Abstract
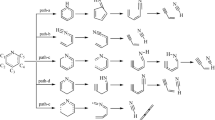
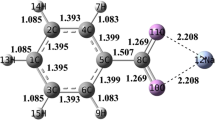
Quantum chemical simulation was used to investigate the catalytic mechanism of Na/K on NO-char heterogeneous reactions during the coal reburning process. Both NO-char and NO-Na/K reactions were considered as three-step processes in this calculation. Based on geometry optimizations made using the UB3LYP/6-31G(d) method, the activation energies of NO-char and NO-Na/K reactions were calculated using the QCISD(T)/6-311G(d, p) method; Results showed that the activation energy of the NO-Na/K reaction (107.9/82.0 kJ/mol) was much lower than that of the NO-char reaction (245.1 kJ/mol). The reactions of NaO/KO and Na2O/K2O reduced by char were also studied, and their thermodynamics were calculated using the UB3LYP/6-31G(d) method; Results showed that both Na and K can be refreshed easily and rapidly by char at high temperature during the coal reburning process. Based on the calculations and analyses, the catalytic mechanism of Na/K on NO-char heterogeneous reactions during the coal reburning process was clarified.
Similar content being viewed by others
References
Aarna, I., Suuberg, E.M., 1997. A review of the kinetics of the nitric oxide-carbon reaction. Fuel, 76(6):475–491. [doi:10.1016/S0016-2361(96)00212-8]
Andersson, S., Marković, N., Nyman, G., 2003. Computational studies of the kinetics of the C+NO and O+CN reactions. The Journal of Physical Chemistry A, 107(28):5439–5447. [doi:10.1021/jp0222604]
Arenillas, A., Josea, J.P., 2002. Nitric oxide reduction in coal combustion: role of char surface complexes in heterogeneous reactions. Environmental Science & Technology, 36(24):5498–5503. [doi:10.1021/es0208198]
Becke, A.D., 1993. Density functional thermochemistry III. The role of exact exchange correlation functions. The Journal of Chemical Physics, 98(7):5648–5652. [doi:10.1063/1.464913]
Chambrion, P., Suzuki, T., Zhang, Z.G., 1996. XPS of nitrogen-containing functional groups formed during the C-NO reaction. Energy and Fuels, 11(3):681–685. [doi:10.1021/ef960109l]
Chen, N., Yang, R.T., 1998. Ab initio molecular orbital study of the unified mechanism and pathways for gas-carbon reactions. The Journal of Physical Chemistry A, 102(31):6348–6356. [doi:10.1021/jp981518g]
Chen, W.Y., Ma, L., 1997. Effect of heterogeneous mechanisms during reburning of nitrogen oxide. AIChE Journal, 42(7):1968–1976. [doi:10.1002/aic.690420717]
Foresman, J.B., Frisch, A., 1996. Exploring Chemistry with Electronic Structure Methods (2nd Ed.). Gaussian, Pittsburgh, PA.
Frisch, M.J., Trucks, G.W., Pople, J.A., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Vreven, J.T., Kudin, K.N., et al., 2003. Gaussian 03. Gaussian, Inc., Pittsburgh, PA.
García-García, A., Illán-Gómez, M.J., Linares-Solano, A., Salinas-Martínez de Lecea, C., 1997. Potassium-containing briquetted coal for the reduction of NO. Fuel, 76(6):499–505. [doi:10.1016/S0016-2361(97)00009-4]
García-García, A., Illán-Gómez, M.J., Linares-Solano, A., Salinas-Martínez de Lecea, C., 2002. NOx reduction by potassium-containing coal briquettes: Effect of preparation procedure and potassium content. Energy and Fuels, 16(3):569–574. [doi:10.1021/ef0101208]
Gauss, J., Cremer, C., 1988. Analytical evaluation of energy gradients in quadratic configuration interaction theory. Chemical Physics Letters, 150(3–4):280–286. [doi:10.1016/0009-2614(88)80042-3]
Gonzalez, C., Schlegel, H.B., 1989. An improved algorithm for reaction path following. The Journal of Chemical Physics, 90(4):2154–2159.
