Abstract
Due to the increase of bacterial resistance, the search for new antibiotics is necessary and the medicinal plants represent its most important source. The aim of this study was to evaluate the antibacterial property of extract and fractions from Protium spruceanum leaves, against pathogenic bacteria. By means of diffusion and microdilution assays, the crude extract was active against the nine bacteria tested being the hydromethanolic fraction the most active. During phytochemical procedures, procyanidin (1) and catechin (2) were identified as the main antibacterial constituents of this fraction. In silico results obtained using PASSonline tool indicated 1 and 2 as having good potential to interact with different targets of currently used antibiotics. These results no indicated potential to none DNA effect and indicated the cell wall as mainly target. Electrophoresis result supported that had no DNA damage. Cell wall damage was confirmed by propidium iodide test that showed increased membrane permeability and by cell surface deformations observed in scanning electronic microscopy. The in vitro assays together with the in silico prediction results establish the potential of P. spruceanum as source of antibacterial compounds that acts on important bacterial targets. These results contribute to the development of natural substances against pathogenic bacteria and to discovery of new antibiotics.
Keywords:
Protium spruceanum; Burseraceae; Procyanidin; Catechin; Antibacterial activity
INTRODUCTION
The emergence of antibiotic resistance bacterial strains has hampered the treatment of infections and represents an important public health problem, being infectious diseases one of the major causes of morbidity and mortality worldwide (Penduka et al., 2018Penduka D, Mthembu W, Cele KH, Mosa RA, Zobolo AM, Opoku AR. Extracts of Ansellia africana and Platycarpha glomerata exhibit antibacterial activities against some respiratory tract, skin and soft tissue infections implicated bacteria. S Afr J Bot. 2018;116:116-122.). Therefore, there is a need to developing new antibiotics using natural products, which are widely prescribed worldwide and are an important source of bioactive compounds (Kumar et al., 2017Kumar S, Mahanti P, Singh NR, Rath SK, Jena PK, Patra JK. Antioxidant activity, antibacterial potential and characterization of active fraction of Dioscorea pentaphylla L. tuber extract collected from Similipal Biosphere Reserve, Odisha, India. Braz J Pharm Sci. 2017;53(4):e17006.; Moro et al., 2017Moro IJ, Gondo GDGA, Pierri EG, Pietro RCLR, Soares CP, Sousa DP, et al. Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives. Braz J Pharm Sci. 2017;53(4):e00076.). Natural compounds such as alkaloids, lectins, flavonoids and terpenoids are considered as an adequate strategy against bacterial resistance and an alternative for treatment of infections (Chandra et al., 2017Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR. Antimicrobial resistance and the alternative resources with special emphasis on plant-Based antimicrobials-A review. Plants. 2017;6(2):16.). Thus, plants are important source of biological active compounds and/or of chemical models for the development of new more effective medicines. In the last decades, natural compounds and its derivatives participated into development of more than 58% of the approved antibiotics by regulatory agencies worldwide (Newman, Cragg, 2016Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629-661.).
In this context, species of the Burseraceae family stand out due to their ethnopharmacological applications. The distribution of family Burseraceae is typically pantropical and it occurs in tropical Africa and America. The Burseraceae family has seventeen genera and 500 species and its most important genera are Bursera, Commiphora, Protium and Canarium. In Brazil, there are seven genera and about sixty species, mostly native to the Amazon region (Rosalem et al., 2017Rosalem PF, Picão TB, Rodrigues-Lisoni FC, Martins AR. Leaf anatomy of Protium ovatum and its antiproliferative potential in cervical cells. Rev Bras Farmacogn. 2017;27(6):673-678. ). Among these genera, species of the genus Protium are important sources of bioactive substances and are predominant in South America, being that more than 80% of the Burseraceae species in the Amazon Biome belong to this genus (Siani et al., 2004Siani AC, Garrido IS, Monteiro SS, Carvalho ES, Ramos MFS. Protium icicariba as a source of volatile essences. Biochem System Ecol. 2004;32(5):477-489.; Marques et al., 2010Marques DD, Sartori RA, Lemos TLG, Machado LL, Souza JSN, Monte FJQ. Chemical composition of the essential oils from two subspecies of Protium heptaphyllum. Acta Amazon. 2010;40(1):227-230.).
Protium spruceanum (Benth.) Engler, popularly known in Brazil as breu-branco is a large canopy tree (up to 20 m tall), native of Atlantic and Amazon rain forests and in riparian forests of the Cerrado, a typical tropical savanna region (Vieira et al., 2010Vieira FA, Appolinário V, Fajardo CG, Carvalho D. Reproductive biology of Protium spruceanum (Burseraceae), a dominant dioecious tree in vegetation corridors in Southeastern Brazil. Rev Bras Bot. 2010;33(4):711-715.; Rodrigues et al., 2013Rodrigues IV, Souza JNP, Silva ACG, Chibli LAA, Cabral VAR, Filho SAV, et al. Antiedematogenic and antinociceptive effects of leaves extracts from Protium spruceanum Benth. (Engler). Pharmacogn J. 2013;5(1):6-12.).
