Abstracts
Hypotheses are presented on the evolution of structural patterns of secondary metabolites (flavonoids and foliar wax alkanes) and fatty acids of families of "campos rupestres". The distribution of fatty acids is given for genera of Lythraceae, with emphasis on Cuphea (supposedly more advanced) and Diplusodon. Compounds with saturated short chains represent a derived condition in Lythraceae although they are probably restricted to Cuphea. It is suggested that evolution selected for more complex flavonoid patterns in Cuphea, with the inclusion of C-glycoflavones and methoxylated flavonols (rhamnetin and isorhamnetin), which are not found in members of Diplusodon and Lafoensia. The supposedly primitive groups of Eriocaulaceae (e.g., Paepalanthus) presented more complex flavonoid patterns characterized by flavones and flavonols, the latter frequently being 6-hydroxylated or methoxylated. More advanced groups of Eriocaulaceae (e.g., Leiothrix and Syngonanthus) apparently possess only flavones, C-glycoflavones are a salient feature of species with smaller habits. In Velloziaceae, members of the primitive subfamily Vellozioideae show distribution of alkanes of foliar epicuticular wax in which C27, C29 or C31 predominate; members of the derived subfamily Barbacenioideae usually show distributions with a predominance of C33 or C35, while species of Pleurostima (Barbacenioideae) have C31 as the main homologue, thus being intermediate between the two subfamilies. It is suggested that the evolution of alkanes in Velloziaceae follows a trend toward elongation of carbon chains. The condition of advanced or primitive chemical patterns is inferred from the results of cladistic analyses based on morphological characters (Eriocaulaceae and Lythraceae), and morphological and molecular characters (Velloziaceae).
Hipóteses são apresentadas sobre a evolução de padrões estruturais de metabólitos secundários (flavonóides e alcanos de ceras foliares) e ácidos graxos, em famílias bem representadas nos campos rupestres. A distribuição de ácidos graxos é comentada no confronto entre os gêneros de Lythraceae, com maior ênfase em Cuphea (supostamente mais avançado) e Diplusodon, com a proposta de que compostos saturados com cadeias curtas representam uma condição derivada em Lythraceae, embora restrita provavelmente a Cuphea. A evolução deve ter gerado perfis flavonoídicos mais complexos em Cuphea, com a inclusão de C-glicoflavonas e flavonóis metoxilados (ramnetina e isoramnetina), ausentes em representantes de Diplusodon e Lafoensia. Ao contrário, grupos supostamente primitivos de Eriocaulaceae (Paepalanthus, por exemplo) apresentam perfis flavonoídicos mais complexos, caracterizados por flavonas e flavonóis, estes últimos freqüentemente com hidroxilação e metoxilação no carbono 6; alguns grupos mais avançados da mesma família (Leiothrix e Syngonanthus) aparentemente possuem apenas flavonas, com uma tendência ao maior acúmulo de C-glicoflavonas nas espécies de menor porte. Em Velloziaceae, os representantes da subfamília supostamente primitiva (Vellozioideae) apresentam preferencialmente distribuições de alcanos da cera foliar epicuticular com modas em C27, C29 ou C31; os representantes da subfamília derivada (Barbacenioideae) apresentam modas preferencialmente em C33 ou C35. No entanto, no gênero Pleurostima (Barbacenioideae) as modas recaem geralmente em C31. As condições de avanço ou primitividade dos perfis de metabólitos secundários são comparadas com resultados de análises cladísticas baseadas em caracteres morfológicos (Eriocaulaceae e Lythraceae) e morfológicos e moleculares (Velloziaceae).
Distribution and evolution of secondary metabolites in Eriocaulaceae, Lythraceae and Velloziaceae from "campos rupestres"
Antonio Salatino, Maria Luiza Faria Salatino, Déborah Yara A.C. dos Santos and Márcia Cristina B. Patrício
Instituto de Biociências, Universidade de São Paulo, Caixa Postal 11461, 05422-970 São Paulo, SP, Brasil. Send correspondence to A.S. Fax: +55-11-818-7416. E-mail: asalatin@ib.usp.br
ABSTRACT
Hypotheses are presented on the evolution of structural patterns of secondary metabolites (flavonoids and foliar wax alkanes) and fatty acids of families of "campos rupestres". The distribution of fatty acids is given for genera of Lythraceae, with emphasis on Cuphea (supposedly more advanced) and Diplusodon. Compounds with saturated short chains represent a derived condition in Lythraceae although they are probably restricted to Cuphea. It is suggested that evolution selected for more complex flavonoid patterns in Cuphea, with the inclusion of C-glycoflavones and methoxylated flavonols (rhamnetin and isorhamnetin), which are not found in members of Diplusodon and Lafoensia. The supposedly primitive groups of Eriocaulaceae (e.g., Paepalanthus) presented more complex flavonoid patterns characterized by flavones and flavonols, the latter frequently being 6-hydroxylated or methoxylated. More advanced groups of Eriocaulaceae (e.g., Leiothrix and Syngonanthus) apparently possess only flavones, C-glycoflavones are a salient feature of species with smaller habits. In Velloziaceae, members of the primitive subfamily Vellozioideae show distribution of alkanes of foliar epicuticular wax in which C27, C29 or C31 predominate; members of the derived subfamily Barbacenioideae usually show distributions with a predominance of C33 or C35, while species of Pleurostima (Barbacenioideae) have C31 as the main homologue, thus being intermediate between the two subfamilies. It is suggested that the evolution of alkanes in Velloziaceae follows a trend toward elongation of carbon chains. The condition of advanced or primitive chemical patterns is inferred from the results of cladistic analyses based on morphological characters (Eriocaulaceae and Lythraceae), and morphological and molecular characters (Velloziaceae).
