Abstract
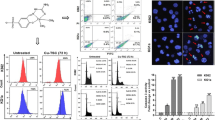
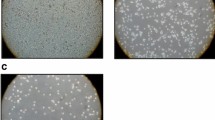
It has been reported that Ethaselen shows inhibitory effects on thioredoxin reductase (TrxR) activity and human tumor cell growth. In order to find an efficient way to reverse cisplatin resistance, we investigated the reversal effects of Ethaselen on cisplatin resistance in K562/cisplatin (CDDP) cells that were established by pulse-inducing human erythrocyte leukemic cell line K562, which are fivefold more resistant to cisplatin compared to K562 cells. The morphology and growth showed that the adhesion of K562/CDDP further decreased while the cell volume increased. The proliferation of K562/CDDP is strengthened. The antitumor activities in vitro were assessed by MTT (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and combination index (CI), showing the significant synergic effects of cisplatin and Ethaselen. Focusing on apoptosis, a series of comparisons was made between K562 and K562/CDDP. Cisplatin induced higher reactive oxygen species (ROS) generation in K562 and subsequently induced the formation of mitochondrial permeability transition pores (PTPs). In addition, cisplatin increased the ratio of Bax to Bcl-2 in K562, which can influence the mitochondrial membrane permeability. PTP formation and mitochondrial membrane permeabilization eventually resulted in the release of cytochrome c and activation of the Caspase pathway. However, these effects were not clearly seen in K562/CDDP, which may be the reason for the acquired CDDP resistance. However, Ethaselen can induce a high level of ROS in K562/CDDP by TrxR activity inhibition and increased ratio of Bax to Bcl-2 in K562/CDDP by nuclear factor κB (NF-κB) suppression, which subsequently induces the release of cytochrome c in K562/CDDP. This response is partly responsible for the reversal of the cisplatin resistance in K562/CDDP cells.
摘 要
目 的
研究凋亡调控相关蛋白来了解顺铂耐药成因, 同 时考察乙烷硒啉(Ethaselen)在K562 耐药细胞 中逆转顺铂耐药的作用, 并初步探讨其作用机 制。
创新点
首次研究乙烷硒啉在逆转顺铂耐药中的作用, 且 此作用与乙烷硒啉诱导细胞凋亡相关。
方 法
通过长时间脉冲诱导得到顺铂耐药K562 细胞, 并观察耐药细胞形态及倍增时间。采用MTT 法 考察乙烷硒啉、顺铂及其联用组在不同细胞株间 的生长抑制作用。流式细胞术分析细胞凋亡情况 以及细胞内活性氧(ROS)水平。最后, 通过蛋 白质免疫印迹(Western blot)考察凋亡调控相关 蛋白水平的变化。
结 论
脉冲诱导得到的K562 耐药细胞对顺铂的耐受性 是原K562 细胞的5.34 倍。形态学观察发现, 耐 药细胞体积增大, 粘附性进一步降低。乙烷硒啉 与顺铂联用表现出协同效应。当加入少量的乙烷 硒啉(顺铂与乙烷硒啉的摩尔比率为10:1), 顺 铂作用K562 耐药细胞的半抑制浓度(IC50)值可 以减少21 倍。流式细胞术及Western blot 表明, 乙烷硒啉能够诱导耐药细胞凋亡。其逆转顺铂耐 药主要是通过调控Bcl-2 及Bax 蛋白比例以及通 过提高细胞内活性氧水平引起线粒体通透转运 孔道(PTP)蛋白孔道的形成来促使释放细胞色 素c, 进而引起Caspase 凋亡途径。
Similar content being viewed by others
References
Bargou, R.C., Bommert, K., Weinmann, P., et al., 1995. Induction of Bax-a precedes apoptosis in a human B lymphoma cell line: potential role of the bcl-2 gene family in surface IgM-mediated apoptosis. Eur. J. Immunol., 25(3):770–775. http://dx.doi.org/10.1002/eji.1830250322
Chao, C.C., Huang, S.L., Huang, H.M., et al., 1991. Crossresistance to UVradiation of a cisplatin-resistant human cell line: overexpression of cellular factors that recognize UV-modified DNA. Mol. Cell. Biol., 11(4):2075–2080. http://dx.doi.org/10.1128/MCB.11.4.2075
Chen, J., Li, H.M., Zhang, X.N., et al., 2014. Dioscin-induced apoptosis of human LNCaP prostate carcinoma cells through activation of caspase-3 and modulation of Bcl-2 protein family. J. Huazhong Univ. Sci. Technol. Med. Sci., 34(1):125–130. http://dx.doi.org/10.1007/s11596-014-1243-y
Chu, G., 1994. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J. Biol. Chem., 269(2):787–790.