In Brazilian traditional medicine P. spruceanum is used as analgesic and anti-inflammatory (Rodrigues et al., 2013Rodrigues IV, Souza JNP, Silva ACG, Chibli LAA, Cabral VAR, Filho SAV, et al. Antiedematogenic and antinociceptive effects of leaves extracts from Protium spruceanum Benth. (Engler). Pharmacogn J. 2013;5(1):6-12.). The antibacterial property of crude extract and fractions from branches of P. spruceanum was previously reported (Amparo et al., 2017Amparo TR, Rodrigues IV, Seibert JB, Souza RHZ, Oliveira AR, Cabral VAR, et al. Antibacterial activity of extract and fractions from branches of Protium spruceanum and cytotoxicity on fibroblasts. Nat Prod Res. 2017;32(16): 1951-54.). However, bioactive compounds from this specie were not reported yet.
Currently, studies related to pharmacological properties involve the use of laboratory experiments associated with in silico studies aiming to optimize the researches. In this context, virtual screening (in silico) have been applied to avoiding the excessive use of animal models, minimizing the high cost of biological experiments and allowing to results in a short time. By means of in silico studies it’s possible to predict more appropriate biological target that adequately directing the laboratorial researches to the medicine discovery (de Oliveira et al., 2014de Oliveira MLG, Assenço RAG, Silva GDF, Lopes JCD, Silva FC, Lanna MCS, et al. Cytotoxicity, anti-poliovirus activity and in silico biological evaluation from Maytenus gonoclada (Celastraceae). Int J Pharm Pharm Sci. 2014;6: 130-137.).
Among in silico tools currently used to predict pharmacological effects of natural compounds stand out “Prediction of Activity Spectra for Substances” (PASS) software available online. Through these procedures the application of public financial resources are pharmacologic and economically more suitable.
The objective of the present work was to analyze the in vitro antibacterial property of crude extract and fractions from leaves of P. spruceanum and identify secondary metabolites present in the active subtractions. Additionally, the chemical structures of procyanidin and catechin constituents of P. spruceanum, were subjected to PASSonline tool, aiming predict mechanisms of antibacterial activity.
MATERIAL AND METHODS
Plant collection and identification
Leaves of P. spruceanum were collected in June 2014 at the region of Lavras municipality, Minas Gerais, Brazil. The specie was identified by Prof. Dr. Vivette Appolinário Rodrigues Cabral of Departamento de Ciências Florestais, Universidade Federal de Lavras. After botanical identification, a voucher of P. spruceanum was deposited in the Herbarium of Universidade Federal de Lavras, under the code No 16399 HESAL. Leaves were dried at room temperature until a constant weight was achieved and then powdered on a knife mill. Activity was registered in SisGen, the National System of Genetic Resource Management and Associated Traditional Knowledge under number A2B5290.
Extraction and fractions obtainment
Fragmented leaves (220.0 g) were subjected to maceration with ethanol 95 ºGL (2 L). After 1 day, the mixture was filtered and the ethanol was recovered in a rotatory evaporator at ≤ 45 ºC under reduced pressure. This extraction process was repeated 5 times, resulting in the crude ethanol extract (CEE, 37.2 g, 16.9% yield). A sample of CEE (20.0 g) was dissolved in methanol-water (1:1) and subjected to liquid-liquid partitions, first with hexane and after with ethyl acetate. After three repetitions of partition process and recovery of extractive solvents were obtained the fractions hexane (HF, 3.6 g, 18.0% yield), ethyl acetate (EAF, 6.0 g, 30.0% yield) and hydromethanolic (HMF, 9.3 g, 46.5% yield).
In vitro antibacterial evaluation
The in vitro antibacterial property of extract and fractions from leaves of P. spruceanum were evaluated against four Gram-positive bacteria (Staphylococcus aureus ATCC 25923, Listeria monocytogenes clinical isolated, Enterococcus faecalis ATCC 15325 and Streptococcus pyogenes ATCC 19615), and five Gram-negative bacteria (Salmonella enteritidis ATCC13076, Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumoniae ATCC 13833, Shigella sonnei ATCC 25931 and Escherichia coli ATCC 25922). Well diffusion method on Mueller-Hinton agar (MHA) was used in the screening for antibacterial activity and the broth microdilution method was to establish the minimum bactericidal concentration (MBC). Bacteria were cultivated in Müeller-Hinton agar (MHA) medium at 37 °C, for 24 h. The inoculums were prepared using the direct colony suspension method by means of a saline (0.9% NaCl) suspension of colonies selected from a 24 h agar plate, before each assay. The suspension was adjusted to reach turbidity equivalent to a 0.5 of the McFarland standard scale (1 x 108 CFU ml-1) (CLSI, 2012CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. In: Clinical and laboratory standards institute. 2 ed. Institute, C.A.L.S; 2012. p. 1-88.).
