Abstracts
PURPOSE: To compare the resistance of skin wound healing of mice submitted to local or systemic hydrocortisone administration, in different postoperative periods. METHODS: An incision and suture was performed on the thoracic skin of 130 male mice: Group 1 (n = 10) resistance of the integer skin; Group 2 (n = 30) submitted only to skin incision and suture; Group 3 (n = 30) skin incision and suture followed by administration of saline fluid; Group 4 (n = 30) skin incision and suture followed by administration of local hydrocortisone; Group 5 (n = 30) skin incision and suture followed by administration of systemic hydrocortisone. The resistance of the wound healing and the weight of the animals were studied on the seventh, 14th and 21st postoperative days. Histological examination was also performed. RESULTS: The mice that received corticoid (groups 4 and 5) presented significant decreasing on their weight (p = 0.02). The Groups 3, 4 and 5 showed lower scar resistance than Group 2 on the seventh postoperative day (p < 0.05). On the 14th and 21st days, there was no difference on the skin would healing resistance (p > 0.05). CONCLUSION: Administration of hydrocortisone in mice is responsible for weight decreasing and reduction of the skin wound healing resistance during the first postoperative week.
Hydrocortisone; Adrenal Cortex Hormones; Wound Healing; Models, Biological; Mice
OBJETIVO: Comparar a resistência cicatricial cutânea de camundongos submetidos a administração de hidrocortisona por diferentes vias e em distintos períodos pós-operatórios. MÉTODOS: Foram estudados 130 camundongos machos submetidos à incisão e sutura de pele da região dorsal do tórax: Grupo 1 (n = 10) resistência da pele íntegra; Grupo 2 (n = 30) incisão da pele e sutura, sem administração de corticóide; Grupo 3 (n = 30) incisão da pele e sutura, seguidas de injeção local de solução salina; Grupo 4 (n = 30) incisão da pele e sutura, seguidas de injeção local de hidrocortisona e Grupo 5 (n = 30) incisão da pele e sutura, seguidas de injeção intraperitoneal de hidrocortisona. Foram avaliadas a resistência cicatricial da pele e a variação ponderal no sétimo, 14º e 21º dias pós-operatórios, bem como sua histologia. RESULTADOS: Os camundongos que receberam hidrocortisona tiveram decréscimo ponderal (p = 0,02). Quanto à resistência cicatricial da pele, os Grupos 3, 4 e 5 apresentaram valor inferior ao Grupo 2, no sétimo dia pós-operatório (p = 0,031). No 14º e 21º dias, não houve diferença entre as tensões cicatriciais. CONCLUSÃO: A administração de hidrocortisona provoca redução ponderal em camundongos e a resistência cicatricial cutânea é menor na primeira semana pós-operatória.
Hidrocortisona; Corticosteróides; Cicatrização; Modelos Biológicos; Camundongos
3 - ORIGINAL ARTICLE
WOUND HEALING
Influence of local or systemic corticosteroids on skin wound healing resistance1 1 Research performed at Laboratory of Experimental Surgery, Department of Surgery, Medicine School, Federal University of Minas Gerais (UFMG), Belo Horizonte-MG, Brazil.
Influência de corticosteróide local e sistêmico no processo cicatricial cutâneo
Luiz Ronaldo AlbertiI; Leonardo de Souza VasconcellosII; Andy PetroianuIII
IPhD, Associate Professor, Department of Surgery, UFMG and Institute of Research, Postgraduate Program of Santa Casa, Belo Horizonte-MG, Brazil. Technical procedures, acquisition and interpretation of data, statistical analysis and manuscript writing
IIPhD, Associate Professor, Department of Propedeutics, UFMG, Minas Gerais, Brazil. Technical procedures, interpretation of data, histopathological examinations, manuscript writing
IIIPhD, Full Professor, Department of Surgery, UFMG, Minas Gerais, Brazil. Tutor. Responsible for conception, design, intellectual and scientific content of the study; critical analysis; final approval of manuscript
Correspondence Correspondence: Prof. Andy Petroianu Avenida Afonso Pena, 1626/1901 30130-005 Belo Horizonte - MG Brasil Tel. /Fax: (55-31)-3274-7744 / 8884-9192 petroian@medicina.ufmg.br petroian@gmail.com
ABSTRACT
PURPOSE: To compare the resistance of skin wound healing of mice submitted to local or systemic hydrocortisone administration, in different postoperative periods.
