Abstracts
HTLV was initially described in association with a form of leukemia in Japan and a neurological disease in the Caribbean. It was soon shown that HTLV-II was endemic among Amerindians and particularly among Brazilian Indians. The Amazon Region of Brazil is presently the largest endemic area for this virus and has allowed several studies concerning virus biology, the search for overt disease, epidemiological data including detailed demographic data on infected individuals, clear-cut geographic distribution, definition of modes of transmission and maintenance within small, epidemiologically-closed groups, and advances in laboratory diagnosis of the infection. A new molecular subtype named HTLV-IIc was further described on the basis of genome sequencing and phylogenetic analysis. This subtype is present in other areas of Brazil, indicating that the virus is additionally both a valuable marker for tracing past human migration routes in the Americas and a probable marker for social habits of the present human population. HIV, the other human retrovirus, is still not prevalent among indigenous communities in the Brazilian Amazon, but these groups are also easy targets for the virus.
Human T-Lymphotropic Virus II; Retroviridae; South American Indians; Amazonian Ecosystem
O HTLV foi descrito inicialmente associado a uma leucemia no Japão e a uma doença neurológica presente no Caribe. Logo foi evidenciado que o HTLV-II era endêmico entre Ameríndios e, particularmente, entre Índios brasileiros. A Amazônia brasileira é a maior área endêmica para o vírus e dessa forma, permitiu que fossem realizados vários estudos relacionados com a sua biologia, a busca de doença, informações epidemiológicas que incluíram uma bem definida distribuição geográfica, a definição dos modos de transmissão e manutenção do vírus em comunidades pequenas, epidemiologicamente fechadas, assim como contribuições para o diagnóstico laboratorial da infecção. Um novo subtipo molecular, denominado HTLV-IIc, foi adicionalmente descrito baseando-se no sequenciamento genético do vírus e na análise filogenética. Esse subtipo está presente em outras áreas do país, indicando que o HTLV também funciona como um marcador precioso das migrações humanas nas Américas no passado e um provável marcador dos costumes das populações atuais. O outro retrovírus humano, o HIV, ainda não é prevalente nas comunidades indígenas, apesar de que elas podem ser facilmente alcançadas em virtude das inúmeras facilidades de transmissão para o vírus.
Vírus Linfotrópico de Células T Humanas II; Retroviridae; Índios Sul-Americanos; Ecossistema Amazônico
REVISÃO REVIEW
Epidemiological aspects of retrovirus (HTLV) infection among Indian populations in the Amazon Region of Brazil
Aspectos epidemiológicos da infecção pelo retrovírus HTLV entre populações indígenas da Amazônia brasileira
Ricardo Ishak; Antonio Carlos Rosário Vallinoto; Vânia Nakauth Azevedo; Marluísa de Oliveira Guimarães Ishak
Laboratório de Virologia, Centro de Ciências Biológicas, Universidade Federal do Pará. C. P. 13005, Belém, PA 66040-970, Brasil. E-mail: rishak@ufpa.br
ABSTRACT
HTLV was initially described in association with a form of leukemia in Japan and a neurological disease in the Caribbean. It was soon shown that HTLV-II was endemic among Amerindians and particularly among Brazilian Indians. The Amazon Region of Brazil is presently the largest endemic area for this virus and has allowed several studies concerning virus biology, the search for overt disease, epidemiological data including detailed demographic data on infected individuals, clear-cut geographic distribution, definition of modes of transmission and maintenance within small, epidemiologically-closed groups, and advances in laboratory diagnosis of the infection. A new molecular subtype named HTLV-IIc was further described on the basis of genome sequencing and phylogenetic analysis. This subtype is present in other areas of Brazil, indicating that the virus is additionally both a valuable marker for tracing past human migration routes in the Americas and a probable marker for social habits of the present human population. HIV, the other human retrovirus, is still not prevalent among indigenous communities in the Brazilian Amazon, but these groups are also easy targets for the virus.