For the agar diffusion method, Petri dishes (15 cm diameter) containing MHA medium (50.0 mL) were punched with a sterile cylinder (6 mm diameter wells) and the microbial inoculum was uniformly spread using sterile cotton swab on agar. The extract and fractions were dissolved (80.0 mg/mL) in dimethylsulfoxide (DMSO). DMSO was used as negative control. As positive control tetracycline (100.0 µg/mL) was used for bacteria and moxifloxacin (100.0 µg/mL) specifically for P. aeruginosa. Then, 50.0 µL of the solution of extracts, fractions and of the controls were added in the wells. The dishes were incubated at 37 °C for 24 h. The bacterial growth inhibition zone around the well was determined in millimeters. The assays were realized in triplicates. Those samples that generated inhibition zone ≥ 10 mm were considered actives (Lima-Filho et al., 2002Lima-Filho JVM, Carvalho AFFU, Freitas SM, Melo VMM. Antibacterial activity of extracts of six macroalgae from the northeastern brazilian coast. Braz J Microbiol. 2002;33(4):311-314.).
For the extract and fractions from leaves of P. spruceanum considered actives in diffusion assay the minimum bactericidal concentration (MBC) was determined through microdilution method (CLSI, 2012CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. In: Clinical and laboratory standards institute. 2 ed. Institute, C.A.L.S; 2012. p. 1-88.; Amparo et al., 2017Amparo TR, Rodrigues IV, Seibert JB, Souza RHZ, Oliveira AR, Cabral VAR, et al. Antibacterial activity of extract and fractions from branches of Protium spruceanum and cytotoxicity on fibroblasts. Nat Prod Res. 2017;32(16): 1951-54.). In 96-well plates, 50.0 µL of methanol were added from the second well of each line on and 100.0 µL of the samples dissolved in methanol (160.0 mg/mL) were added in the first well. Thus, 50.0 µL from the first well were transferred to the next (serial dilutions 1:2) to obtain extract and fractions concentrations from 80.00 to 0.15 mg/mL. Then 75.0 µL of Müeller-Hinton broth were added in each well. In the negative control were added 50 µL of methanol and 75 µL of broth. As growth control 75 µL of broth were added in a well. To verify the methodology efficacy, tetracycline or moxifloxacin (100.0 µg/mL) were used as positive control. To methanol withdraw opened plates were placed in a vacuum desiccator at 25 °C for one hour. Then, the plates were sterilized by ultraviolet germicidal irradiation for 20 minutes. The inoculum (1 x 108 CFU/mL) were diluted 1:50 in broth in order to obtain a final assay with 5 x 105 CFU/mL. From these diluted inoculum 25 µL were added in the wells (except in the medium control). The plates were closed and incubated at 37 °C, for 24 h. After the incubation period, 30 µL of triphenyl tetrazolium chloride (TTC, 0.25 mg/mL) were added and the plates were incubated for aditional 3 h. After TTC addition the wells without visible color was peaked to Petri dishes using inoculation loop (10 µL). These Petri dishes were incubated for 24 h. MBC was established as the smallest concentration in which no bacterial growth was detected.
Cytotoxicity and selectivity index
Cytotoxicity of the samples was evaluated using murine fibroblasts L929, cultivated in RPMI 1640 medium. Extract and fractions were dissolved in 2% DMSO, at concentrations ranging from 4.00 to 0.01 mg/mL. Cells were distributed in 96-well microtiter (1 x 105 cell/well) and were incubated at 37 °C with 5% of CO2 for 24 h. Cells were treated with the samples and after 24 h incubation, the cell viability was evaluated using the sulforhodamine B assay (SRB) (Skehan et al., 1990Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107-1112.). The media was removed and cells were fixed with cold 20% trichloroacetic acid for 1 h at 4 °C. The microtiter plate was washed with distilled water and dried. Thereafter, fixed cells were stained for 30 min with 0.1% SRB dissolved in 1% acetic acid. The plate washed again with 1% acetic acid, again allowed to dry and 200 µL of 10 mM TRIS buffer (pH 10.5) were added to stain solubilization at room temperature for ~30 min. Samples absorbance was read in the spectrophotometer (490 nm) and the values were expressed as the cytotoxic concentration of sample for 50% of the cell (CC50), compared to control. CC50 values were calculated by a polynomial regression equation. Selectivity index (SI) was calculated by the ration CC50/MBC.