METHODS: An incision and suture was performed on the thoracic skin of 130 male mice: Group 1 (n = 10) resistance of the integer skin; Group 2 (n = 30) submitted only to skin incision and suture; Group 3 (n = 30) skin incision and suture followed by administration of saline fluid; Group 4 (n = 30) skin incision and suture followed by administration of local hydrocortisone; Group 5 (n = 30) skin incision and suture followed by administration of systemic hydrocortisone. The resistance of the wound healing and the weight of the animals were studied on the seventh, 14th and 21st postoperative days. Histological examination was also performed.
RESULTS: The mice that received corticoid (groups 4 and 5) presented significant decreasing on their weight (p = 0.02). The Groups 3, 4 and 5 showed lower scar resistance than Group 2 on the seventh postoperative day (p < 0.05). On the 14th and 21st days, there was no difference on the skin would healing resistance (p > 0.05).
CONCLUSION: Administration of hydrocortisone in mice is responsible for weight decreasing and reduction of the skin wound healing resistance during the first postoperative week.
Key words: Hydrocortisone/administration & dosage. Adrenal Cortex Hormones/administration and dosage. Wound Healing/drug effects. Models, Biological. Mice.
RESUMO
OBJETIVO: Comparar a resistência cicatricial cutânea de camundongos submetidos a administração de hidrocortisona por diferentes vias e em distintos períodos pós-operatórios.
MÉTODOS: Foram estudados 130 camundongos machos submetidos à incisão e sutura de pele da região dorsal do tórax: Grupo 1 (n = 10) resistência da pele íntegra; Grupo 2 (n = 30) incisão da pele e sutura, sem administração de corticóide; Grupo 3 (n = 30) incisão da pele e sutura, seguidas de injeção local de solução salina; Grupo 4 (n = 30) incisão da pele e sutura, seguidas de injeção local de hidrocortisona e Grupo 5 (n = 30) incisão da pele e sutura, seguidas de injeção intraperitoneal de hidrocortisona. Foram avaliadas a resistência cicatricial da pele e a variação ponderal no sétimo, 14º e 21º dias pós-operatórios, bem como sua histologia.
RESULTADOS: Os camundongos que receberam hidrocortisona tiveram decréscimo ponderal (p = 0,02). Quanto à resistência cicatricial da pele, os Grupos 3, 4 e 5 apresentaram valor inferior ao Grupo 2, no sétimo dia pós-operatório (p = 0,031). No 14º e 21º dias, não houve diferença entre as tensões cicatriciais.
CONCLUSÃO: A administração de hidrocortisona provoca redução ponderal em camundongos e a resistência cicatricial cutânea é menor na primeira semana pós-operatória.
Descritores: Hidrocortisona/administração & dosagem. Corticosteróides/administração & dosagem. Cicatrização/efeitos de drogas. Modelos Biológicos. Camundongos.
Introduction
Skin wound healing is a complex process that involves inflammation, reepithelization, angiogenesis, granulation tissue formation, and deposition of interstitial matrix, besides other events carried out by different types of cells, such as keratinocytes, fibroblasts, inflammatory and endothelial cells. These phenomena are influenced by the interstitial matrix, growth factors, and other mediators1.
Glucocorticoids seems to hind the wound healing process, causing a decreasing in cellular proliferation, in neovascularization, and in matrix production2. In animals, a delay in the afflux of macrophages, neutrophils, and fibroblasts was reported. It is owned that corticosteroids reduce the inflammatory phase of wound healing2. The chronic use of corticoids may influence negatively reepithelization, neovascularization, and collagen synthesis3.