INTRODUCTION
Plant secondary metabolites have been widely used as taxonomic characters for comparisons at all hierarchic levels (Harborne and Turner, 1984), and certain classes of secondary metabolites such as benzylisoquinoline alkaloids, betalaines, glucosinolates, iridoids and polyacetylenes have had a great influence in the establishment of all recent systems of angiosperm classification (e.g., Cronquist, 1981; Dahlgren, 1989; Takhtajan, 1997).
Other classes of secondary metabolites, most importantly flavonoids (Woodland, 1997), have been more frequently used for comparisons at lower hierarchic levels such as genera, species and infraspecific categories. Another class of compounds that has deserved much attention, not only in plant but also in insect taxonomy, are hydrocarbons, in particular alkanes (Hamilton, 1995). Both classes of secondary metabolites combine the advantages of universal occurrence in vascular plants, chemical stability and the availability of rapid isolation and identification methods (Harborne, 1998).
In spite of the widespread use of secondary metabolites in taxonomy they have never achieved the same status as characters for the establishment of phyletic relationships among plant taxa as macromolecules such as proteins and nucleic acids, which have recently received much attention in molecular systematics (Hillis et al., 1996). Inferences regarding the evolution of secondary metabolites depend on comparisons with dendrograms obtained through cladistic techniques based on wide sets of "traditional" morphologic or molecular characters.
This paper discusses the development of secondary metabolite patterns in taxa of three families, Eriocaulaceae, Lythraceae and Velloziaceae, which are widely distributed in the campos rupestres, mountainous ecosystems found in the Espinhaço Mountains of the Brazilian States of Minas Gerais and Bahia. We also discuss the evolution of the distribution of secondary metabolites based on recent cladistic analyses, taking into account the distribution of secondary metabolites such as the flavonoids of Eriocaulaceae and Lythraceae, the alkanes of the foliar waxes of Velloziaceae and the fatty acids of Lythraceae.
FLAVONOIDS
Eriocaulaceae
The Eriocaulaceae are pantropical monocotyledons readily characterized by congested capitulla. The family comprises about 1100 species of which 700 occur in the New World, particularly on higher altitude mountains in Minas Gerais and Bahia (Giulietti and Pirani, 1988). Genera particularly well represented in the campos rupestres are Eriocaulon, Leiothrix, Paepalanthus and Syngonanthus.
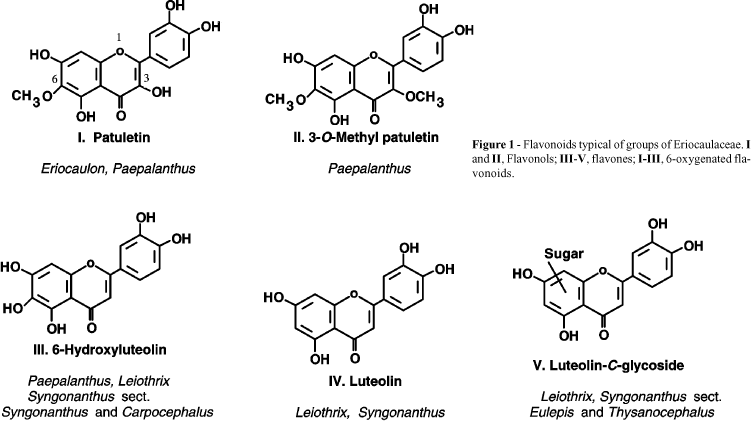
Our knowledge about the distribution of flavonoids in Eriocaulaceae derives from the works of Bate-Smith and Harborne (1969) on six species of Eriocaulon, Dokkedal and Salatino (1992) on six species of Leiothrix, Mayworm and Salatino (1993) on four species of Paepalanthus and Ricci et al. (1996) on 22 species of Syngonanthus. Figure 1 shows some examples of flavonoid aglycones found in Eriocaulaceae and the genera and some infrageneric categories in which the structural types predominate.
A cladistic analysis based on 49 predominantly morphologic characters (Giulietti et al., 2000) suggests that Paepalanthus is polyphyletic and should be divided into smaller monophyletic genera. Eriocaulon is closely related to some groups of Paepalanthus, while Leiothrix and Syngonanthus appear as more advanced sister groups (Figure 2). The cladogram shows the evolution in Syngonanthus at the sectional level: the Eulepis and Thysanocephalus sections seem to constitute a more advanced monophyletic group than the Carpocephalus and Syngonanthus sections (Figure 2).
Some of these relationships are reflected by the amount of phenolic substances in the capitulla of Eriocaulaceae, with specimens of Paepalanthus and Eriocaulon tending to have higher amounts while the more advanced genera Leiothrix and Syngonanthus have lower amounts (Salatino et al., 1990) (Figure 2). It seems that evolution has selected for a reduction in the amount of phenolic compounds in capitulla of Eriocaulaceae.
In the case of flavonoid distribution in Eriocaulaceae, there seems to be a relationship between high phenolic content and the predominance of flavonols, and low phenolic content and the predominance of flavones. In fact, in Paepalanthus and Eriocaulon species, almost always with more than 1% soluble phenols in their capitulla, flavonols are highly predominant, while in Leiothrix and Syngonanthus species, always with less than 1% phenols, there are apparently only flavones (Figures 1 and 2).