Chromatographic fractionation and analysis
The most active fraction, HMF was fractionated aiming to identify the antibacterial compounds. Thus, a sample of HMF was dissolved in methanol reaching 15.0 mg /mL. An aliquot (20 µl) of this solution was injected in a Shimadzu LC-6AD® chromatographer equipped with Phenomenex Luna® 5 µm C18(2) 100 Å, LC Column 250 x 4.6 mm. Milli-Q® purified water (A) and methanol (B) were used as mobile phase at flow rate of 0.7 mL/min. The following gradient was used: 5% B at 0 min, 80% B at 20.0 min, 100% B at 30 to 35 min and 5% B at 40.0 min. Injections were repeated 75 times and each run elution was monitored at 210 and 350 nm. The fractions were collected at the interval of 15 to 20 min (F2) and of 20 to 25 min (F3). These retention times were chosen according to the UV spectra profile. To identify the respective constituents, after dried, fraction F2 (X.0 mg) and F3 (Y.0 mg) were analysed by LC-DAD-ESI/MS. Waters. Milli-Q® purified water with 0.1% methanoic acid and acetonitrile (ACN) with 0.1% methanoic acid were used as mobile phase. The flow rate was 0.3 mL/min in a gradient elution from 5% to 95% ACN in 10 min, held until 11 min. Aliquot of 4.0 µL of each sample solution (1.0 mg/mL) was injected. ESI-MS/MS analysis was performed in following conditions: 3.5 kV capillary voltage, positive and negative ion mode, 320 °C capillary temperature, 5 kV source voltage, 320 °C vaporizer temperature, 5 mA corona needles current and 27 psi gas and nitrogen pressure. Analyses were run in a full scan mode (100-2000 u). The UV spectra were registered from 190 to 450 nm.
The antibacterial property of collected fractions F2 and F3 were evaluated using S. aureus.
In silico antimicrobial activity evaluation
The identified constituents in the bioactive fraction, procyanidin and catechin, from leaves of P. spruceanum were subjected to in silico analysis by means of PASSonline tool aiming to predict its antibacterial action mechanisms (available at http://www.pharmaexpert.ru/passonline/). The Software Chemdraw 10.0 (ChemBiodraw, Cambridge Soft) was used to draw the chemical structures and they were saved in sdf file. PASS online tool decompose the chemical structure into descriptors and realize a comparison with a database containing more than 250,000 biologically active targets. The results are expressed as the probability of the compound under analysis be active (Pa) or inactive (Pi) (de Oliveira et al., 2014de Oliveira MLG, Assenço RAG, Silva GDF, Lopes JCD, Silva FC, Lanna MCS, et al. Cytotoxicity, anti-poliovirus activity and in silico biological evaluation from Maytenus gonoclada (Celastraceae). Int J Pharm Pharm Sci. 2014;6: 130-137.). For the in silico analysis were included four classical targets corresponding to those reached by antibiotics actually used in the medicine, and two considered as new bacterial targets described in the scientific literature. The potential antibacterial action targets of compounds were evaluated based on the difference (Pa-Pi). Thus, the potential was considered as unsatisfactory (Pa < Pi), low [(Pa-Pi) < 0.2], moderate [(Pa - Pi) ≥ 0.2 and < 0.5] or high [(Pa - Pi) ≥ 0.5].
DNA damage
HMF was dissolved in broth with 2% DMSO to obtain a final concentration equivalent to MBC. Then 5.0 mL of S. aureus inoculum (5 x 107 CFU/mL) and 5.0 mL of sample were added in tubes and incubated for 24 h at 37 °C. For the control, were added broth and inoculum. The tubes were centrifuged for 5 minutes at 15000 x g, The pellet were resuspended in 300 µL of TE buffer (50 mM glycose, 25 mM Tris, 10 mM EDTA, pH 8,0), 5 µL of proteinase k (2 mg/mL) and 30 µL sodium dodecyl sulfate (SDS) 10%. After incubation 37 ºC for 2 h, and two extractions were made with 300 µL of chloroform. The aqueous phase were combined with 0.1 volume of NaCl 3 M and equal volume of isopropanol and the samples were freezer incubated for 1 h. After centrifugation the pellet were washed with ethanol 70%, dried and resuspended in 25 µL TE buffer. Test samples and DNA marker were analyzed by electrophoresis in TAE buffer (4.84 g Tris base, pH 8.0, 0.5M EDTA/1 L) using 1.8% agarose gel containing ethidium bromide, subjected to 100 V for 90 min (Jin et al., 2006Jin JD, Lee DS, Shin EK, Kim SJ, Jung R, Hahn TW. Molecular typing by random amplification of polymorphic DNA (RAPD) and detection of virulence genes of Salmonella enterica subspecies enterica serovar Gallinarum biovar gallinarum. J Vet Med Sci. 2006;68(12):1321-1326.).
