardiac assessments and study echocardiograms. This will not be considered as meeting the primary endpoint. If a patient develops symptomatic heart failure at any time that is confirmed by the cardiologist, the patient will go off study. This will be named a cardiac event and considered a primary endpoint.
Cardiac monitoring. Echocardiograms will be performed at baseline and after starting HER2 targeted therapy every 6 weeks for 2 assessments and then every 3 months while in the study. All echocardiograms will be analyzed by an independent investigator at the Core Lab blinded to any clinical data and following standard procedures as recommended by the American Society of Echocardiography, and results will be used to determine when HER2 targeted therapy should be held, rechallenged, or stopped.
Abbreviations: *, LVEF read by core lab; HER2, human epidermal growth receptor 2; HF, heart failure; LVEF, left ventricular ejection fraction; q3, every 3; q6, every 6.
Standard cTnI and high‐sensitivity cardiac troponin T will be measured at enrollment, every 6 weeks after starting HER2 therapy for two assessments, and every 12 weeks thereafter. Cardiac troponin I will be performed in clinical laboratories using standard commercial assays. Results will be available to the treating physician but will not be used to make clinical decisions in the absence of clinical symptoms of cardiac ischemia or significantly elevated values higher than 1 ng/mL [34]. High‐sensitivity cardiac troponin T assay will be performed by central laboratory (Roche Diagnostics) and will be used for research purposes only; the results will not be available to the treating physician.
Initiation and Titration of Cardiac Medications
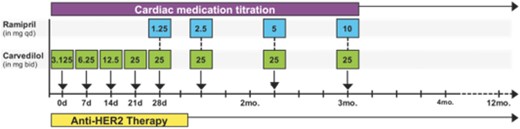
After study enrollment, cardiac treatment with beta‐blockers and ACE inhibitors will be started in all patients who do not have contraindications. Carvedilol will be initiated at a starting dose of 3.125 mg twice a day with dose increases as tolerated. Carvedilol was chosen based on its favorable effects in patients with chronic HF [35]. Once a patient reaches the maximum tolerated dose of carvedilol twice daily, ramipril will be added at 1.25 mg daily and increased as tolerated up to the maximum dose of 10 mg. Patients who are on different ACE inhibitors, including lisinopril and enalapril, for the treatment of blood pressure or other causes will be allowed to continue. Figure 3 depicts study events in patients initially not on beta‐blocker, ACE inhibitor, or angiotensin receptor blockers (ARBs) at the time of study entry and who are able to tolerate dose increases without developing hypotension, bradycardia, or other side effects. In patients who have a history of allergy or intolerance to ACE inhibitors, an ARB will be started instead of ACE inhibitors. Candesartan will be considered the first ARB of choice. Other approved ARBs can be used depending on clinical circumstances or insurance coverage at the discretion of the study cardiologist(s). If a patient cannot receive any of the cardiac medications due to existing contraindications or intolerance, the patient can still participate in the study. At present there is no evidence that any cardiac medication prevents or slows the mild LVEF decline that occurs with HER2 targeted agents that would warrant exclusion of patients who cannot tolerate a specific cardiac medication. Therefore, the design of our study is based on clinical strategy of optimization of risk factors (high blood pressure, hypercholesterolemia) and neurohormonal blockade in doses that are tolerated.
Flow diagram of cardiac medication titration. After study enrollment, cardiac treatment with beta blockers and angiotensin‐converting‐enzyme inhibitors will be started in all patients who do not have contraindications. Once a patient reaches the maximum tolerated dose of carvedilol twice daily, ramipril will be added at 1.25 mg daily and increased as tolerated up to the maximum dose of 10 mg.
Abbreviations: bid, twice a day; HER2, human epidermal growth receptor 2; qd, once a day.
Assessment of Safety
Safety assessments will consist of monitoring and recording adverse events (AE) of special interest and serious adverse events (SAE). AE of special interest for this study include symptoms attributable to HF (shortness of breath, orthopnea, paroxysmal nocturnal dyspnea, bilateral ankle swelling), cardiac arrhythmia requiring pharmacological or electrical treatment or/and myocardial infarction. All patients will be followed for SAE and AE of special interest for 6 months after being off study. Asymptomatic declines in LVEF will not be reported, except in the case of an asymptomatic decline in LVEF ≥ 10% points from baseline to an LVEF ≤35%. As an investigator‐initiated study that utilizes FDA approved agents, this trial is considered of moderate risk and the following safety procedures have been implemented: (a) real‐time safety monitoring by the Principal Investigator, coinvestigators, and study team during weekly study group meetings and monthly breast cancer network disease teleconferences; (b) semi‐annual reviews by the Lombardi Comprehensive Cancer Center Data and Safety Monitoring Committee (DSMC); (c) addition of an external cardiologist with expertise in cardiotoxicity to the DSMC; and (d) formation of an internal cardiac review panel that reviews all cardiac safety data quarterly and independently assesses any cardiac events within 3 weeks of any CE.