Other structural aspects of the flavonoids seem to have paralleled the evolution of Eriocaulaceae because in the supposedly most primitive groups of the family 6-oxygenated compounds frequently occur, while flavonoids with this characteristic, such as quercetagetin and patuletin (Figure 1), are rarer in angiosperms than flavonoids like quercetin and luteolin, which have no oxygen at position 6 (Figure 1). Evidence suggests that in the Eriocaulaceae 6-oxygenation has been superseded during evolution: in primitive groups (flavonol bearers), like Paepalanthus and Eriocaulon, 6-oxygenated compounds predominate. In the more advanced groups (flavone bearers), like Syngonanthus, the highly advanced sections Eulepis and Thysanocephalus lack 6-oxygenated derivatives, while in the more primitive sections Carpocephalus and Syngonanthus 6-hydroxyluteolin predominates (Figures 1 and 2). Surprisingly, the advanced sections Eulepis and Thysanocephalus show a prevalence of C-glycoflavones, a character suggested as an indicator of chemical primitivity in angiosperms (Harborne, 1972).
So far, the available data suggest that flavonoid evolution in Eriocaulaceae has followed the path:
Lythraceae
Among the Myrtales the Lythraceae represent a family with 31 genera and about 600 species widely distributed in tropic and subtropic regions of the Old and New World, mainly in mesophytic and humid habitats, with the habits of Lythraceae varying from trees to annual herbs.
Relatively little is known about flavonoid distribution in the family. Graham et al. (1980) reported the flavonoids of Ammania coccinea; Blatt et al. (1994) the flavonoids of 27 species of Diplusodon; Santos et al. (1995), 16 species of Cuphea, and Santos et al. (2000), 3 species of Lafoensia. Figure 3 shows some examples of flavonoids and the corresponding Lythraceae taxa.
Cuphea is predominantly herbaceous and the largest genus of Lythraceae, comprising 250 species of the New World. Diplusodon is the second largest genus in the family, with 74 shrubby or subshrubby species, all endemic to Brazil. Lafoensia is a small South American genus, with 9 arboreal or shrubby species.
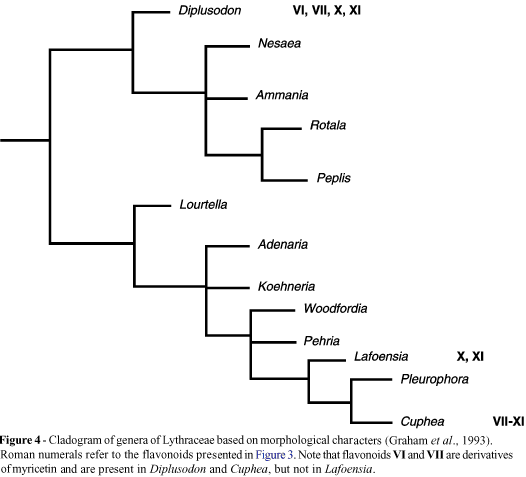
Graham et al. (1993) carried out a cladistic analysis embracing all genera of Lythraceae. Unfortunatelly the two genera about which most information on flavonoid chemistry is available (Cuphea and Diplusodon) are not closely related, occurring in relatively distant clades (Figure 4). The cladistic analysis reveals that the evolution of Lythraceae followed the trend arboreal ® shrubby (woody) ® herbaceous habits (including herbs of swampy habitats), and it can be seen that in Cuphea, an evolutionary dead end in the cladogram (Figure 4), herbaceous habits prevail. By contrast, in Diplusodon shrubby and subshrubby plants predominate, suggesting a more primitive condition relative to Cuphea. Diplusodon emerges from the base of a clade, which has at its ends very advanced aquatic groups of flooded areas, like Ammania, Nesaea, Peplis and Rotala (Graham et al., 1993; Figure 4). As no more information about the flavonoid chemistry of the family is available, we will adopt Diplusodon and Cuphea as representatives of more primitive and more advanced groups, respectively, of Lythraceae, while Lafoensia will be regarded as more primitive and closely related to Cuphea (Figure 4).
Chemically, Diplusodon is remarkable for the very frequent occurrence of mono- and diglycosides of myricetin (Figures 3 and 4). Harborne (1972) assigned to myricetin a role of primitivity indicator because it is a characteristic compound of woody dicotyledons, including Myrtales (Giannasi, 1988). Perhaps even more remarkable is the frequent occurrence in Diplusodon of the glycosidic combination of myricetin and glucuronic acid (Figure 3), the latter figuring as the rarest sugar in flavonoids, such association making the glycosides relatively unstable and readily liable to oxydation. Methylation may be viewed as a protection against oxydation of phenolic hydroxyls (Pugialli et al., 1994), but no methoxylated flavonoids were found in Diplusodon.
Assuming Cuphea as more advanced than Diplusodon the following attributes could be regarded as evolutionary advances of the flavonoid chemistry of the Lythraceae: a) the absence of glycosidic combinations involving myricetin and glucuronic acid; b) the rare occurrence of glucuronic acid; c) the presence of methoxylated flavonoids such as rhamnetin and isorhamnetin and d) the more frequent occurrence of galactose glycosides. However, the present knowledge of the chemistry of the Lythraceae is too sketchy to accept these assumptions as definite proof of either primitivity or chemical advance in the family because the differences observed between the two genera might not be reproduced in other taxa with a similar distance of relative advancement.