Membrane permeability test
The inoculum (1 x 108 CFU/mL) was diluted in Mueller-Hinton broth in order to obtain a final assay with 1 x 106 CFU/mL and the HMF was dissolved in broth with 2% DMSO to obtain concentrations equivalent to 2x MBC, MBC and ½ MBC. Then into a 96-well plate were added 100 µL of inoculum and 100 µL of sample and for the control were added 100 µL of inoculum and 100 µL of Mueller-Hinton broth. This assay was performed in triplicate for each of the samples and the plate was incubated for 24 h at 37 °C. After incubation, samples were taken from the wells and were added in tubes, washed with 2 mL of phosphate buffered saline (PBS) and centrifuged for 10 minutes at 2500 xg. The supernatant was discarded and the sample were resuspended in 100 µl of PBS and incubated with 1 µL propidium iodide solution (1 mg/mL). The samples were incubated at room temperature and protected from light for 30 min. Flow cytometry analysis were conducted using BD FACS Calibur (BD Bioscence®) and data were analyzed by Flow Jo v.10® (Chopra et al., 2015Chopra L, Singh G, Kumar K, Sahoo DK. Sonorensin: A new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Sci Rep. 2015;5:13412.). Results are presented as mean ± standard deviation (SD) and data were evaluated by one-way analysis of variance (ANOVA) followed by Dunn’s test, using GraphPad Prism software. P values less than 0.05 (p < 0.05) were considered as indicative of significance.
Scanning electronic microscopy (SEM)
HMF was dissolved in broth with 2% DMSO to obtain a final concentration equivalent to MBC. Then 500 µL of S. aureus inoculum (1 x 108 CFU/mL) and 500 µL of sample were added in microtubes and incubated for 24 h at 37 °C. For the control, were added 500 µL of broth and 500 µL of inoculum. After incubation, the microtubes were centrifuged for 10 minutes at 2500 xg and the supernatant was discarded. Glutaraldehyde fixative 3% in 0.1M phosphate buffer pH 7.3 was added (1 mL) and incubated for 1 h at room temperature. The samples were centrifuged again and resuspended with 100 µL PBS. The suspension (50 µL) was spread in a coverslip and dried at 50 ºC for 1 h. Then, samples serially dehydrated in ethanol (70%, 95% and 100%, 2 min in each) and dried again at 50 ºC (Brudzynski, Sjaarda, 2014Brudzynski K, Sjaarda C. Antibacterial compounds of Canadian honeys target bacterial cell wall inducing phenotype changes, growth inhibition and cell lysis that resemble action of β-lactam antibiotics. PLoS One. 2014;9(9):e106967.). A small amount of carbon was sputtered on the samples and the coverslips observed in scanning electron microscope (JSM - 6010LA®). Secondary electron images were taken at 5.0 kV.
RESULTS AND DISCUSSION
The antibacterial property against nine pathogenic bacteria was observed for the extract CEE and its fractions EAF and HMF (Table I). HF not presented antibacterial activity.
Cytotoxicity and antibacterial activity of ethanol extract (CEE) from leaves of P. spruceanum and its fractions
Greater growth inhibition activity was observed against S. aureus (MBC 0.6 mg/mL). Species of the Staphylococcus genus have been reported as cause of skin infections, sepsis, pneumonia, toxic shock syndrome, endocarditis and urinary tract infections (Otto, 2010Otto M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol. 2010;5(2): 183-195.). Studies involving these bacteria are important also because of resistant strains, that difficult the treatment, as S. aureus MRSA (Lopez et al., 2015Lopez BGC, de Lourenço CC, Alves DA, Machado D, Lancellotti M, Sawaya ACHF. Antimicrobial and cytotoxic activity of red propolis: an alert for its safe use. J Appl Microbiol. 2015;119(3):677-687.).
Among fractions from CEE, the HMF showed the lowest MBC values indicating that the mainly antimicrobial compounds responsible for antibacterial activity are polar metabolites. HMF also showed the lowest cytotoxicity (CC50 3.7 mg/mL) and the greater selectivity (SI 6.17), considered satisfactory (Makhafola et al., 2012Makhafola TJ, Samuel BB, Elgorashi EE, Eloff JN. Ochnaflavone and ochnaflavone 7-O-methyl ether two antibacterial biflavonoids from Ochna pretoriensis (Ochnaceae). Nat Prod Commun. 2012;7(12):1601-1604.; Elisha et al., 2017Elisha IL, Botha FS, Mcgaw LJ, Eloff JN. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement Altern Med. 2017;17(1):133.). Thus, HMF was fractionated obtaining the sub-fractions F2 and F3. One tannin and one flavan-3-ol were identified in F2 and three flavonols glycosides were identified in F3 (Table II).