The effect of prolonged use of corticotherapy on surgical wound healing shows conflicting results in literature. Several factors participate in this controversy, depending on the type and dosage of corticosteroids used, species of animals, period of treatment, and methods of evaluation of healing efficacy4. Diethelm5 observed that pre- or intraoperative administration of hydrocortisone is associated with a greater incidence of infections and delay in healing time. Rizzo et al.6 verified that the adverse effects of the adrenocorticotropic hormone (ACTH) and cortisone were dose-dependent. According to Vogel7, high doses of cortisol and prednisolone reduce the healing resistance of the skin, but small doses, even for a prolonged time, increase healing resistance. On the other hand, Jalali and Bayat8 found unspecific effects with moderate and low doses of corticosteroids, whereas Kletsas et al.9 noted an increased collagen synthesis in fibroblast cultures of human embryo skin by adding small concentrations of corticoids.
The need for further studies in order to understand the effects of corticoids on skin healing motivated this project, which is part of a line of research on wound healing10-12. The objective of this study was to compare the skin wound healing resistance after local and systemic administration of hydrocortisone in distinct postoperative periods.
Methods
This project was carried out according to the recommendations of the international legislations on animal protection and was approved by the Chamber of the Department of Surgery, School of Medicine, Federal University of Minas Gerais (UFMG) and by the Experimental Research Ethics Committee (CETEA) of the UFMG.
One hundred and thirty male albino CF-1 Swiss mice were used, with an average body weight of 40 ± 5g. The animals were randomly distributed into five groups:
Group 1 (n = 10): control, not operated;
Group 2 (n = 30): submitted only to thoracic skin incision and suture;
Group 3 (n = 30): submitted to thoracic skin incision and suture, completed by local daily administration of 0.9% saline solution;
Group 4 (n = 30): submitted to thoracic skin incision and suture, completed by daily local administration of hydrocortisone at 10 mg/kg;
Group 5 (n = 30): submitted to thoracic skin incision and suture, completed by daily intraperitoneal administration of hydrocortisone at 10 mg/kg.
In the Control Group, the resistance of intact skin was evaluated. For the study of skin wound healing resistance, groups 2, 3, 4, and 5 were divided into three subgroups each, as per- and postoperative periods studied:
Subgroup A (n = 10): seventh postoperative day;
Subgroup B (n = 10): 14th postoperative day;
Subgroup C (n = 10): 21st postoperative day.
The animals were daily followed, they received the same nutrition and remained allocated in cages with identical numbers of animals (n = 5).
The operations were conducted under general anesthesia with ketamine hydrochloride (90 mg/kg) and xylazine hydrochloride (10 mg/kg), both intraperitoneally. After shaving the dorsal region, the skin of the median dorsal portion of the thorax was incised longitudinally, measuring 3 cm length, sparing the subjacent muscles. Next, the borders of the wound were sutured with four simple stitches, being used 4-0 monofilament polypropylene.
After the follow-up time, the animals were killed with a lethal inhaled dose of ether after general anaesthesia with ketamine hydrochloride and xylazine hydrochloride with the same doses as previously used. The following parameters were studied:
Body weight variation of the animals during the follow-up period;
Presence of general and local skin complications;
Measures the resistance of rupture tensions of intact skin and scar;
Histological evaluation of the skin wound healing.
The mice were weighed at the onset of the experiment and immediately after death.
In each animal from groups 4 and 5, a 0.1 ml solution was injected daily containing hydrocortisone at 10 mg/kg/day, either at the site of the scar (Group 4) or intraperitoneally (Group 5), as of two days before the operation and continuing until evaluation of skin wound healing resistance. In the group that received only local saline, the volume injected was also 0.1 ml. The application of these injections had the objective of comparing the systemic and local effects of the corticoid, as well as the role of tissue distention at the incision site in the wound healing process.
Resistance of the skin wound healing was assessed by removing a skin sample transversal to the scar, measuring 4 x 1 cm, with scar in the middle portion. The stitches were carefully removed and the fragment was submitted to a tensile resistance testing by electronic tensiometer.