An intriguing aspect emerges if one attempts to include the flavonoid pattern of Lafoensia (Figure 3) into the cladogram of Figure 4, bearing in mind the flavonoid profiles of Cuphea and Diplusodon already commented upon. Despite its woody habit (arboreal in several species), Lafoensia appears in close association with the predominantly herbaceous Cuphea in the cladogram. The flavonoid patterns of Lafoensia and Cuphea are substantially different (Figure 3) and up to the present, myricetin, O-methylated flavonols and flavonoids with glucuronic acid have not been reported for Lafoensia. Taking into account the existence of myricetin and glucuronic acid as constituent flavonoids of Diplusodon and Cuphea, it becomes difficult to accommodate such a simple flavonoid profile as that of Lafoensia in the topology of the Figure 4: Lafoensia should have some characteristics common to the other two genera. The flavonoid chemistry suggests that Lafoensia belongs to an evolutionary line different from Cuphea, or is a more advanced group, an alternative, however, which is not compatible with the distribution of fatty acids in Lafoensia (see below).
At the infrageneric level, there is an interesting aspect linking flavonoid chemistry and the evolution of Diplusodon. This genus is divided into sections according to patterns of foliar venation. The Penninerves section is presumably the primitive group, with the more common venation pattern, while the Palmatinerves and Diplusodon sections (the latter with a single prominent vein in the center of the lamina) can be regarded as derived groups. Flavonols and flavones are mutually exclusive in species of Diplusodon; yet flavones have been observed only in representatives of the Penninerves section, and in species of this section two flavonoid profiles are observed, exclusively flavonols or exclusively flavones, while in representatives of the other sections only flavonols have been found. Flavonoid evolution in Diplusodon can be envisaged according to the scheme depicted in Figure 5, where the primitive condition (Penninerves section) is characterized by a more complex profile, with flavonols and flavones, the two groups of phenols probably occurring together in an ancestral group with pennate venation. The evolution that led to derived patterns of venation was paralleled by a blockage of flavone synthesis, leading to the present patterns (Figure 5).
SEED FATTY ACIDS
Lythraceae
In the seed triglycerides of most dicotyledons, unsaturated fatty acids with 18 carbon atoms (C18) predominate, among which the most common are oleic (C18:1,one unsaturation) and linoleic acids (C18:2, two unsaturations). More rarely, species can be found with substantial amounts of fatty acids with longer carbon chains such as arachidic (C20:0) and behenic (C22:0), or shorter chains such as palmitic (C16:0), myristic (C14:0) and lauric (C12:0) acids (Figure 6).
In Arecaceae (palm trees), the seed fatty acids present short and saturated chains (C10-C14). Triglycerides with such fatty acids have high commercial value because of their utility in the production of cosmetics and shampoos. In temperate countries the commercial exploitation of palm trees is not possible, so other vegetable sources of oils with similar characteristics are important, and the discovery of species of Cuphea with seed oils resembling coconut and palma oils (Graham et al., 1981; Wolf et al., 1983; Graham and Kleiman, 1987) raised considerable interest. Fatty acid patterns based on palmitic (C16:0), oleic and linoleic acids are the rule in dicotyledons and the Cuphea pattern of seed fatty acids is regarded as a derived condition (apomorphy). All the evidence thus far available indicates that the patterns of seed fatty acids in Cuphea represent an autapomorphy, because similar profiles have not been found in genera of other Lythraceae (Graham and Kleiman, 1987, Lythraceae in general; Santos and Salatino, 1998, Diplusodon).
ALKANES OF FOLIAR EPICUTICULAR WAX
Velloziaceae
The Velloziaceae constitute a family of predominantly South American tropical monocotyledons, with approximately 200 species, most of which occur in Brazil, especially in the campos rupestres. Other species occur in Venezuela, Bolivia, Argentina and Africa.
The family is divided into two subfamilies, Vellozioideae (supposedly the most primitive) and Barbacenioideae (advanced). The number and delimitation of the genera are controversial subjects, and two systems of classification (Smith and Ayensu, 1976; Menezes, 1980) with several conflicting points have been proposed for the family (see Mello-Silva, 1994, for a detailed discussion). The classification of Menezes (1980) will be adopted in this paper, with the inclusion of the genera Barbaceniopsis, Nanuza and Talbotia from the system of Smith and Ayensu (1976). According to Menezes' system, the Vellozioideae comprise the genera Vellozia (South America) and Xerophyta, the latter corresponding to South American and African species, plus the genera Barbaceniopsis (Venezuela, Bolivia and Argentina), Talbotia (Africa, monotypic) and Nanuza (Brazil, monotypic) from Smith and Ayensu's (1976) system. The Barbacenioideae comprise the genera Aylthonia, Barbacenia, Burlemarxia and Pleurostima.
Cytogenetic analyses (Melo et al., 1997) revealed that Vellozia and South American Xerophyta are diploid, while the Barbacenioideae Aylthonia, Barbacenia, Burlemarxia and Pleurostima are tetraploid. Since the African groups Talbotia and Xerophyta sensu Smith and Ayensu (1976) are hexaploid, Melo et al. (1997) suggested that the Velloziaceae originated in South America.
A cladistic analysis based predominantly on morphologic characters (Menezes et al., 1994) supports the view (evidenced by morphology and citogenetics) that the Vellozioideae constitute the primitive group of the family, and revealed that they are paraphyletic. The monotypic condition of Nanuza and Talbotia is reinforced by the cladistic analysis, as well as the inclusion of Talbotia, Barbaceniopsis and African Xerophyta among the Barbacenioideae, which appear in the analysis as a consistently monophyletic group. From the base of the Barbacenioideae, Burlemarxia emerges, while Pleurostima is the most advanced genus and consistently monophyletic.