Compounds identified by means of UV and LC-DAD-ESI/MS analysis of sub-fraction F2 and F3 from hydromethanolic fraction of P. spruceanum leaves
The fraction F2, where identified the tannin procyanidin and the flavan-3-ol catechin, presented antibacterial activity against S. aureus (MBC 0.6 mg/mL) and for fraction F3 was not observed any effect in the assay conditions. Antibacterial activity of catechin (Bais et al., 2002Bais HP, Walker TS, Stermitz FR, Hufbauer RA, Vivanco JM. Enantiomeric-dependent phytotoxic and antimicrobial activity of (±)-Catechin. A rhizosecreted racemic mixture from spotted knapweed. Plant Physiol. 2002;128(4):1173-1179.) and procyanidin (Wang et al., 2015Wang C, Hsu Y, Jhan Y, Tsai S, Lin S, Su C, Chou C. Structure Elucidation of procyanidins isolated from Rhododendron formosanum and their anti-oxidative and anti-bacterial activities. Molecules. 2015;20(7):12787-12803.) was already described.
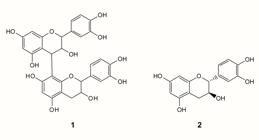
Therefore, it was possible to consider that procyanidin (1) and catechin (2) (Figure 1) as the main constituents responsible for the antibacterial activity of P. spruceanum. For this reason, the chemical structure of these compounds was subjected to PASSonline analysis.
Chemical structures of procyanidin (1) and catechin (2) identified in antibacterial fraction (F2) from leaves of P. spruceanum and subjected to in silico PASS online tool, to predict its action mechanisms.
Through PASSonline it was possible to predict pharmacological effects, mechanisms of action and specific toxicity of the studied compounds by comparison with similar structure regions of biologically active substances found in its data bank, with 95% accuracy. Based on the results, in vitro and in vivo assays are selected, realized and the pharmacologic effect can be validated (Goel et al., 2011Goel RK, Singh D, Lagunin A, Poroikov V. PASS-assisted exploration of new therapeutic potential of natural products. Med Chem Res. 2011;20(9):1509-1514.).
For the in silico activity prediction linked to classical targets reached by the antibiotics currently prescribed by physicians, compound 1 and 2 were considered as inadequate (Pa < Pi) or of low potential (Pa - Pi < 0.2). However, in relation to the considered new bacterial targets, these compounds were classified as having moderate [(Pa - Pi) ≥ 0.2 and < 0.5] or high [(Pa - Pi) ≥ 0.5] potential activity (Table III).
In silico test prediction of potential bacterial targets of procyanidin and catechin using PASSonline tool
An important target for blocking the development of bacteria is your cell wall. The majority of compounds that act on the cellular wall inhibit the peptidoglycan synthesis involving transpeptidase and glycosyltransferase. Transpeptidase is a target of -lactam antibiotics, but inhibitors of glycosyltransferase remains to be developed (Derouaux, Sauvage, Terrak, 2013Derouaux A, Sauvage E, Terrak M. Peptidoglycan glycosyltransferase substrate mimics as templates for the design of new antibacterial drugs. Front Immunol. 2013;4:78.). Other new target involved on the cellular wall integrity is the CDP-glycerol glycerophosphotransferase that catalyzes the teichoic acid synthesis, an essential cell wall component (Fitzgerald, Foster, 2000Fitzgerald SN, Foster TJ. Molecular analysis of the tagF gene, encoding CDP-Glycerol:Poly(glycerophosphate) glycerophosphotransferase of Staphylococcus epidermidis ATCC 14990. J Bacteriol. 2000;182(4): 1046-1052.).
The in silico results showed no potential to action mechanisms involved with DNA damage and indicated the bacterial cell wall as a potential action target (Table III). The absence DNA effect was supported by the electrophoresis result (Figure 2A), that showed no changes in relation to the control (Soyingbe et al., 2013Soyingbe OS, Oyedeji A, Basson AK, Opoku AR. The essential oil of Eucalyptus grandis W. Hill ex Maiden inhibits microbial growth by inducing membrane damage. Chin Med. 2013;4(1):7-14. ).
Effects caused in S. aureus treated with hidromethanolic fraction (FHM) from P. spruceanum leaves. Analysis of bacterial DNA using 1.8% agarose gel electrophoresis (A), membrane permeability by proppidium iodide, where *p<0.05 versus control (B) and cell shape by scanning electronic microscopy (20000x, SS20), where white arrows indicate FHM precipitated and black arrows indicate cell surface deformations (C).