Statistical Considerations
There is no data about the current proportion of patients with reduced cardiac function who are receiving HER2 targeted therapy, but we estimate it to be fewer than 10%. We propose that, if 30% or more of patients with reduced LVEF and HER2 positive breast cancer are able to safely receive HER2 targeted therapy on study, this would represent a clinically meaningful increase in patients receiving treatment with documented oncologic benefit. Therefore, we define a completion rate of 30% as clinically relevant, and a completion rate of 10% as similar to current practice.
A sample size of 30 patients is planned based on the primary endpoint of completion of HER2 targeted therapy without a CE. A two‐stage design is planned to test if the completion rate is at least 30% versus below 10% with 80% power at a significance level of 5% . At the first stage, 15 patients will be entered. If one or more patients complete therapy in the absence of CE, then an additional 15 patients will be enrolled in the second stage; if none of the 15 patients in the first stage complete the therapy, we will conclude that the therapy is not feasible in this patient population. The chance for a reversal of the conclusion either for efficacy or for futility based on this decision rule is less than 2% (the discordance probability). At the completion of the second stage, if more than 6 of the 30 patients complete the therapy, we will conclude that the therapy is feasible in this patient population. Early stopping rules are incorporated for safety based on cardiac death and symptomatic HF separately. If one cardiac death related to treatment is observed, the trial will be terminated. If three patients experience symptoms of HF confirmed by the cardiologist, then the trial will be terminated.
Discussion
Cardiac toxicity associated with breast cancer therapies poses cardiac and oncological challenges. In patients with HER2 positive breast cancer, left ventricular dysfunction, even if it is asymptomatic, continues to limit the use of HER2 targeted therapies that are associated with substantial oncological benefits . In an era of an increasing survival in the population of patients with HER2 positive breast cancer, it is fundamental that oncologists and cardiologists work together to achieve the best outcomes. To our knowledge, SAFE‐HEaRt is the first clinical trial that prospectively tests the hypothesis that HER2 targeted therapies may be safely administered in patients with mildly reduced cardiac function in the setting of ongoing cardiac treatment and monitoring. Its design incorporates cardiovascular assessments before and during HER2 targeted treatment to assure individualized management of cardiovascular risk factors and initiation of beta‐blockers and renin‐angiotensin‐aldosterone system (RAAS) inhibitors for treatment of early cardiomyopathy. The results of this study will provide cardiac safety data and inform consideration of clinical practice changes in patients with HER2 positive breast cancer and reduced cardiac function, as well as provide information regarding cardiovascular monitoring and treatment in this population.
Mechanisms of HER2‐targeted‐therapy‐associated cardiac dysfunction have not been fully elucidated but are proposed to include a cardiac injury that initiates ventricular remodeling and ultimately leads to LV function decline. Contemporary clinical guidelines for heart failure [38] recognize development of structural abnormalities, including asymptomatic LV function decline, as stage B HF and recommend use of neurohormonal agents, including beta‐adrenergic receptor blockers and RAAS inhibitors, to prevent progression and HF symptoms. The evidence for this approach stems mostly from cardiovascular studies that did not include patients on cancer therapies, but newer trials indicate safety and potential benefit of these agents in patients undergoing cancer treatment . Our choice of the specific agents used in this trial, carvedilol and ramipril, was driven by extrapolation from HF trials that included symptomatic, stage C HF patients since large randomized studies in stage B are not available. It is important to note that our trial was designed to test the strategy of initiation and optimization of HF treatment based on individual patient tolerability, rather than benefit of any specific HF agent. For example, in patients with low heart rates at risk for symptomatic bradycardia, carvedilol may be omitted, patients with poor renal function may not receive ACE‐inhibitors, and patients with significant hypertension may require additional agents for optimal blood pressure control all while continuing HER2 targeted therapy. We believe that this approach strengthens the study as it allows us to maximize the number of eligible patients and address potentially heterogeneous CV risk factors.