Histological studies were conducted in preparations stained by hematoxylin-eosin and by Gomori's trichrome. The thickness of the wound healing fibrosis neoformation was measured in three different regions: near the cranial extremity, in the mid portion, and near the caudal border.
One-way ANOVA tests were used, followed by the Tukey-Kramer test so the body weights and skin wound healing resistance among the groups could be compared. The differences were considered significant for values corresponding to p<0.05.
Results
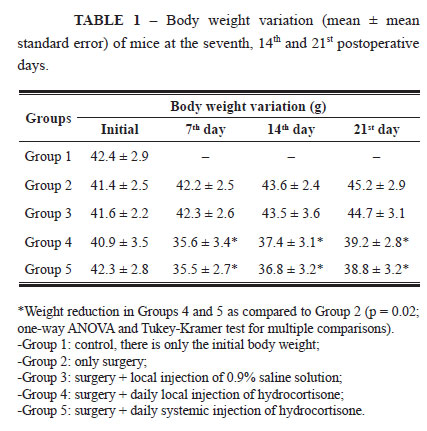
All mice survived the experiment and progressed satisfactorily. The weight variation of all groups studied is shown on Table 1.
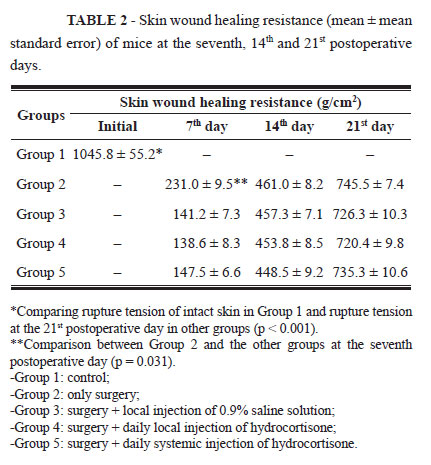
The mice that received hydrocortisone, groups 4 and 5, presented body weight reduction (p = 0.02) during the three postoperative periods, while the animals from the Control Group and those that received only saline solution showed increased body weight. The results of mean skin wound healing resistance are shown on Table 2.
Mice from Groups 3, 4, and 5 displayed values lower than those of Group 2 only on the seventh postoperative day (p = 0.031). During the other periods, there were no differences in skin wound healing resistance in the presence or absence of hydrocortisone.
Administration of corticosteroid, whether local (Group 4) or systemic (Group 5), did not interfere in wound healing resistance when compared to Group 2.
In all mice that were underwent surgery (Groups 2, 3, 4, and 5), the skin wound healing resistance on the seventh postoperative day was lower than that found on the 14th and 21st postoperative days (p<0.05). On the 14th postoperative day, the resistance was lower than that found on the 21st day (p<0.05). Even after 21 days, the skin wound healing resistance was lower than the one of the intact skin in all groups (p<0.01).
With the macroscopic study of the healing process, on the seventh postoperative day, in groups 4 and 5, it was verified that the scar area was looser. In the other groups, on the other hand, healing was firmer. There was no infection or other cicatricial anomaly.
Upon histological assessment, it was verified that in Groups 2 and 3, on the seventh postoperative day there was an increase in collagen bundles, presence of an inflammatory infiltrate composed of polymorphonuclear cells, plasmocytes, lymphocytes, and macrophages, besides vascular congestion. In groups 4 and 5, still during the seventh day, a more heterogeneous pattern was identified, with an inflammatory reaction in addition to the smaller number of fibroblasts and collagen bundles. On the 14th postoperative day, in all the groups, a more homogeneous morphological pattern was observed relative to the first week, with a better structural arrangement of fibroblasts interspersed with firmer fibers that are more ordered. There were no differences between the animals that received and did not receive hydrocortisone. On the 21st postoperative day, collagen bundles were thicker in comparison to the two initial weeks, which shows a more ordered and parallel distribution, with no differences in the aspects of the four groups evaluated.
Discussion
Factors that affect skin wound healing are continually researched, and scar delay is one of the frequently discussed and controversial effects of corticosteroids. Although there are several experimental studies on the relation between wound healing phenomena and the use of corticosteroids, methodological variation makes its evaluation difficult2-5.