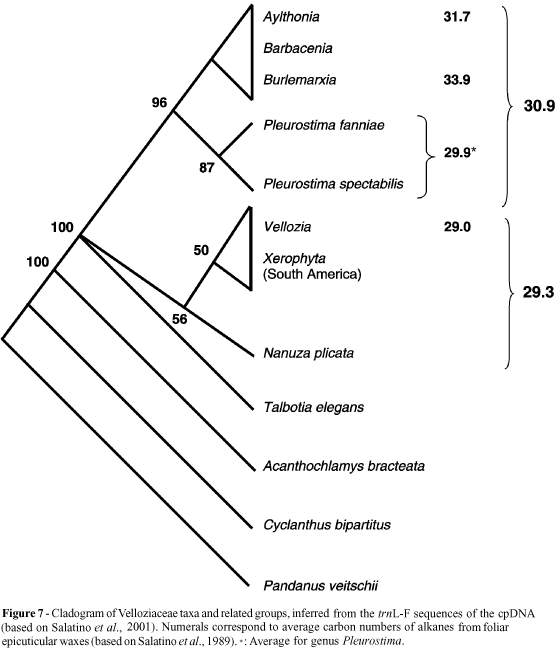
Phylogenetic analysis of South American Velloziaceae (Barbaceniopsis not included), plus Talbotia elegans and Acanthochlamys bracteata (an Asian species with close affinities with Velloziaceae, based on rbcL sequencing; Chase et al., 1995), based on sequencing of the trnL-F region of the chloroplast DNA (Salatino et al., 2001), supports some points raised by the cladistic analysis commented on above but disagrees with other points (Figure 7). The Vellozioideae emerge from the base of the system and are paraphyletic. Nanuza plicata does not group with other species, emerging from the base of the system of the Vellozioideae. However, Talbotia figures in a polytomy at the base of the whole system, with no indication as to which subfamily it belongs. The Barbacenioideae are monophyletic, but from its base emerges Pleurostima rather than Burlemarxia (compare with the results of the morphologic analysis in the previous paragraph). Furthermore, trnL-F sequencing suggests that Burlemarxia is a very recent group, closely related and genetically indistinguishable from Barbacenia.
This section continues with comments on the possible evolutionary trends of the distribution of alkanes in Velloziaceae. No discussion will be made relative to some groups of secondary metabolites relatively well studied in the family, such as flavonoids (see, for example, Williams et al., 1994) and diterpenoids (see, for example, Pinto et al., 1997).
Alkanes of foliar epicuticular wax
Plant cutinized organs present a surface coating formed by amorphous or crystalline deposits of waxes (Baker et al., 1982), which are termed epicuticular, as opposed to the intracuticular waxes which impregnate the cutin lattice of the external cell walls. Epicuticular waxes may have rather complex composition, and different classes of substances such as hydrocarbons, esters, ketones, aldehydes, alcohols, free carboxylic acids, triterpenes and flavonoids may occur in different combinations (Bianchi, 1995). Among these classes of constituents, the alkanes (a class of hydrocarbons) are the most efficient as barriers against water loss, and are seemingly universal in vascular plants.
Alkanes are obtained from epicuticular waxes in general as mixtures of substances with normal carbon chains. Depending on the plant, the alkane distribution can vary from compounds with 15 (C15) to compounds with 37 (C37) carbon atoms, but in most angiosperms the most abundant compounds are C27, C29 or C31.
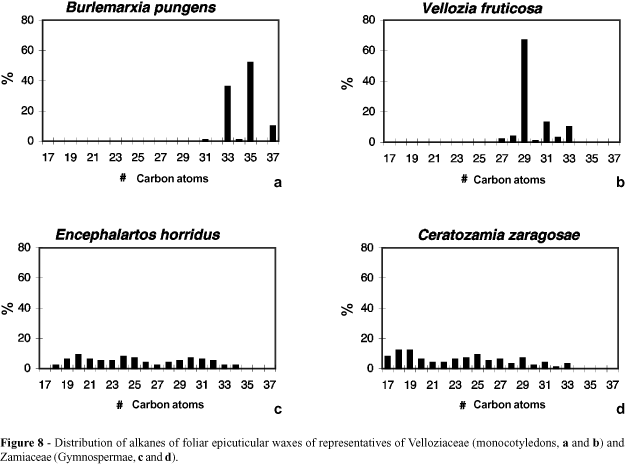
An inventory of the alkane distribution of foliar epicuticular waxes and its contribution to the taxonomy of Velloziaceae has been produced by Salatino et al. (1989) and Figure 8 (a, b) shows the distribution of alkanes of two species of Velloziaceae, where a predominance of homologues with odd numbers of carbon atoms is clearly noticed. The distribution relative to Vellozia fruticosa, with a peak at C29, is in agreement with the general trend in angiosperms, although the alkane distribution of Burlemarxia pungens, which peaks at C35, represents a remarkable deviation from the general tendency. Table I presents the average and confidence interval for alkane distribution for groups of Velloziaceae. Groups within the Vellozioideae have alkanes with numbers of carbon atoms significantly lower than those of Barbacenioideae groups. Among the Vellozioideae there is no significant difference between Vellozia and the South American Xerophyta. In Barbacenioideae, two groups stand out: Pleurostima, with shorter alkane chains than the average (with peaks predominantly at C31), and Burlemarxia which has longer chains (two out of three species of the genus presenting peaks at C35).
Taking into account the alkane distribution of Velloziaceae and the evidence on the evolution of the family, there seems to be a tendency of elongation of the carbon chains which parallels the evolution of the group. Among the Barbacenioideae the direction of elongation of the carbon chains - Pleurostima ® Barbacenia/Aylthonia ® Burlemarxia (Table I) - is more coherent with the phylogeny inferred from chloroplast DNA sequencing (Figure 7) than the phylogeny obtained from morphologic evidence (see above for the conflict between the relative positioning of Barbacenia and Pleurostima between both phylogenies).