Three recent randomized placebo‐controlled trials investigated the use of cardioprotective strategies to prevent LVEF decline and assure continuation of HER2 targeted therapy in patients with HER2‐positive breast cancer (Table 2). Overall, they demonstrated safety of neurohormonal agents, beta‐blockers or/and RAAS inhibitors, and two trials showed positive, although small, improvements in LV function in patients receiving cardioprotection compared to placebo. Unlike SAFE‐HEaRt, these placebo‐controlled trials were designed to test the efficacy of a specific agent in preventing LVEF decline. Interestingly, in the report by Pituskin et al. [25], bisoprolol (beta‐blocker) was associated with less LVEF decline and a higher number of patients receiving trastuzumab without interruption, compared to perindopril (ACE inhibitor) and placebo. In contrast, Gulati et al. [26] showed the beneficial effect of candesartan (ARB), but did not find any significant effect of metoprolol or placebo on preventing LVEF decline in patients receiving epirubicin and trastuzumab. Most recently, a randomized trial in 210 women with HER2 positive, early breast cancer reported no significant effect of candesartan compared to placebo on trastuzumab‐associated cardiotoxicity [27], leaving open questions about a single, evidence‐based cardioprevention drug. All three noted trials focused on primary prevention in which patients who were eligible had normal cardiac function, and the event rates were low compared to historic estimates, thus suggesting that the risk of trastuzumab‐associated cardiotoxicity in this population may be too low to warrant primary prevention. In contrast, the SAFE‐HEaRt is the first trial to investigate a strategy of continuing or initiating HER2 targeted therapy in higher risk patients with mild cardiac dysfunction and concomitant cardiac treatment using beta‐blockers, ACE inhibitors, and/or ARBs, based on individual tolerability.
Studies investigating cardiac pharmacologic intervention to prevent cardiotoxicity associated with HER2‐targeted therapy
| ClinicalTrials.gov number . | Study name . | Oncology setting/eligibility criteria . | Cardiac intervention (all primary prevention) . | Primary outcome measure . |
|---|---|---|---|---|
| NCT01434134 | Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA) | Early breast cancer and plan to administer epirubicin‐based adjuvant therapy with or without HER2 targeted therapy | Randomized, double blind placebo‐controlled trial of candesartan and metoprolol | Change in left ventricular ejection fraction, as assessed by cardiac MRI |
| NCT01016886 | Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research (MANTICORE) | Diagnosis of HER2 positive breast cancer and eligible to receive trastuzumab | Randomized, double blind, placebo‐controlled, trial of perindopril and bisoprolol | Left ventricular remodeling measured by cardiac MRI |
| NCT00459771 | Evaluating the Effect of Candesartan vs. Placebo in Prevention of Trastuzumab‐associated Cardiotoxicity | HER2 positive breast cancer and plan for trastuzumab treatment | Randomized, double blind, placebo‐controlled trial of candesartan | The occurrence of cardiotoxicity, defined as a decline in LVEF (MUGA) of more than 15% or a decrease of less than 15% to an absolute value below 45% |
| NCT01009918 | Lisinopril or Coreg CR in Reducing Side Effects in Women with Breast Cancer Receiving Trastuzumab | HER2 positive breast cancer with plan to administer neoadjuvant or adjuvant trastuzumab therapy | Phase II placebo‐controlled trial of lisinopril and carvedilol | Reduction in incidence of trastuzumab‐induced cardiotoxicity after 52 weeks of treatment as measured by preservation of LVEF |
| NCT02177175 | Carvedilol for the Prevention of Anthracycline/Anti‐HER2 Therapy Associated Cardiotoxicity Among Women with HER2‐Positive Breast Cancer Using Myocardial Strain Imaging for Early Risk Stratification | HER2‐positive breast cancer and planned to receive anthracycline chemotherapy followed by anti‐HER2 therapy | Phase II, placebo‐controlled study of carvedilol | Maximum change in LVEF (measured by echocardiography) |
| ClinicalTrials.gov number . | Study name . | Oncology setting/eligibility criteria . | Cardiac intervention (all primary prevention) . | Primary outcome measure . |
|---|---|---|---|---|
| NCT01434134 | Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA) | Early breast cancer and plan to administer epirubicin‐based adjuvant therapy with or without HER2 targeted therapy | Randomized, double blind placebo‐controlled trial of candesartan and metoprolol | Change in left ventricular ejection fraction, as assessed by cardiac MRI |
| NCT01016886 | Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research (MANTICORE) | Diagnosis of HER2 positive breast cancer and eligible to receive trastuzumab | Randomized, double blind, placebo‐controlled, trial of perindopril and bisoprolol | Left ventricular remodeling measured by cardiac MRI |
| NCT00459771 | Evaluating the Effect of Candesartan vs. Placebo in Prevention of Trastuzumab‐associated Cardiotoxicity | HER2 positive breast cancer and plan for trastuzumab treatment | Randomized, double blind, placebo‐controlled trial of candesartan | The occurrence of cardiotoxicity, defined as a decline in LVEF (MUGA) of more than 15% or a decrease of less than 15% to an absolute value below 45% |
| NCT01009918 | Lisinopril or Coreg CR in Reducing Side Effects in Women with Breast Cancer Receiving Trastuzumab | HER2 positive breast cancer with plan to administer neoadjuvant or adjuvant trastuzumab therapy | Phase II placebo‐controlled trial of lisinopril and carvedilol | Reduction in incidence of trastuzumab‐induced cardiotoxicity after 52 weeks of treatment as measured by preservation of LVEF |
| NCT02177175 | Carvedilol for the Prevention of Anthracycline/Anti‐HER2 Therapy Associated Cardiotoxicity Among Women with HER2‐Positive Breast Cancer Using Myocardial Strain Imaging for Early Risk Stratification | HER2‐positive breast cancer and planned to receive anthracycline chemotherapy followed by anti‐HER2 therapy | Phase II, placebo‐controlled study of carvedilol | Maximum change in LVEF (measured by echocardiography) |
Abbreviations: HER2, human epidermal growth receptor 2; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; MUGA, multigated acquisition scan.