The loss of the bacterial cell wall integrity and the inhibition of peptidoglycan turnover enhance membrane permeability, since the outer membrane is cross-bridged to the peptidoglycan of the cell wall (Brudzynski, Sjaarda, 2014Brudzynski K, Sjaarda C. Antibacterial compounds of Canadian honeys target bacterial cell wall inducing phenotype changes, growth inhibition and cell lysis that resemble action of β-lactam antibiotics. PLoS One. 2014;9(9):e106967.; Kim et al., 2015Kim S, Lee H, Lee S, Yoon Y, Choi KH. Antimicrobial action of oleanolic acid on Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis. PLoS One. 2015;10(3):e0118800.; Hoerr et al., 2016Hoerr V, Duggan GE, Zbytnuik L, Poon KK, Große C, Neugebauer U, et al. Characterization and prediction of the mechanism of action of antibiotics through NMR metabolomics. BMC Microbiol. 2016;16:82.). To investigate bacterial cell membrane damage, propidium iodide uptake was measure. Propidium iodide only pass through damaged membranes, binds non-specically to DNA and emits fluorescence. Thus greater ratio of propidium iodide uptake cells (% positive IP) indicates membrane damage. FHM treatment increased the fluorescent S. aureus cells (Figure 2B), showing that this fraction damage the cell membrane of this bacteria. Bacterial wall cell damage by FHM was also visually confirmed by SEM (Figure 2C). S. aureus treated with FHM (Figure 2C2) showed cell surface deformations in comparison to the control (Figure 2C1).
In conclusion, antibacterial property against nine pathogenic bacteria was observed for the extract CEE from leaves of P. spruceanum and its fractions EAF and HMF. Catechin and procyanidin were considered as antibacterial constituents of HMF. In silico analyses indicated the potential of these compounds to act in new antimicrobial targets and indicated no DNA effect and cell wall as mainly target. The in silico results were supported by in vitro tests that showed no DNA damage and showed cell wall damage, with increase in membrane permeability and changes in cell shape. Substances from P. spruceanum may contribute to treatment of infections caused by resistant bacteria. The results open perspectives to new studies using HMF or F2 as components of medicines against bacteria.
ACKNOWLEDGEMENT
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG- CDS-APQ-02004-14).
REFERENCES
- Amparo TR, Rodrigues IV, Seibert JB, Souza RHZ, Oliveira AR, Cabral VAR, et al. Antibacterial activity of extract and fractions from branches of Protium spruceanum and cytotoxicity on fibroblasts. Nat Prod Res. 2017;32(16): 1951-54.
- Bais HP, Walker TS, Stermitz FR, Hufbauer RA, Vivanco JM. Enantiomeric-dependent phytotoxic and antimicrobial activity of (±)-Catechin. A rhizosecreted racemic mixture from spotted knapweed. Plant Physiol. 2002;128(4):1173-1179.
- Brudzynski K, Sjaarda C. Antibacterial compounds of Canadian honeys target bacterial cell wall inducing phenotype changes, growth inhibition and cell lysis that resemble action of β-lactam antibiotics. PLoS One. 2014;9(9):e106967.
- Callemien D, Collin S. Use of RP-HPLC-ESI(-)-MS/MS to differentiate various proanthocyanidin isomers in lager beer extracts. J Am Soc Brew Chem. 2008;66(2):109-115.
- Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR. Antimicrobial resistance and the alternative resources with special emphasis on plant-Based antimicrobials-A review. Plants. 2017;6(2):16.
- Chopra L, Singh G, Kumar K, Sahoo DK. Sonorensin: A new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Sci Rep. 2015;5:13412.
- CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. In: Clinical and laboratory standards institute. 2 ed. Institute, C.A.L.S; 2012. p. 1-88.
- de Oliveira MLG, Assenço RAG, Silva GDF, Lopes JCD, Silva FC, Lanna MCS, et al. Cytotoxicity, anti-poliovirus activity and in silico biological evaluation from Maytenus gonoclada (Celastraceae). Int J Pharm Pharm Sci. 2014;6: 130-137.
- Derouaux A, Sauvage E, Terrak M. Peptidoglycan glycosyltransferase substrate mimics as templates for the design of new antibacterial drugs. Front Immunol. 2013;4:78.
- Elisha IL, Botha FS, Mcgaw LJ, Eloff JN. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement Altern Med. 2017;17(1):133.
- Fabre N, Rustan I, Hoffmann E, Quetin-Leclercq J. Determination of flavone, flavonol, and flavanone aglycones by negative Ion liquid chromatography electrospray ion trap mass spectrometry. J Am Soc Mass Spectrom. 2001;12(6): 707-715.
- Fitzgerald SN, Foster TJ. Molecular analysis of the tagF gene, encoding CDP-Glycerol:Poly(glycerophosphate) glycerophosphotransferase of Staphylococcus epidermidis ATCC 14990. J Bacteriol. 2000;182(4): 1046-1052.
- Goel RK, Singh D, Lagunin A, Poroikov V. PASS-assisted exploration of new therapeutic potential of natural products. Med Chem Res. 2011;20(9):1509-1514.