Key words: Human T-Lymphotropic Virus II; Retroviridae; South American Indians; Amazonian Ecosystem
RESUMO
O HTLV foi descrito inicialmente associado a uma leucemia no Japão e a uma doença neurológica presente no Caribe. Logo foi evidenciado que o HTLV-II era endêmico entre Ameríndios e, particularmente, entre Índios brasileiros. A Amazônia brasileira é a maior área endêmica para o vírus e dessa forma, permitiu que fossem realizados vários estudos relacionados com a sua biologia, a busca de doença, informações epidemiológicas que incluíram uma bem definida distribuição geográfica, a definição dos modos de transmissão e manutenção do vírus em comunidades pequenas, epidemiologicamente fechadas, assim como contribuições para o diagnóstico laboratorial da infecção. Um novo subtipo molecular, denominado HTLV-IIc, foi adicionalmente descrito baseando-se no sequenciamento genético do vírus e na análise filogenética. Esse subtipo está presente em outras áreas do país, indicando que o HTLV também funciona como um marcador precioso das migrações humanas nas Américas no passado e um provável marcador dos costumes das populações atuais. O outro retrovírus humano, o HIV, ainda não é prevalente nas comunidades indígenas, apesar de que elas podem ser facilmente alcançadas em virtude das inúmeras facilidades de transmissão para o vírus.
Palavras-chave: Vírus Linfotrópico de Células T Humanas II; Retroviridae; Índios Sul-Americanos; Ecossistema Amazônico
A brief history of the retroviruses and HTLV
The viruses of the Retroviridae family have become notorious to the lay public since the description of the human immunodeficiency virus (HIV), but the group as a whole had already been familiar to the scientific community since the beginning of the last century.
Pioneering work by Rous (1911) on the induction of sarcomas in birds (inoculated with cell-free filtrates) generated great interest in the possibility of the infectious causation of human neoplasms. Viral agents of the Retroviridae family were subsequently described following isolation from fishes, reptiles, birds, and mammals. Although the pathogenic potential of the retroviruses initially studied was not fully convincing, the hypothesis became an attractive subject for study, since it raised the possibility of reducing tumor incidence by preventive methods such as vaccination.
The enormous research efforts devoted to the retroviruses led to the description of several oncogenic animal viruses, spawning new concepts of virus-host interactions, including the magnificent evolutionary aspect of viral and cell nucleic acid integration, vertical transmission of the virus to offspring, viral persistence, and viral latency. Within the virus family, the presence of oncogenes was described (genome sequences capable of inducing the appearance of neoplasms), as was an enzyme with the function of reading an RNA molecule and producing a DNA copy (reverse transcriptase). The presence of such an enzyme gave rise to the present name of the family, Retroviridae (from reverse transcriptase). The scientific importance of the retroviruses is clearly reflected by the two Nobel prizes awarded for work in relation to these descriptions.
In the early 1980s the association was demonstrated between a retroviral infection and human cancer with the successful isolation of the first human retrovirus, human T-cell lymphotropic virus (HTLV), from patients with cutaneous T-cell lymphomas and leukemia (Poiesz et al., 1980, 1981) and from patients with adult T-cell leukemia/lymphoma (ATLL) (Miyoshi et al., 1981).
In 1982, a second human retrovirus was described (Kalyanaraman et al., 1982). The biological and molecular characterization of these agents showed that they were closely related but distinct agents named HTLV-I and HTLV-II (Hall et al., 1994), which presented different T-cell tropism, for CD4+ and CD8+, respectively (Hall et al., 1996; Rosenblatt et al., 1988; Trejo & Ratner, 2000).
Subsequent seroepidemiologic studies indicated the association of HTLV-I with an endemic encephalo-neuromyelopathy in the Caribbean known as tropical spastic paraparesis (Gessain et al., 1985), now referred to as HTLV-I-associated myelopathy, TSP/HAM (Gessain & Gout, 1992).
Biological characteristics of the virus
HTLV is a typical C-type retrovirus, with an electron-dense centrally located core, medium-sized (100-120nm) enveloped virus, classified in the Retroviridae family, genus Deltaretrovirus, and like all other retroviruses containing two positive-sense, equal, single-stranded RNA (ssRNA) molecules with similar replication strategies (Coffin, 1996).
Apart from the structural genes that are common to the other retroviruses, (gag, env and pol/pro), HTLV also presents a coding region named pX and two long terminal repeats (LTR) without coding function flanking the two ends of the genome. The gag gene encodes structural proteins p19, p24, and p15. The pro/pol genes encode protease and reverse transcriptase, respectively. The env gene encodes the transmembrane and external envelope glycoproteins gp21 and gp46. The pX gene (3' end) presents four open reading frames which encode the spliced, regulatory proteins Tax, Rex (two different molecular presentations) and three other proteins with unknown functions (Johnson et al., 2001; Shimotohno et al., 1985).
