Abstract
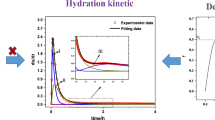
The thermal behavior of magnesium potassium phosphate cement (MKPC) during the early reaction period has been monitored by isothermal conduction calorimetry at 298 K. It is found that the typical heat evolution curve of the MKPC hydration has one endothermic valley and two distinct exothermic peaks. It is believed that the endothermic valley corresponds to the dissolution of potassium dihydrogen phosphate and the exothermic peaks are related to the dissolution of magnesia and the formation of magnesium potassium phosphate hexahydrate, respectively. The influences of the water to powder ratio, the magnesium to phosphate molar ratio and the setting retarder content on the reaction can be reflected in a change of shape and intensity of the peaks on the heat flow curve. The variation trend is consistent with the change of the setting time of MKPC pastes.












Similar content being viewed by others
References
Mabie CP Jr (1973) Petrographic study of the refractory performance of high-fusing dental alloy investments. I. High-fired, phosphate-bonded investments. J Dent Res 52(1):96–110
Yang Q, Zhu B, Wu X (2000) Characteristics and durability test of magnesium phosphate cement-based material for rapid repair of concrete. Mater Struct 33(228):229–234
Neiman R, Sarma AC (1980) Setting and thermal reactions of phosphate investments. J Dent Res 59(9):1478–1485
Abdelrazig BEI, Sharp JH, EI-Jazairi B (1988) The chemical composition of mortars made from magnesia-phosphate cement. Cem Concr Res 18(3):415–425
Hall DA, Stevens R, EI-Jazairi B (1998) Effect of water content on the structure and mechanical properties of magnesia-phosphate cement mortar. J Am Ceram Soc 81(6):1550–1556
Yang Q, Zhang S, Wu X (2002) Deicer-scaling resistance of phosphate cement-based binder for rapid repair of concrete. Cem Concr Res 32(1):165–168
Soudée E, Péra J (2002) Influence of magnesia surface on the setting time of magnesia–phosphate cement. Cem Concr Res 32(1):153–157
Paceagiu J, Georgescu M (2008) The influence of curing conditions on the physical and mechanical properties of magnesia phosphate cements. Rev Chim 59(2):135–139
Soudée E, Péra J (2000) Mechanism of setting reaction in magnesia-phosphate cements. Cem Concr Res 30(2):315–321
Yang Q, Wu X (1999) Factors influencing properties of phosphate cement-based binder for rapid repair of concrete. Cem Concr Res 29(3):389–396
Thomas NL, Jameson DA, Double DD (1981) The effect of lead nitrate on the early hydration of Portland cement. Cem Concr Res 11(1):143–153
Hall DA, Stevens R, El-Jazairi B (2001) The effect of retarders on the microstructure and mechanical properties of magnesia-phosphate cement mortar. Cem Concr Res 31(3):455–465
Ding Z, Li Z (2005) Effect of aggregates and water contents on the properties of magnesium phospho-silicate cement. Cem Concr Compos 27(1):11–18
Wagh AS (2004) Chemically bonded phosphate ceramics: 21st century materials with diverse applications. Elsevier, Oxford
Qiao F, Lin W, Chau CK, Li Z (2009) Property assessment of magnesium phosphate cement. Key Eng Mater 400–402:115–120
Qiao F, Chau CK, Li Z (2009) Setting and compressive strength characteristics of magnesium phosphate cement paste. Adv Cem Res 21(4):175–180
Iyengar SR, Al-Tabbaa A (2007) Developmental study of a low pH magnesium phosphate cement for environmental applications. Environ Technol 28(12):1387–1401
Cahil A, Šoptrajanov B, Najdoski M, Lutz HD, Engelen B, Stefov V (2008) Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (struvite) and its isomorphous analogues. Part VI: FT-IR spectra of isomorphously isolated species. NH4 + ions isolated in MKPO4·6H2O (M = Mg; Ni) and PO4 3− ions isolated in MgNH4AsO4·6H2O. J Mol Struct 876(1–3):255–259
Alonso S, Palomo A (2001) Calorimetric study of alkaline activation of calcium hydroxide–metakaolin solid mixtures. Cem Concr Res 31(1):25–30
Nocuń-Wczelik W, Pytel Z (2004) Heat evolution in hydrated cementitious systems admixtured with different set controlling components. J Therm Anal Calorim 77(1):159–164
Gao WY, Wang YW, Dong LM, Yu ZW (2006) Thermokinetic analysis of the hydration process of calcium phosphate cement. J Therm Anal Calorim 85(3):785–789
Gruyaert E, Robeyst N, De Belie N (2010) Study of the hydration of Portland cement blended with blast-furnace slag by calorimetry and thermogravimetry. J Therm Anal Calorim 102(3):941–951
Qiao F, Chau CK, Li Z (2010) Property evaluation of magnesium phosphate cement mortar as patch repair material. Constr Build Mater 24(5):695–700
Carvalho Arlete M, Segadaes Ana M (2008) The hydration of magnesium phosphate cements: effect of powder characteristics on the reaction kinetics. Mater Sci Forum 591–593:833–838
Qiao F (2010) Reaction mechanisms of magnesium potassium phosphate cement and its application. PhD thesis. Hong Kong University of Science and Technology
Acknowledgments
The financial support from the Hong Kong Research Grant Council under grant 615810 and the China Ministry of Science and Technology under grant 2009CB623200 are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiao, F., Chau, C.K. & Li, Z. Calorimetric study of magnesium potassium phosphate cement. Mater Struct 45, 447–456 (2012). https://doi.org/10.1617/s11527-011-9776-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1617/s11527-011-9776-z