Hampartsoumian, E., Folayan, O.O., Nimmo, W., 2003. Optimisation of NOx reduction in advanced coal reburning systems and the effect of coal type. Fuel, 82(4):373–384. [doi:10.1016/S0016-2361(02)00311-3]
Illán-Gómez, M.J., Raymundo-Piñero, E., García-García, A., Linares-Solano, A., Salinas-Martínez de Lecea, C., 1999. Catalytic NOx reduction by carbon supporting metals. Applied Catalysis B: Environmental, 20(4):267–275. [doi:10.1016/S0926-3373(98)00119-2]
Illán-Gómez, M.J., Brandán, S., Linares-Solano, A., Salinas-Martínez de Lecea, C., 2000. NOx reduction by carbon supporting potassium-bimetallic catalysts. Applied Catalysis B: Environmental, 25(1):11–18. [doi:10.1016/ S0926-3373(99)00120-4]
Illán-Gómez, M.J., Brandán, S., Salinas-Martínez de Lecea, C., Linares-Solano, A., 2001. Improvements in NOx reduction by carbon using bimetallic catalysts. Fuel, 80(14):2001–2005. [doi:10.1016/S0016-2361(01)00091-6]
Kyotani, T., Tomita, A., 1999. Analysis of the reaction of carbon with NO/N2O using Ab initio molecular orbital theory. The Journal of Physical Chemistry B, 103(17):3434–3441. [doi:10.1021/jp9845928]
Lee, C., Yang, W., Parr, R.G., 1988. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B, 37(2):785–789. [doi:10.1103/PhysRevB.37.785]
Liu, H., Hampartsoumian, E., Gibbs, B.M., 1997. Evaluation of the optimal fuel characteristics for efficient NO reduction by coal reburning. Fuel, 76(11):985–993. [doi:10.1016/S0016-2361(97)00114-2]
Maly, P.M., Zamansky, V.M., Locho, R.P., 1999. Alternative fuel reburning. Fuel, 78(3):327–334. [doi:10.1016/S0016-2361(98)00161-6]
Moyeda, D.K., Li, B., Maly, P., 1995. Experimental/Modeling Studies of the Use of Coal-based Reburning Fuels for NOx Control. 12th International Pittsburgh Coal Conference, the Combustion Institute, Pittsburgh, USA, p.1119–1124.
Noda, K., Chambrion, P., Kyotani, T., Tomita, A., 1999. A study of the N2 formation mechanism in carbon-N2O reaction by using isotope gases. Energy and Fuels, 13(4):941–946. [doi:10.1021/ef9900132]
Salter, E.A., Trucks, G.W., Bartlett, R.J., 1989. Analytic energy derivatives in many-body methods: First derivatives. The Journal of Chemical Physics, 90(3):1752–1766. [doi:10.1063/1.456069]
Scott, A.P., Random, L., 1996. Harmonic vibrational frequencies: an evaluation of Hartree-Fock, Moller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. The Journal of Physical Chemistry, 100(41):16502–16513. [doi:10.1021/jp960976r]
Smoot, L.D., Hill, S.C., Xu, H., 1998. NOx control through reburning. Progress in Energy and Combustion on Science, 24(5):385–408. [doi:10.1016/S0360-1285(97)00022-1]
Suzuki, T., Kyotani, T., Tomita, A., 1994. Study on the carbon-nitric oxide reaction in the presence of oxygen. Industrial & Engineering Chemistry Research, 33(11):2840–2845. [doi:10.1021/ie00035a038]
Takuwa, T., Naruse, I., 2007. Detailed kinetic and control of alkali metal compounds during coal combustion. Fuel Processing Technology, 88(11–12):1029–1034. [doi:10.1016/j.fuproc.2007.06.010]
Wang, Z.H., Zhou, J.H., Wen, Z.C., Cen, K.F., 2007. Effect of mineral matter on NO reduction in coal reburning process. Energy and Fuels, 21(4):2038–2043. [doi:10.1021/ ef0604902]
Yang, J., Mestl, G., Herein, D., 2000. Reaction of NO with carbonaceous materials1: 1. Reaction and adsorption of NO on ashless carbon black. Carbon, 38(5):715–727. [doi:10.1016/S0008-6223(99)00150-5]
Zhao, Z.B., Li, W., Li, B.Q., 2002a. Catalytic reduction of NO by coal chars loaded with Ca and Fe in various atmospheres. Fuel, 81(11–12):1559–1564. [doi:10.1016/ S0016-2361(02)00077-7]
Zhao, Z.B., Li, W., Qiu, J.S., Li, B.Q., 2002b. Catalytic effect of Na-Fe on NO-char reaction and NO emission during coal char combustion. Fuel, 81(18):2343–2348. [doi:10.1016/S0016-2361(02)00174-6]
Zhao, Z.B., Qiu, J.S., Wen L., Li, B.Q., 2003. Influence of mineral matter in coal on decomposition of NO over chars and emissions of NO during char combustion. Fuel, 82(8):949–957. [doi:10.1016/S0016-2361(02)00394-0]
Zhong, B.J., Tang, H., 2007. Catalytic effect NO reduction at high temperature by de-ashed chars with catalysts. Combustion and Flame, 149(1–2):234–243. [doi:10.1016/j.combustflame.2006.04.004]
Zhong, B.J., Zhang, H.S., Fu, W.B., 2003. Catalytic effect of KOH on the reaction NO with char. Combustion and Flame, 132(3):364–373. [doi:10.1016/S0010-2180(02)00480-7]
Zhou, H., Qiu, K.Z., Wang, Z.H., Cen, K.F., 2004. Study of coal rank and fineness on NOx reduction with coal reburning technology. Journal of Fuel Chemistry and Technology, 32(2):147–150 (in Chinese).
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the National Science Fund for Distinguished Young Scholars (No. 50525620), and the Key Project of Chinese National Programs for Fundamental Research and Development (No. 2006CB200303), China
Rights and permissions
About this article
Cite this article
Wen, Zc., Wang, Zh., Zhou, Jh. et al. Quantum chemical study on the catalytic mechanism of Na/K on NO-char heterogeneous reactions during the coal reburning process. J. Zhejiang Univ. Sci. A 10, 423–433 (2009). https://doi.org/10.1631/jzus.A0820345
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.A0820345