HTLV-I and HTLV-II homology varies according to the coding region. They share approximately 85% of their nucleotide sequences in the gag region, but only 65% of the env gene. LTR is commonly used for genotyping HTLV molecular subtypes.
Although the HTLV cell receptor identity has still not been clearly described, it is assumed that there is one cell surface protein acting as a receptor that helps the virus initiate infection (Trejo & Ratner, 2000). HTLV-I displays cell tropism for the T, CD4+ lymphocyte population, whereas HTLV-II preferentially infects the T, CD8+ population.
The peculiar replication of HTLV-I/II provides its biological maintenance in nature and its adaptive advantage in which the ssRNA genome is converted by reverse transcriptase to a dsDNA which is integrated within the host cell nucleus DNA. Such persistent infection subsequently alternates a latency process and a productive cycle, with the production of viral macromolecules and viral particles capable of infecting other cells. Furthermore, such diverse interaction favors an effective mechanism of vertical and horizontal viral transmission.
HTLV-I shows six major genetic subtypes based on the geographic origin of the virus, comparison of the sequences, and phylogenetic analyses of the gp21 and LTR region, named HTLV-Ia (Cosmopolitan subtype), HTLV-Ib (Central African subtype), HTLV-Ic (Australo-Melanesian subtype), HTLV-Id (New Central African subtype), HTLV-Ie, and HTLV-If (Miura et al., 1994; Ureta-Vidal et al., 1994; van Dooren et al., 2001). Likewise, HTLV-II has four molecular subtypes, IIa, IIb, IIc, and IId. This genetic heterogeneity within HTLV-I and -II has provided valuable information on geographic clustering and viral transmission (Hall et al., 1994; Ishak et al., 1995; Slattery et al., 1999; Vandamme et al., 1998).
Epidemiologic aspects of HTLV
Both HTLV-I and HTLV-II have shown an epidemiologic characteristic of prevalence in distinct geographic areas. HTLV-I is maintained at low prevalence (3% to 6%) among specific population groups (in Europe, the Americas, the Caribbean, and sub-Saharan Africa) ranging to more than 30% in Southern Japan (Echeverría-De Perez et al., 1993; Maloney et al., 1992; Mueller et al., 1996; Taylor, 1996).
HTLV-II is endemic among injecting drug users (IDU) in the United States, Europe, and Southeast Asia, and particularly among Amerindian population groups throughout North, Central, and South America, as well as in Pygmy tribes in Central Africa (Egan et al., 1999; Feigal et al., 1991; Fujiyoshi et al., 1999; Fukushima et al., 1998; Gessain & De Thé, 1996; Góngora-Biachi et al., 1997; Goubau et al., 1993; Heneine et al., 1991; Ishak et al., 1995; Salemi et al., 1998; Taylor, 1996). Infection rates are generally low, but high-risk population groups such as IDUs and several Indian tribes in Brazil who are predominantly HTLV-II-infected have a seroprevalence ranging from 1% to more than 40% (Hall et al., 1990, 1996; Ishak et al., 1995, 1998).
In Brazil, HTLV-I/II is present in different geographic areas as confirmed through the several seroepidemiologic studies among the general population as well as in specific groups such as blood donors and patients with hematological and neurological diseases (Andrada-Serpa et al., 1989; Araújo et al., 1993; Castro-Costa et al., 1989; Farias-de-Carvalho et al., 1997; Ferreira Jr. et al., 1995; Ishak et al., 1998, 2002; Lessa et al., 1993; Moreira Jr. et al., 1993; Pombo-de-Oliveira et al., 1990).
Current transmission of HTLV-I/II varies according to the geographic settings and certain behavioral risk factors associated with the dissemination of blood (injected drug use and blood transfusion) and other biological fluids exchanged during sexual relations (male-to-female and female-to-male), as well as vertical (mother-to-child) transmission (Hall et al., 1996).
Local and nationwide regulations controlling blood banks in many parts of the world have substantially reduced the risk of acquiring infection through transfusion of cellular blood products and consequently decreased the current routes of virus transmission. Efficiency of HTLV-II transmission via transfusion ranges from 20% (United States) to 60% (Japan/United States). HTLV is currently spread among human populations through mother-to-child transmission, illicit drug injection, and sexual relations.