- Hoerr V, Duggan GE, Zbytnuik L, Poon KK, Große C, Neugebauer U, et al. Characterization and prediction of the mechanism of action of antibiotics through NMR metabolomics. BMC Microbiol. 2016;16:82.
- Jin JD, Lee DS, Shin EK, Kim SJ, Jung R, Hahn TW. Molecular typing by random amplification of polymorphic DNA (RAPD) and detection of virulence genes of Salmonella enterica subspecies enterica serovar Gallinarum biovar gallinarum. J Vet Med Sci. 2006;68(12):1321-1326.
- Kim S, Lee H, Lee S, Yoon Y, Choi KH. Antimicrobial action of oleanolic acid on Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis PLoS One. 2015;10(3):e0118800.
- Kumar S, Mahanti P, Singh NR, Rath SK, Jena PK, Patra JK. Antioxidant activity, antibacterial potential and characterization of active fraction of Dioscorea pentaphylla L. tuber extract collected from Similipal Biosphere Reserve, Odisha, India. Braz J Pharm Sci. 2017;53(4):e17006.
- Lima-Filho JVM, Carvalho AFFU, Freitas SM, Melo VMM. Antibacterial activity of extracts of six macroalgae from the northeastern brazilian coast. Braz J Microbiol. 2002;33(4):311-314.
- Lopez BGC, de Lourenço CC, Alves DA, Machado D, Lancellotti M, Sawaya ACHF. Antimicrobial and cytotoxic activity of red propolis: an alert for its safe use. J Appl Microbiol. 2015;119(3):677-687.
- Makhafola TJ, Samuel BB, Elgorashi EE, Eloff JN. Ochnaflavone and ochnaflavone 7-O-methyl ether two antibacterial biflavonoids from Ochna pretoriensis (Ochnaceae). Nat Prod Commun. 2012;7(12):1601-1604.
- March RE, Miao XS. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int J Mass Spectrom. 2004;231(2-3):157-167.
- Marques DD, Sartori RA, Lemos TLG, Machado LL, Souza JSN, Monte FJQ. Chemical composition of the essential oils from two subspecies of Protium heptaphyllum Acta Amazon. 2010;40(1):227-230.
- Martucci MEP, De Vos RCH. Carollo CA, Gobbo-Neto L. Metabolomics as a Potential Chemotaxonomical Tool: Application in the genus Vernonia Schreb Plos One. 2014;9(4):1-8.
- Moro IJ, Gondo GDGA, Pierri EG, Pietro RCLR, Soares CP, Sousa DP, et al. Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives. Braz J Pharm Sci. 2017;53(4):e00076.
- Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629-661.
- Otto M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol. 2010;5(2): 183-195.
- Penduka D, Mthembu W, Cele KH, Mosa RA, Zobolo AM, Opoku AR. Extracts of Ansellia africana and Platycarpha glomerata exhibit antibacterial activities against some respiratory tract, skin and soft tissue infections implicated bacteria. S Afr J Bot. 2018;116:116-122.
- Rodrigues IV, Souza JNP, Silva ACG, Chibli LAA, Cabral VAR, Filho SAV, et al. Antiedematogenic and antinociceptive effects of leaves extracts from Protium spruceanum Benth. (Engler). Pharmacogn J. 2013;5(1):6-12.
- Rosalem PF, Picão TB, Rodrigues-Lisoni FC, Martins AR. Leaf anatomy of Protium ovatum and its antiproliferative potential in cervical cells. Rev Bras Farmacogn. 2017;27(6):673-678.
- Siani AC, Garrido IS, Monteiro SS, Carvalho ES, Ramos MFS. Protium icicariba as a source of volatile essences. Biochem System Ecol. 2004;32(5):477-489.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107-1112.
- Soyingbe OS, Oyedeji A, Basson AK, Opoku AR. The essential oil of Eucalyptus grandis W. Hill ex Maiden inhibits microbial growth by inducing membrane damage. Chin Med. 2013;4(1):7-14.
- Tiberti LA, Yariwake JH, Ndjoko K, Hostettmann K. On-line LC/UV/MS analysis of flavonols in the three apple varieties most widely cultivated in Brazil. J Braz Chem Soc. 2007;18(1):100-105.
- Vieira FA, Appolinário V, Fajardo CG, Carvalho D. Reproductive biology of Protium spruceanum (Burseraceae), a dominant dioecious tree in vegetation corridors in Southeastern Brazil. Rev Bras Bot. 2010;33(4):711-715.
- Wang C, Hsu Y, Jhan Y, Tsai S, Lin S, Su C, Chou C. Structure Elucidation of procyanidins isolated from Rhododendron formosanum and their anti-oxidative and anti-bacterial activities. Molecules. 2015;20(7):12787-12803.
Publication Dates
-
Publication in this collection
26 Apr 2021 -
Date of issue
2020
History
-
Received
15 June 2018 -
Accepted
15 Jan 2019