The mouse was chosen as the experimental animal due to easy of acquisition, handling, accommodations, resistance to surgical aggression, and low mortality due to infectious processes. Only adult males were used to avoid the hormonal influence of the estrus cycle of females, which could interfere in the process of skin wound healing13.
The choice of hydrocortisone dose for this study was defined on previous studies that verified that 10 mg/kg/day is the minimal concentration necessary to affect wound healing, and it is also proportional to doses prescribed in several clinical treatments10,11. Following other authors7,13, the administration of the drug was initiated in the preoperative phase and continued until the day the animals were killed. According to literature7,8, when corticosteroids are administered before surgery and maintained postoperatively, their harmful effects are more evident.
The body weight reduction of mice submitted to corticoids is also a known fact. According to previous studies3,14, corticotherapy used in animals participates in a complex metabolic process that results in malnutrition. Even though no satisfactory explanation has been found for this phenomenon, this worsening of the animal general condition may have contributed to delay wound healing. Contrary to some studies3,8 in which there has been body weight loss and interference in wound healing process after seven weeks, in this study, the follow-up period of the mice was a lot shorter than those that had been described, and there was a reduction in body weight and skin wound healing resistance as early as the first week of corticoid administration.
This result may be due to the delay in collagen deposition, with a consequent reduction of skin wound healing resistance. During the first week, all groups displayed a healing resistance lower than that posteriorly found. In the beginning, the collagen bundles are still very thin and the skin wound healing resistance is low, which hinders evaluation of the hydrocortisone effect. Even so, it was noted that the groups treated with this drug had a lower wound healing resistance than the Control Group. During the subsequent periods, the skin wound healing resistance of all groups was organized, and there was no difference related to the drug.
As to administration route, it is known that in wound healing processes, topical corticotherapy tends to be used more than systemic administration, especially because of its milder side effects. Nevertheless, in this study, the systemic effects of corticosteroids did not differ from the local effects
It is possible that the comparison of an injection of corticosteroid with its use as an ointment might produce different results, but it is still difficult to obtain results experimentally.
The immediate inflammatory response to trauma is characterized by the presence of hemorrhage, edema, vascular congestion, and inflammatory infiltrate, lasting, on average, for five days. Posteriorly, the fibroplasia phase was accompanied by vascular neoformation and proliferation of fibroblasts, extending, on average, until the 14th day. Histology revealed a delay in the skin wound healing of the mice that received hydrocortisone. On the seventh postoperative day, the Control Group was already in the fibroplasia phase, with intense fibroblasts cells and deposition of collagen fibers, while the groups receiving corticosteroid were still in the inflammatory phase, with scarce fibroblast cells and thin disarrayed collagen fibers13. After the second postoperative week, the healing process of all animals was better organized, with no differences among the groups, displaying well-formed connective tissue rich in ordered collagen fibers.
Despite the observation of a decrease in skin wound healing resistance in mice receiving corticoids during the first postoperative week, it is not possible yet to explain its pathophysiology. The immediate inflammatory response to trauma is characterized by the presence of hemorrhage, edema, vascular congestion, and inflammatory infiltrate, lasting about five days. Later, the fibroplasia phase is followed by vascular neoformation and fibroblast proliferation, extending, on average, until the 14th day. Finally, the maturation cycle, which can last up to two years, arranges the collagen and devascularizes the granulation tissue4.
The action of the corticosteroid may interfere in any one of these processes. According to some authors, this drug promotes stabilization of the lysosome membrane, protecting it against its lysis, and consequently reducing the initial inflammatory reaction4,5,7. In antagonizing angiogenesis, these drugs inhibit fibroblast proliferation, reducing collagen synthesis3,4. This action could possibly explain the reduction in skin wound healing tension, but others studies are still necessary to clarify this issue.