In the case of Velloziaceae it is interesting to speculate on the adaptive meaning of longer chains for constituents of foliar waxes. A high proportion of the representatives of the family consists of xerophytic plants, many of them growing on rocky or extremely sandy soil, and in such cases it is reasonable to assume that efficient waxes act as barriers against water loss and are important adaptive attributes, which tend to be strongly fixed genetically, which is probably one of the reasons for the low intraspecific variation of alkane patterns among Velloziaceae (Salatino et al., 1991; Salatino, 1998). It has also been shown that the elongation of carbon chains increases the efficiency of wax constituents as waterproofing agents (Schönherr, 1982), thus alkanes with longer carbon chains could be viewed as a biochemical adaptive advantage related to the reduction of cuticular transpiration, which evolved preferentially in the advanced groups of the family in habitats with limited moisture availability. Perhaps this is the reason for the existence of alkanes with relatively long carbon chains (peaks at C33 or higher) in foliar waxes of other obviously xerophytic families, such as Crassulaceae (Eglinton et al., 1962), Portulacaceae (Tuloch, 1974) and Euphorbiaceae (Proksch et al., 1981). It is noteworthy that the leaves of some South American Vellozioideae, like Nanuza plicata (syn. Xerophyta plicata), dehydrate under prolonged water stress and remain biologically inactive until they receive water (Meguro et al., 1977).
Evolutionarily, the alkanes of Velloziaceae and other xerophytic groups represent the extreme of a biosynthetic development that must have begun with the algae. The latter present alkanes with carbon chains C15-C19, without a clear prevalence between homologues with odd and even numbers of carbon atoms (Blumer et al., 1971). In these groups, alkanes are formed from decarboxylation of fatty acids C16-C20. In land plants, the development of the elongation-decarboxylation system ensued (Wettstein-Knowles, 1995), leading to the synthesis of C28-C32 fatty acids, which give rise, after decarboxylation, to C27-C31 alkanes, with a clear predominance of odd over even homologues, this being the general rule in the angiosperms. The Cycadales form an intermediate step between the more primitive condition of the alkane patterns of the algae and the more advanced pattern of the angiosperms. In these gymnosperms, complex patterns covering the range C18-C33 are common, sometimes bimodal (Figure 8c, d), and without a clear predominance of odd over even alkanes (Osborne et al., 1989, 1993).
In Velloziaceae and some other groups, the elongation system added some additional steps, leading to the synthesis of fatty acids up to C38, which consequently gave rise to alkanes of up to C37 (Figure 8b). Taking all the evidence into consideration, it seems reasonable to assume that, in Velloziaceae, alkanes with longer carbon chains in the foliar waxes represent an evolutionarily advanced state.
CONCLUDING REMARKS
Although phylogeny reconstruction has not been attainable through analyses of the distribution of secondary metabolites, it is scientifically exciting and taxonomically useful to know the evolutionary trends of these plant substances. In addition to plant taxonomy, other areas can benefit from the progress in studies about the evolution of secondary metabolites. Evolutionary ecology is one of these fields, since it takes into account aspects of the evolution of the organisms in connection with the processes of their adaptation to the environment. A particularly fruitful area in evolutionary ecology refers to plant-insect relationships, and studies in this field frequently reveal that secondary metabolites play a key role as intermediaries in mutualistic and parasitic interactions. In this way the understanding of the evolution of secondary metabolites in plant groups has the potential to help achieve a better understanding of the evolution of such interactions and, consequently the evolution of herbivorous insects.
ACKNOWLEDGMENTS
Most results discussed in this paper were obtained through investigations partially financed by one or more of the following Institutions: CAPES (Fund. Coord. Aperfeiçoamento do Pessoal de Nível Superior), CNPq (Cons. Nac. do Desenv. Científico e Tecnológico) and FAPESP (Fund. Amparo à Pesq. do Estado de S. Paulo). Publication supported by FAPESP.
RESUMO
Hipóteses são apresentadas sobre a evolução de padrões estruturais de metabólitos secundários (flavonóides e alcanos de ceras foliares) e ácidos graxos, em famílias bem representadas nos campos rupestres. A distribuição de ácidos graxos é comentada no confronto entre os gêneros de Lythraceae, com maior ênfase em Cuphea (supostamente mais avançado) e Diplusodon, com a proposta de que compostos saturados com cadeias curtas representam uma condição derivada em Lythraceae, embora restrita provavelmente a Cuphea. A evolução deve ter gerado perfis flavonoídicos mais complexos em Cuphea, com a inclusão de C-glicoflavonas e flavonóis metoxilados (ramnetina e isoramnetina), ausentes em representantes de Diplusodon e Lafoensia. Ao contrário, grupos supostamente primitivos de Eriocaulaceae (Paepalanthus, por exemplo) apresentam perfis flavonoídicos mais complexos, caracterizados por flavonas e flavonóis, estes últimos freqüentemente com hidroxilação e metoxilação no carbono 6; alguns grupos mais avançados da mesma família (Leiothrix e Syngonanthus) aparentemente possuem apenas flavonas, com uma tendência ao maior acúmulo de C-glicoflavonas nas espécies de menor porte. Em Velloziaceae, os representantes da subfamília supostamente primitiva (Vellozioideae) apresentam preferencialmente distribuições de alcanos da cera foliar epicuticular com modas em C27, C29 ou C31; os representantes da subfamília derivada (Barbacenioideae) apresentam modas preferencialmente em C33 ou C35. No entanto, no gênero Pleurostima (Barbacenioideae) as modas recaem geralmente em C31. As condições de avanço ou primitividade dos perfis de metabólitos secundários são comparadas com resultados de análises cladísticas baseadas em caracteres morfológicos (Eriocaulaceae e Lythraceae) e morfológicos e moleculares (Velloziaceae).
