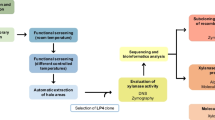
Abstract
Objective
This study aimed to clone and characterize the oxiranedicarboxylate hydrolase (ORCH) from Labrys sp. WH-1.
Methods
Purification by column chromatography, characterization of enzymatic properties, gene cloning by protein terminal sequencing and polymerase chain reaction (PCR), and sequence analysis by secondary structure prediction and multiple sequence alignment were performed.
Results
The ORCH from Labrys sp. WH-1 was purified 26-fold with a yield of 12.7%. It is a monomer with an isoelectric point (pl) of 8.57 and molecular mass of 30.2 kDa. It was stable up to 55 °C with temperature at which the activity of the enzyme decreased by 50% in 15 min (T5015) of 61 °C and the half-life at 50 °C (t1/2, 50 °c) of 51 min and was also stable from pH 4 to 10, with maximum activity at 55 °C and pH 8.5. It is a metal-independent enzyme and strongly inhibited by Cu2+, Ag+, and anionic surfactants. Its kinetic parameters (Km, kcat, and kcat/Km) were 18.7 mmol/L, 222.3 s−1, and 11.9 mmol/(L·s), respectively. The ORCH gene, which contained an open reading frame (ORF) of 825 bp encoding 274 amino acid residues, was overexpressed in Escherichia coli and the enzyme activity was 33 times higher than that of the wild strain.
Conclusions
The catalytic efficiency and thermal stability of the ORCH from Labrys sp. WH-1 were the best among the reported ORCHs, and it provides an alternative catalyst for preparation of l(+)-2,3-dihydrobutanedioic acid.
概要
目 的
克隆双头菌 WH-1 环氧乙烷二酸水解酶(ORCH) 的基因并研究其酶学性质。
创新点
首次获得双头菌 ORCH 的基因, 且该酶催化效率 高, 热稳定性好。
方 法
柱层析纯化 ORCH 后, 进行酶学性质研究; 通过 蛋白末端测序和PCR 获得其基因序列; 通过二级 结构预测和多序列比对进行 ORCH 序列分析。
结 论
来源于双头菌 WH-1 的 ORCH 是迄今报道的催化 效率和热稳定性最好的 ORCH, 为 l(+)-2,3-二羟 基丁二酸的生产提供了新的催化剂。
Similar content being viewed by others
References
Bao WN, Pan HF, Zhang ZH, et al., 2014. Analysis of essential amino acid residues for catalytic activity of cis-epoxysuccinate hydrolase from Bordetella sp. BK-52. Appl Microbiol Biotechnol 98(4): 1641–1649. https://doi.org/10.1007/s00253-013-5019-2
Bao WN, Pan HF, Zhang ZH, et al., 2015. Isolation of the stable strain Labrys sp. BK-8 for L(+)-tartaric acid production. J Biosci Bioeng 119(5): 538–542. https://doi.org/10.1016/j.jbiosc.2014.10.013
Cheng YQ, Wang L, Pan HF, et al., 2014a. Purification and characterization of a novel cis-epoxysuccinate hydrolase from Klebsiella sp. that produces L(+)-tartaric acid. Biotechnol Lett 36(11): 2325–2330. https://doi.org/10.1007/s10529-014-1614-2
Cheng YQ, Pan HF, Bao WN, et al., 2014b. Cloning, homology modeling, and reaction mechanism analysis of a novel cis-epoxysuccinate hydrolase from Klebsiella sp. Biotechnol Lett 36(12): 2537–2544. https://doi.org/10.1007/s10529-014-1638-7
Dong S, Liu X, Cui G, et al., 2018. Structural insight into the catalytic mechanism of a cis-epoxysuccinate hydrolase producing enantiomerically pure D(−)-tartaric acid. Chem Commun (Camb) 54(61): 8482–8485. https://doi.org/10.1039/c8cc04398a
Liu ZQ, Li Y, Ping LF, et al., 2007a. Isolation and identification of a novel Rhodococcus sp. ML-0004 producing epoxide hydrolase and optimization of enzyme production. Process Biochem 42(5): 889–894. https://doi.org/10.1016/j.procbio.2007.01.009
Liu ZQ, Li Y, Xu YY, et al., 2007b. Cloning, sequencing, and expression of a novel epoxide hydrolase gene from Rhodococcus opacus in Escherichia coli and characterization of enzyme. Appl Microbiol Biotechnol 74(1): 99–106. https://doi.org/10.1007/s00253-006-0635-8
Martin WR, Foster JW, 1955. Production of trans-L-epoxysuccinic acid by fungi and its microbiological conversion to meso-tartartic acid. J Bacteriol 70(4): 405–414.