Du, J.P., Chen, P., Fei, H.X., et al., 2007. Establishment of adriamycin-resistant and cisplatin-resistant gastric cancer cell lines and assessment on their sustainability of drug resistance. Chin. J. Gastroenterol. Hepatol., 16(4):368–372 (in Chinese). http://dx.doi.org/10.3969/j.issn.1006-5709.2007.04.022
Dumay, A., Laulier, C., Bertrand, P., et al., 2006. Bax and Bid, two proapoptotic Bcl-2 family members, inhibit homologous recombination, independently of apoptosis regulation. Oncogene, 25(22):3196–3205. http://dx.doi.org/10.1038/sj.onc.1209344
Fu, J.N., Li, J., Tan, Q., et al., 2011. Thioredoxin reductase inhibitor ethaselen increases the drug sensitivity of the colon cancer cell line LoVo towards cisplatin via regulation of G1 phase and reversal of G2/M phase arrest. Invest. New Drugs, 29(4):627–636. http://dx.doi.org/10.1007/s10637-010-9401-y
Hahn, P., Lindsten, T., Ying, G.S., et al., 2003. Proapoptotic Bcl-2 family members, Bax and Bak, are essential for developmental photoreceptor apoptosis. Invest. Ophthalmol. Vis. Sci., 44(8):3598–3605. http://dx.doi.org/10.1167/iovs.02-1113
Harrington, C.F., le Pla, R.C., Jones, G.D., et al., 2010. Determination of cisplatin 1,2-intrastrand guanine-guanine DNA adducts in human leukocytes by high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry. Chem. Res. Toxicol., 23(8): 1313–1321. http://dx.doi.org/10.1021/tx100023c
Haslehurst, A.M., Koti, M., Dharsee, M., et al., 2012. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer, 12:91. http://dx.doi.org/10.1186/1471-2407-12-91
Heath-Engel, H.M., Chang, N.C., Shore, G.C., 2008. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene, 27(50):6419–6433. http://dx.doi.org/10.1038/onc.2008.309
Jia, G., Wang, Q., Wang, R., et al., 2015. Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome C/Caspase-3 pathway. Onco-Targets Ther., 8:303–311. http://dx.doi.org/10.2147/OTT.S76063
Lan, L., Zhao, F., Wang, Y., et al., 2007. The mechanism of apoptosis induced by a novel thioredoxin reductase inhibitor in A549 cells: possible involvement of nuclear factor-?B-dependent pathway. Eur. J. Pharmacol., 555(2-3): 83–92. http://dx.doi.org/10.1016/j.ejphar.2006.10.037
Mantri, Y., Lippard, S.J., Baik, M.H., 2007. Bifunctional binding of cisplatin to DNA: why does cisplatin form 1,2-intrastrand cross-links with AGbut not with GA? J. Am. Chem. Soc., 129(16):5023–5030. http://dx.doi.org/10.1021/ja067631z
Miyata, Y., Nomata, K., Ohba, K., et al., 2012. Use of lowdose combined therapy with gemcitabine and paclitaxel for advanced urothelial cancer patients with resistance to cisplatin-containing therapy: a retrospective analysis. Cancer Chemother. Pharmacol., 70(3):451–459. http://dx.doi.org/10.1007/s00280-012-1938-3
Oiso, S., Takayama, Y., Nakazaki, R., et al., 2014. Factors involved in the cisplatin resistance of KCP-4 human epidermoid carcinoma cells. Oncol. Rep., 31(2):719–726. http://dx.doi.org/10.3892/or.2013.2896
Rossé, T., Olivier, R., Monney, L., et al., 1998. Bcl-2 prolongs cell survival after Bax-induced release of cytochromec. Nature, 391(6666):496–499. http://dx.doi.org/10.1038/35160
Sainz, R.M., Mayo, J.C., Tan, D.X., et al., 2003. Antioxidant activity of melatonin in Chinese hamster ovarian cells: changes in cellular proliferation and differentiation. Biochem. Biophys. Res. Commun., 302(3):625–634. http://dx.doi.org/10.1016/S0006-291X(03)00230-4
Shi, C., Yu, L., Yang, F., et al., 2003. A novel organoselenium compound induces cell cycle arrest and apoptosis in prostate cancer cell lines. Biochem. Biophys. Res. Commun., 309(3):578–583. http://dx.doi.org/10.1016/j.bbrc.2003.08.032