HTLV among Amerindians
HTLV, particularly HTLV-II, is widespread among Amerindian population groups. The physical isolation of several communities until recently, maintaining limited contacts with other Indian and urban groups, suggests that the virus is an ancient endemic infection of humans that persisted as presently known or evolved from a primitive virus accompanying the various human migration routes from the African continent (Black, 1997; Gessain & De Thé, 1996). HTLV is remarkably adapted to its human host, and HTLV-II is frequently used as a model of prehistoric human movements throughout the American continent.
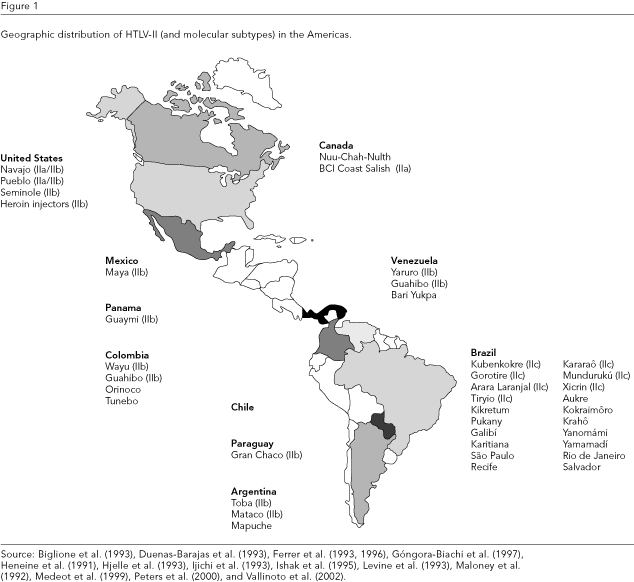
The infection is endemic and widespread within Indian population groups (Figure 1) in North America (Navajo and Pueblo in New Mexico and Seminole in Florida, United States; Nuu-Chah-Nult in Canada; and Maya in Mexico), Central America (Guaymi in Panama), and South America (Wayu, Guahibo, Orinoco, and Tunebo in Colombia; Yaruro, Guahibo, and Bari Yukpa in Venezuela; Toba, Mataco, and Mapuche in Argentina; the Gran Chaco in Paraguay; and in small groups in Chile).
In Brazil, HTLV-II shows a geographic distribution with a strong endemic area within the Indian population groups of the Amazon region, many of whom still living in epidemiologically closed communities (Ishak et al., 1995; Maloney et al., 1992).
HTLV among the indigenous populations of the Brazilian Amazon region
HTLV-I/II prevalence is generally low among the general urban population and it varies according to the behavioral risk factors associated with transmission. Geographic distribution of the virus is remarkably circumscribed to well-defined areas of the world which display different levels of dissemination.
Seroepidemiologic studies have defined primary prevalence rates, but few further studies have been performed to confirm the data mostly published initially in the 1980s and 1990s, that would allow the evaluation of possible trends in the current incidence and trends in the dissemination of HTLV worldwide.
In the Amazon region of Brazil there are several linguistic branches of indigenous groups distributed in eight States and occupying more than three million square kilometers. Access to these areas often poses difficulties ranging from the absence of paved roads, presence of an exuberantly dense native tropical forest, absence of river navigation facilities, other geographic difficulties, and numerous infectious agents (both known and unknown) in the ecosystem.
Initial investigation of HTLV among Indian populations in the Brazilian Amazon was based on retrospective seroepidemiologic studies aimed at finding markers for previous infection by the virus in human populations within the area. The studies used stored serum samples which were tested using appropriate serologic tests with sensitivity and specificity to detect the presence of antibodies to the virus.
HTLV-II infection was shown to be endemic in an indeterminate collection of sera from the Kayapó and Krahô (Maloney et al., 1992). Further serologic studies used a collection with more precisely defined demographic information on 1,382 subjects belonging to 26 Indian communities distributed in six States from the North of Brazil (Ishak et al., 1995). Their geographic distribution was:
Maranhão: Urubú-Kaapór.
Amapá: Galibí, Palikúr and Wayampí.
Roraima: Yanomámi.
Amazonas: Yamamadí.
Rondônia: Cinta-Larga, Suruí, and Karitiána.
Pará: Wayána-Apalaí, Tiriyó, Assuriní Kuatinemo, Asuriní Trocará, Zoé, Arára Laranjal, Arára Kurambê, Arára, Irirí, Araweté, Parakanã, Mundurukú, and six different Kayapó villages (Kararaô, Aukre, Kubenkokre, Pukany, Kikretum, and Kokraimôro).