Studies investigating cardiac pharmacologic intervention to prevent cardiotoxicity associated with HER2‐targeted therapy
| ClinicalTrials.gov number . | Study name . | Oncology setting/eligibility criteria . | Cardiac intervention (all primary prevention) . | Primary outcome measure . |
|---|---|---|---|---|
| NCT01434134 | Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA) | Early breast cancer and plan to administer epirubicin‐based adjuvant therapy with or without HER2 targeted therapy | Randomized, double blind placebo‐controlled trial of candesartan and metoprolol | Change in left ventricular ejection fraction, as assessed by cardiac MRI |
| NCT01016886 | Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research (MANTICORE) | Diagnosis of HER2 positive breast cancer and eligible to receive trastuzumab | Randomized, double blind, placebo‐controlled, trial of perindopril and bisoprolol | Left ventricular remodeling measured by cardiac MRI |
| NCT00459771 | Evaluating the Effect of Candesartan vs. Placebo in Prevention of Trastuzumab‐associated Cardiotoxicity | HER2 positive breast cancer and plan for trastuzumab treatment | Randomized, double blind, placebo‐controlled trial of candesartan | The occurrence of cardiotoxicity, defined as a decline in LVEF (MUGA) of more than 15% or a decrease of less than 15% to an absolute value below 45% |
| NCT01009918 | Lisinopril or Coreg CR in Reducing Side Effects in Women with Breast Cancer Receiving Trastuzumab | HER2 positive breast cancer with plan to administer neoadjuvant or adjuvant trastuzumab therapy | Phase II placebo‐controlled trial of lisinopril and carvedilol | Reduction in incidence of trastuzumab‐induced cardiotoxicity after 52 weeks of treatment as measured by preservation of LVEF |
| NCT02177175 | Carvedilol for the Prevention of Anthracycline/Anti‐HER2 Therapy Associated Cardiotoxicity Among Women with HER2‐Positive Breast Cancer Using Myocardial Strain Imaging for Early Risk Stratification | HER2‐positive breast cancer and planned to receive anthracycline chemotherapy followed by anti‐HER2 therapy | Phase II, placebo‐controlled study of carvedilol | Maximum change in LVEF (measured by echocardiography) |
| ClinicalTrials.gov number . | Study name . | Oncology setting/eligibility criteria . | Cardiac intervention (all primary prevention) . | Primary outcome measure . |
|---|---|---|---|---|
| NCT01434134 | Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA) | Early breast cancer and plan to administer epirubicin‐based adjuvant therapy with or without HER2 targeted therapy | Randomized, double blind placebo‐controlled trial of candesartan and metoprolol | Change in left ventricular ejection fraction, as assessed by cardiac MRI |
| NCT01016886 | Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research (MANTICORE) | Diagnosis of HER2 positive breast cancer and eligible to receive trastuzumab | Randomized, double blind, placebo‐controlled, trial of perindopril and bisoprolol | Left ventricular remodeling measured by cardiac MRI |
| NCT00459771 | Evaluating the Effect of Candesartan vs. Placebo in Prevention of Trastuzumab‐associated Cardiotoxicity | HER2 positive breast cancer and plan for trastuzumab treatment | Randomized, double blind, placebo‐controlled trial of candesartan | The occurrence of cardiotoxicity, defined as a decline in LVEF (MUGA) of more than 15% or a decrease of less than 15% to an absolute value below 45% |
| NCT01009918 | Lisinopril or Coreg CR in Reducing Side Effects in Women with Breast Cancer Receiving Trastuzumab | HER2 positive breast cancer with plan to administer neoadjuvant or adjuvant trastuzumab therapy | Phase II placebo‐controlled trial of lisinopril and carvedilol | Reduction in incidence of trastuzumab‐induced cardiotoxicity after 52 weeks of treatment as measured by preservation of LVEF |
| NCT02177175 | Carvedilol for the Prevention of Anthracycline/Anti‐HER2 Therapy Associated Cardiotoxicity Among Women with HER2‐Positive Breast Cancer Using Myocardial Strain Imaging for Early Risk Stratification | HER2‐positive breast cancer and planned to receive anthracycline chemotherapy followed by anti‐HER2 therapy | Phase II, placebo‐controlled study of carvedilol | Maximum change in LVEF (measured by echocardiography) |
Abbreviations: HER2, human epidermal growth receptor 2; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; MUGA, multigated acquisition scan.