REFERENCES
- Baker, E.A., Bukovac, M.J. and Hunt, G.M. (1982). Composition of tomato fruit cuticle as related to fruit growth and development. In: The Plant Cuticle (Cutler, D.F., Alvin, K.L. and Price, C.E., eds). Academic Press, London, pp. 33-44.
- Bate-Smith, E.C. and Harborne, J.B. (1969). Quercetagetin and patuletin in Eriocaulon Phytochemistry 8: 1035.
- Bianchi, G. (1995). Plant waxes. In: Waxes: Chemistry, Molecular Biology and Functions (Hamilton, R.J., ed.). The Oily Press, Dundee, pp. 175-222.
- Blatt, C.T.T., Salatino, A., Salatino, M.L.F., del Pero Martinez, M.A. and Cavalcanti, T.B. (1994). Flavonoids of Diplusodon (Lythraceae). Biochem. Syst. Ecol. 22: 101-107.
- Blumer, M., Guillard, R.R.L. and Chase, T. (1971). Hydrocarbons of marine phytoplankton. Mar. Biol. 8: 183-189.
- Chase, M.W.,Stevenson, D.W., Wilkin, P. and Rudall, P.J. (1995). Monocot systematics: a combined analysis. In: Monocotyledons: Systematics and Evolution (Rudall, P.J., Cribb, D.F., Cutler, D.F. and Humphries, C.J., eds). Whitstable Litho Printers Ltd., London, pp. 685-730.
- Cronquist, A. (1981). An Integrated System of Classification of Flowering Plants Columbia University Press, New York.
- Dahlgren, G. (1989). An updated angiosperm classification. Bot. J. Linn. Soc. 100: 197-203.
- Dokkedal, A.L. and Salatino, A. (1992). Flavonoids of Brazilian species of Leiothrix Biochem. Syst. Ecol. 20: 31-32.
- Eglinton, G., Hamilton, R.J., Raphael, R.A. and Gonzalez, A.G. (1962). Hydrocarbon constituents of the wax coatings of plant leaves: a taxonomic survey. Phytochemistry 1: 89-102.
- Giannasi, D.E. (1988). Flavonoids and evolution in the dicotyledons. In: The Flavonoids - Advances in Research Since 1980 (Harborne, J.B., ed.). Chapman and Hall, London, pp. 479-504.
- Giulietti, A.M. and Pirani, J.R. (1988). Patterns of geographic distribution of some plant species from the Espinhaço Range, MG - BA, Brazil. In: Proceedings of the Workshop on Neotropical Distribution Patterns (Vanzolini, P.E. and Heyer, W.R., eds.). Academia Brasileira de Ciências, Rio de Janeiro, pp. 39-69.
- Giulietti, A.M., Scatena, V.L., Sano, P.T., Parra, L.R., Queiroz, L.P., Harley, R.M., Menezes, N.L., Isepon, A.M.B., Salatino, A., Salatino, M.L.F., Vilegas, W., Santos, L.C., Ricci, C.V., Bomfim-Patrício, M.C. and Miranda, E.B. (2000). Multidisciplinary studies on Neotropical Eriocaulaceae. In: Monocots: Systematics and Evolution (Wilson, K.L. and Morrison, D.A., eds.). CSIRO, Melbourne, pp. 580-589.
- Graham, S.A. and Kleiman, R. (1987). Seed lipids of the Lythraceae. Biochem. Syst. Ecol. 15: 433-439.
- Graham, S.A., Timmermann, B.N. and Mabry, T.J. (1980). Flavonoids glycosides in Ammania coccinea (Lythraceae). J. Nat. Prod. 34: 644-645.
- Graham, S.A., Hirsinger, F. and Röbbelen, G. (1981). Cuphea seed lipids and their systematic significance. BioScience 31: 244-246.
- Graham, S.A., Crisci, J.V. and Hoch, P.C. (1993). Cladistic analysis of the Lythraceae sensu lato based on morphological characters. Bot. J. Linn. Soc. 133: 1-33.
- Hamilton, R.J. (1995). Waxes: Chemistry, Molecular Biology and Functions. The Oily Press Ltd., Dundee.
- Harborne, J.B. (1972). Evolution and function of flavonoids in plants. Recent Adv. Phytochem. 4: 107-141.
- Harborne, J.B. (1998). Phytochemical Methods. Chapman & Hall, London.
- Harborne, J.B. and Turner, B.L. (1984). Plant Chemosystematics Academic Press, London.
- Hillis, D.M., Moritz, G. and Mable, B.K. (1996). Molecular Systematics Sinauer Associates, Inc., Sunderland.
- Mayworm, M.A.S. and Salatino, A. (1993). Flavonoids de quatro espécies de Paepalanthus Ruhl. (Eriocaulaceae). Acta Bot. Bras. 7: 129-133.
- Meguro, M., Joly, C.A. and Bittencourt, M.M. (1977). "Stress" hídrico e alguns aspectos do comportamento fisiológico em Xerophyta plicata Spreng. - Velloziaceae. Bolm Bot. Univ. S. Paulo 5: 27-42.