Pan HF, Xie ZP, Bao WN, et al., 2008. Isolation and identification of a novel cis-epoxysuccinate hydrolase-producing Bordetella sp. BK-52 and optimization of enzyme production. Acta Microbiol Sin 48(8): 1075–1081 (in Chinese). https://doi.org/10.13343/j.cnki.wsxb.2008.08.012
Pan HF, Bao WN, Xie ZP, et al., 2010. Molecular cloning and characterization of a cis-epoxysuccinate hydrolase from Bordetella sp. BK-52. J Microbiol Biotechnol 20(4): 659–665. https://doi.org/10.4014/jmb.0905.05059
Pan HF, Xie ZP, Bao WN, et al., 2011. Site-directed mutagenesis of epoxide hydrolase to probe catalytic amino acid residues and reaction mechanism. FEBS Lett 585(15): 2545–2550. https://doi.org/10.1016/j.febslet.2011.07.006
Qiao P, Wu MB, Zhu L, et al., 2015. Enhancing the thermal tolerance of a cis-epoxysuccinate hydrolase via combining directed evolution with various semi-rational redesign methods. J Mol Catal B Enzym 121: 96–103. https://doi.org/10.1016/j.molcatb.2015.08.010
Salmanowicz BP, Langner M, Franaszek S, 2014. Charge-based characterisation of high-molecular-weight glutenin subunits from common wheat by capillary isoelectric focusing. Talanta 129: 9–14. https://doi.org/10.1016/j.talanta.2014.04.055
Wang ZQ, Wang YS, Su ZG, 2013. Purification and characterization of a cis-epoxysuccinic acid hydrolase from Nocardia tartaricans CAS-52, and expression in Escherichia coli. Appl Microbiol Biotechnol 97(6): 2433–2441. https://doi.org/10.1007/s00253-012-4102-4
Yuan L, Sadiq FA, Liu TJ, et al., 2018. Spoilage potential of psychrotrophic bacteria isolated from raw milk and the thermo-stability of their enzymes. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 19(8): 630–642. https://doi.org/10.1631/jzus.B1700352
Zhu J, Zhu LWS, Yang JH, et al., 2018. Proteomic analysis of human umbilical vein endothelial cells exposed to PM2.5. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 19(6): 458–470. https://doi.org/10.1631/jzus.B1700103
Author information
Authors and Affiliations
Contributions
Wen-na BAO and Zi-sheng LUO designed the research and wrote the manuscript. Shi-wang LIU polished the English. Yuan-feng WU and Gong-nian XIAO analyzed the data. Pei-lian WEI and Yong LIU performed the experiments. All authors read and approved the final manuscript. Therefore, all authors have full access to all the data in the study and take responsibility for the integrity and security of the data.
Corresponding author
Ethics declarations
Wen-na BAO, Zi-sheng LUO, Shi-wang LIU, Yuan-feng WU, Pei-lian WEI, Gong-nian XIAO, and Yong LIU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LQ19C200001), the Education Department of Zhejiang Province Scientific Research Project (No. Y201737925), the Zhejiang Provincial Key Laboratory for Chemical and Biological Processing Technology of Farm Products (No. 2016KF0048), and the Key Laboratory of Bioorganic Synthesis of Zhejiang Province (No. 20170110), China
Electronic supplementary materials
Rights and permissions
About this article
Cite this article
Bao, Wn., Luo, Zs., Liu, Sw. et al. Cloning and characterization of an oxiranedicarboxylate hydrolase from Labrys sp. WH-1. J. Zhejiang Univ. Sci. B 20, 995–1002 (2019). https://doi.org/10.1631/jzus.B1900392
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1900392