Tan, Q., Li, J., Yin, H.W., et al., 2010. Augmented antitumor effects of combination therapy of cisplatin with ethaselen as a novel thioredoxin reductase inhibitor on human A549 cell in vivo. Invest. New Drugs, 28(3):205–215. http://dx.doi.org/10.1007/s10637-009-9235-7
Tanaka, S., Wakeyama, H., Akiyama, T., et al., 2010. Regulation of osteoclast apoptosis by Bcl-2 family protein Bim and Caspase-3. Adv. Exp. Med. Biol., 658:111–116. http://dx.doi.org/10.1007/978-1-4419-1050-9_12
Tao, X.J., Tilly, K.I., Maravei, D.V., et al., 1997. Differential expression of members of the bcl-2 gene family in proliferative and secretory human endometrium: glandular epithelial cell apoptosis is associated with increased expression of bax. J. Clin. Endocrinol. Metab., 82(8):2738–2746. http://dx.doi.org/10.1210/jcem.82.8.4146
Tilly, J.L., Tilly, K.I., Kenton, M.L., et al., 1995. Expression of members of the bcl-2 gene family in the immature rat ovary: equine chorionic gonadotropin-mediated inhibition of granulosa cell apoptosis is associated with decreased bax and constitutive bcl-2 and bcl-xlong messenger ribonucleic acid levels. Endocrinology, 136(1): 232–241. http://dx.doi.org/10.1210/endo.136.1.7828536
Torigoe, T., Izumi, H., Ishiguchi, H., et al., 2005. Cisplatin resistance and transcription factors. Curr. Med. Chem. Anticancer Agents, 5(1):15–27. http://dx.doi.org/10.2174/1568011053352587
Wang, L., Fu, J.N., Wang, J.Y., et al., 2011. Seleniumcontaining thioredoxin reductase inhibitor ethaselen sensitizes non-small cell lung cancer to radiotherapy. Anticancer Drugs, 22(8):732–740. http://dx.doi.org/10.1097/CAD.0b013e32834618bc
Wang, L., Yang, Z., Fu, J., et al., 2012. Ethaselen: a potent mammalian thioredoxin reductase 1 inhibitor and novel organoselenium anticancer agent. Free Radic. Biol. Med., 52(5):898–908. http://dx.doi.org/10.1016/j.freeradbiomed.2011.11.034
Xing, F., Li, S., Ge, X., et al., 2008. The inhibitory effect of a novel organoselenium compound BBSKE on the tongue cancer Tca8113 in vitro and in vivo. Oral Oncol., 44(10):963–969. http://dx.doi.org/10.1016/j.oraloncology.2007.12.001
Yaneva, J.N., Paneva, E.G., Zacharieva, S.I., et al., 2007. Histone H1 interacts preferentially with DNA fragments containing a cisplatin-induced 1,2-intrastrand cross-link. Z. Naturforsch. C, 62(11–12):905–908. http://dx.doi.org/10.1515/znc-2007-11-1220
Yeh, K.H., Cheng, A.L., 1997. High-dose tamoxifen reverses drug resistance to cisplatin and etoposide in a patient with advanced large cell carcinoma of lung. Anticancer Res., 17(2B):1341–1343.
Zhao, F., Yan, J., Deng, S., et al., 2006. A thioredoxin reductase inhibitor induces growth inhibition and apoptosis in five cultured human carcinoma cell lines. Cancer Lett., 236(1):46–53. http://dx.doi.org/10.1016/j.canlet.2005.05.010
Zheng, S., Zhao, M., Ren, Y., et al., 2015. Sesamin suppresses STZ induced INS-1 cell apoptosis through inhibition of NF-κB activation and regulation of Bcl-2 family protein expression. Eur. J. Pharmacol., 750:52–58. http://dx.doi.org/10.1016/j.ejphar.2015.01.031
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported in part by the National Natural Science Foundation of China (No. 81372266) and the National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2011zx09101-001-03)
ORCID: Suo-fu YE, http://orcid.org/0000-0001-7661-6272
Rights and permissions
About this article
Cite this article
Ye, Sf., Yang, Y., Wu, L. et al. Ethaselen: a novel organoselenium anticancer agent targeting thioredoxin reductase 1 reverses cisplatin resistance in drug-resistant K562 cells by inducing apoptosis. J. Zhejiang Univ. Sci. B 18, 373–382 (2017). https://doi.org/10.1631/jzus.B1600073
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1600073