- Mello-Silva, R. (1994). A new species, new synonyms, and a new combination in Brazilian Velloziaceae. Novon 4: 271-275.
- Melo, N.F., Guerra, M., Benko-Iseppon, A.M. and Menezes, N.L. (1997). Cytogenetics and cytotaxonomy of Velloziaceae. Plant Syst. Evol. 204: 257-273.
- Menezes, N.L. (1980). Evolution in Velloziaceae, with special reference to androecial characters. In: Petaloid Monocotyledons: Horticultural and Botanical Research (Brickell, C.D., Cutler, D.F. and Gregory, M., eds). Academic Press, London, pp. 117-137.
- Menezes, N.L., Mello-Silva, R. and Mayo, S.J. (1994). A cladistic analysis of the Velloziaceae. Kew Bull. 49: 71-92.
- Osborne, R., Salatino, M.L.F. and Salatino, A. (1989). Alkanes of foliar epicuticular waxes of the genus Encephalartos. Phytochemistry 11: 3027-3030.
- Osborne, R., Salatino, M.L.F., Sekiya, C.M. and Vazquez-Torres, M. (1993). Alkanes of foliar epicuticular waxes from five cycad genera in the Zamiaceae. Phytochemistry 32: 607-608.
- Pinto, A.C., Pizzolatti, M.G. and Epifanio, R.D.A. (1997). The isolation of novel diterpenoids, including a C-40 bis-diterpenoid, from the Brazilian plant Vellozia magdalenae (Velloziaceae). Tetrahedron 53: 2005-2012.
- Proksch, P., Sternburg, C. and Rodriguez, E. (1981). Epicuticular alkanes from desert plants of Baja California. Biochem. Syst. Ecol. 9: 205-206.
- Pugialli, H.R.L., Kaplan, M.A. and Gottlieb, O.R. (1994). Evolução flavonoídica em Zingiberales. An. Acad. Bras. Cienc. 66: 491-494.
- Ricci, C.V., Patrício, M.C.B., Salatino, M.L.F., Salatino, A. and Giulietti, A.M. (1996). Flavonoids of Syngonanthus Ruhl. (Eriocaulaceae): Taxonomic implications. Biochem. Syst. Ecol. 24: 577-583.
- Salatino, A. (1998). Importância ecológica e taxonômica dos constituintes das ceras vegetais. In: Proceedings of the VI Congreso Latinoamericano de Bótanica (Fortunato, R. and Bacigalupo, N., eds). Missouri Botanical Garden Press, St. Louis, pp. 505-514.
- Salatino, M.L.F., Salatino, A., Menezes, N.L. and Mello-Silva, R. (1989). Alkanes of foliar epicuticular waxes of Velloziaceae. Phytochemistry 28: 1105-1114.
- Salatino, A., Salatino, M.L.F. and Giulietti, A.M. (1990). Contents of soluble phenolic compounds of capitula of Eriocaulaceae. Química Nova 13: 289-292.
- Salatino, A., Salatino, M.L.F., Mello-Silva, R. and Duerholt-Oliveira, I. (1991). An appraisal of the plasticity of alkane profiles of some species of Velloziaceae. Biochem. Syst. Ecol. 19: 241-248.
- Salatino, A., Salatino, M.L.F., Mello-Silva, R., van Sluys, M.-A., Giannasi, D.E. and Price, R.A. (2001). Phylogenetic inference in Velloziaceae using chloroplast trnL-F sequences. Syst. Bot. (in press).
- Santos, D.Y.A.C. and Salatino, A. (1998). Fatty acids of seed oils of species of Diplusodon Pohl (Lythraceae). Biochem. Syst. Ecol. 26: 109-115.
- Santos, D.Y.A.C., Salatino, M.L.F. and Salatino, A. (1995). Flavonoids of species of Cuphea (Lythraceae) from Brazil. Biochem. Syst. Ecol. 23: 99-103.
- Santos, D.Y.A.C., Salatino, M.L.F. and Salatino, A. (2000). Flavonoids of Lafoensia (Lythraceae). Biochem. Syst. Ecol. 28: 487-488.
- Schönherr, J. (1982). Resistance of plant surfaces to water loss: transport properties of cutin, suberin, associated lipids. In: Physiological Plant Ecology II Encyclopedia of Plant Physiology (Lange, O.L., Nobel, P.S., Osmond, C.B. and Ziegler, H., eds). New Series, Vol. 12B. Springer-Verlag, Berlin, pp. 154-179.
- Smith, L.B. and Ayensu, E.S. (1976). A revision of American Velloziaceae. Smithson. Contr. Bot. 30: 1-172.
- Takhtajan, A. (1997). Diversity and Classification of Flowering Plants Columbia University Press, New York.
- Tulloch, A.P. (1974). Leaf wax of Portulaca oleracea. Lipids 9: 664-668.
- Wettstein-Knowles, P. von (1995). Biosynthesis and genetics of waxes. In: Waxes: Chemistry, Molecular Biology and Functions (Hamilton, R.J., ed.). The Oily Press, Dundee, pp. 131-174.
- Williams, C.A., Harborne, J.B., Greenham, J. and Eagles, J. (1994). Differences in flavonoid patterns between genera within the Velloziaceae. Phytochemistry 36: 931-940.
- Wolf, R.B., Graham, S.A. and Kleiman, R. (1983). Fatty acid composition of Cuphea seed oils. J. Am. Oil Chem. Soc. 60: 103-104.
- Woodland, D.W. (1997). Contemporary Plant Systematics Andrews University Press, Michigan.
Publication Dates
-
Publication in this collection
13 Nov 2001 -
Date of issue
Dec 2000