Our choice of primary endpoint, the proportion of patients who complete planned oncological therapy, was based on the clinical rationale that at the present time very few patients with HER2 positive breast cancer and decreased cardiac function receive HER2 targeted therapy and are therefore at increased risk for adverse oncologic outcomes. We are using further LVEF decline of ≥10% points from baseline or to ≤35%, corroborated by confirmatory echocardiogram, in 2–4 weeks as a criterion for asymptomatic worsening of cardiac function. As our primary outcome and clinical decision making rely on echocardiography, we have included centralized image review and reporting through the Cardiovascular Imaging Core Lab. This approach assures standardized LVEF assessment and reduces inter‐reader variability that is a known challenge in clinical echocardiography laboratories. While relatively novel in oncology trials (we are not aware of another randomized clinical oncology trial that has used Core Lab reporting for decision making), Core Labs are an intrinsic part of most cardiovascular large trials where echocardiographic measurements represent key or important outcomes [33].
Conclusion
In summary, the SAFE‐HEaRt trial will provide the first prospective data about the safety of HER2 targeted therapies in patients with HER2 positive breast cancer and cardiac dysfunction. Its design implements early initiation and optimization of beta‐blockers, ACE inhibitors and/or ARBs, and close cardiac clinical and echocardiographic monitoring with Core Lab confirmation and reporting. The results of our trial will have the potential to impact clinical practice and inform the design of future clinical trials aimed at improving cardiovascular and oncological health of patients with cancer and reduced LV function.
Trial Registration: ClinicalTrials.gov NCT01904903
Acknowledgments
This trial is partially supported by Genentech, Inc. and funded by a Young Investigator Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology. Biostatistics and bioinformatics data reported in this publication was supported by National Cancer Institute of the National Institutes of Health under award number P30CA051008 (PI Weiner) as a shared resource. Neither F. Hoffmann‐La Roche AG, nor Genentech, Inc., nor the Conquer Cancer Foundation had a role in the design of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
Conception/Design: Filipa Lynce, Ana Barac, Ming T. Tan, Federico M. Asch, Karen L. Smith, Chau Dang, Claudine Isaacs, Sandra M. Swain
Provision of study material or patients: Filipa Lynce, Ana Barac, Ming T. Tan, Federico M. Asch, Karen L. Smith, Chau Dang, Claudine Isaacs, Sandra M. Swain
Collection and/or assembly of data: Filipa Lynce, Ana Barac, Ming T. Tan, Federico M. Asch, Karen L. Smith, Chau Dang, Claudine Isaacs, Sandra M. Swain
Data analysis and interpretation: Filipa Lynce, Ana Barac, Ming T. Tan, Federico M. Asch, Karen L. Smith, Chau Dang, Claudine Isaacs, Sandra M. Swain
Manuscript writing: Filipa Lynce, Ana Barac, Ming T. Tan, Federico M. Asch, Karen L. Smith, Chau Dang, Claudine Isaacs, Sandra M. Swain
Final approval of manuscript: Filipa Lynce, Ana Barac, Ming T. Tan, Federico M. Asch, Karen L. Smith, Chau Dang, Claudine Isaacs, Sandra M. Swain
Disclosures
Filipa Lynce: Genentech, Pfizer (RF); Karen L. Smith: Abbvie, Abbott Labs (OI [spouse]); Chau Dang: GlaxoSmithKline, Genentech, Roche (RF); Claudine Isaacs: Genentech (H, RF); Sandra M. Swain: Genentech (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
Author notes
† Contributed equally.
Disclosures of potential conflicts of interest may be found at the end of this article.