The transposition of the experimental findings to clinical practice should be carefully performed. Riobó et al.15, analyzed patients with intestinal inflammatory disease under treatment with systemic corticotherapy and submitted to surgical treatment, and did not observe changes in complications of ileorectal anastomoses. Fleshner et al.16, on the other hand, verified postoperative adverse effects in patients receiving corticoids.
Conclusion
There has been a decrease of body weight variation and a reduction of skin wound healing resistance during the first postoperative week in mice submitted to the effects of hydrocortisone.
Received: November 10, 2011
Review: January 12, 2012
Accepted: February 14, 2012
Conflict of interest: none
Financial source: National Council for Scientific and Technological Development (CNPq) and FAPEMIG
- 1. Leonard AL, Hanke CW. Second intention healing for intermediate and large postsurgical defects of the lip. J Am Acad Dermatol. 2007;57:832-5.
- 2. Rosen DJ, Patel MK, Freeman K, Weiss PR. A primary protocol for the management of ear keloids: results of excision combined with intraoperative and postoperative steroid injections. Plast Reconstr Surg. 2007;120:1395-400.
- 3. Gupta A, Jain GK, Raghubir R. A time course study for the development of an immunocompromised wound model, using hydrocortisone. J Pharmacol Toxicol Methods. 1999;41:183-7.
- 4. Cohen IK, Diegelmann RF, Johnson ML. Effect of corti costeroids on collagen synthesis. Surgery. 1977;82:15-20.
- 5. Diethelm AG. Surgical management of complications of steroid therapy. Ann Surg. 1977;185(3):251-63.
- 6. Rizzo MC, Solé D, Naspitz CK. Corticosteroids (inhaled and/or intranasal) in the treatment of respiratory allergy in children: safety vs. efficacy. Allergol Immunopathol. 2007;35:197-208.
- 7. Vogel HG. Tensile strength of skin wounds in rats after treatment with corticosteroids. Acta Endocrinol. 1970;64:295-303.
- 8. Jalali M, Bayat A. Current use of steroids in management of abnormal raised skin scars. Surgeon. 2007;5(3):175-80.
- 9. Kletsas D, Pratsinis H, Gioni V, Pilichos K, Yiacoumettis AM, Tsagarakis S. Prior chronic in vivo glucocorticoid excess leads to an anabolic phenotype and an extension of cellular life span of skin fibroblasts in vitro. Ann N Y Acad Sci. 2007;1100:449-54.
- 10. Melo MAB, Almeida LM, Petroianu A. Cicatrização de anastomose colônica em ratos submetidos a diferentes preparos colônicos. Rev Bras Colo-Proctol. 1996;16:19-22.
- 11. Arantes VA, Okawa RY, Petroianu A, Araújo ID. Influência da icterícia obstrutiva na cicatrização de pele e de anastomose jejunal em ratos. Rev Col Bras Cir. 1999;26:269-73.
- 12. Arantes VN, Okawa RY, Petroianu A. Efeito da metilprednisolona sobre a tensão anastomótica jejunal. Arq Gastroenterol. 1994;31:97-101.
- 13. Martins NLP, Malafaia O, Ribas-Filho JM, Heibel M, Baldez N, Vasconcelos PRL, Moreira H, Mazza M, Nunes PA. Análise comparativa da cicatrização da pele com o uso intraperitoneal de extrato aquoso de orbignya phalerata (babaçu). Acta Cir Bras. 2006;21:66-75.
- 14. Pessoa ES, Melhado RM, Theodoro LH, Garcia VG. A histologic assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals. Photomed Laser Surg. 2004;22:199-204.
- 15. Riobó P, Sánchez Vilar O, Burgos R, Sanz A. Colectomy management. Nutr Hosp. 2007;22:135-44.
- 16. Fleshner P, Ippoliti A, Dubinsky M, Ognibene S, Vasiliauskas E, Chelly M, Mei L, Papadakis KA, Landers C, Targan S. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2007;5:952-8
Publication Dates
-
Publication in this collection
24 Apr 2012 -
Date of issue
Apr 2012
History
-
Received
10 Nov 2011 -
Accepted
14 Feb 2012 -
Reviewed
12 Jan 2012