HTLV was shown to be present in 17 of the 26 communities. Presence of antibodies to HTLV-I was limited to five individuals from the Galibí, Yanomámi, and the Aukre. HTLV-II-positive samples were found to have a widespread geographic distribution and involved culturally distinct and isolated communities. The results are summarized in Table 1.
These results represented the first seroepidemiologic evidence that the Amazon region of Brazil (i) was the largest endemic area of the world for the occurrence of HTLV-II, (ii) presented a unique opportunity to clarify key aspects of virus transmission and pathogenicity, and (iii) provided a chance to correlate these factors with the circulating subtype(s) in the area.
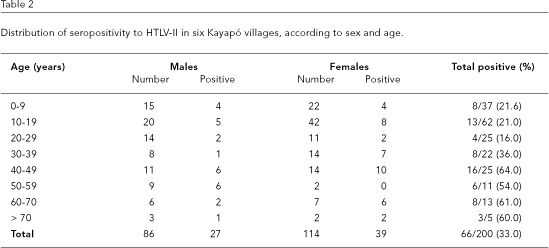
In relation to HTLV-II transmission, six of the Kayapó villages (Kararaô, Aukre, Kubenkokre, Pukany, Kikretum, and Kokraimôro) were grouped (Table 2), and of the 207 subjects (49.8% males and 50.2% females, with ages from three to 74 years of age), 67 (32.3%) were reactive to HTLV-II. The importance of sexual transmission was evidenced by the gradual increase of antibody prevalence with age. The observation of seven couples from the Kubenkokre village demonstrated the constant and continuous transmission between husband and wife (Figure 2). Differently from Black et al. (1996), no difference was found in HTLV-II prevalence between males and females (31.4% vs. 34.2%; p < 0.05), suggesting that sexual transmission of the virus may be equally efficient between the sexes.
For the first time, the occurrence and importance of vertical HTLV-II transmission were also shown. This was indirectly supported by the finding of high seropositivity rates in children (more than 20% of children under nine years were already infected by the virus, including a six-year-old boy) and directly by familial studies among the Kubenkokre showing evidence of HTLV-II infection in three generations (Figure 2). Furthermore, for the Kararaô, it was possible to show the genetic similarity of the virus transmitted from mother to child (Ishak et al., 2001). Such molecular evidence of vertical HTLV-IIc transmission in isolated tribes, as in the Kararaô, highlights both this transmission route and its importance in maintaining the viral infection in small communities.
Although vertical transmission has already been evidenced, the mechanism remains to be elucidated. HTLV-II was isolated from breast milk of mothers harboring the virus (Lal et al., 1993), and a higher transmission level was shown among Guaymi children born of HTLV-II-positive mothers as compared to those with uninfected mothers (Pardi et al., 1993; Vitels et al., 1995). In the seven different Kayapó villages and in families with more than one child, the chances of HTLV-II infection in children with seropositive mothers ranged from 33% to 50% (Ishak et al., 1995); however, these data should be viewed with caution, because the true transmission rate by breastfeeding may be difficult to assess accurately in indigenous Amazonian peoples, among whom it is common for children to be breastfed by other lactating women in the community.
The importance of in utero or perinatal transmission in the maintenance of HTLV-II is still unknown within these epidemiologically closed or partially closed indigenous communities.
More recently, HTLV has been confirmed among the Tiriyó (HTLV-II) and Wayampí (HTLV-I) (Shindo et al., 2002). However, the Amazon region of French Guiana has only shown the presence of HTLV-I among the Arawack (1/54) and Palikúr (2/78; both belonging to the Arawack linguistic branch) and the Wayampí (2/138) of the Tupí-Guaraní linguistic branch (Talarmin et al., 1999). Not surprisingly, HTLV-IIc was found in a single Brazilian Amerindian woman but in none of the urban immigrants from Brazil to French Guiana (Kazanji et al., 2001).
HTLV-II and disease among Amerindians in the Brazilian Amazon
Following the retrospective seroepidemiologic approach, six communities (Mundurukú, Tiriyó, Arára Laranjal, and three Kayapó villages: Kararaô, Kubenkokre, and Gorotire) were revisited to perform an active search for clinical and laboratory information that would allow associations between HTLV-II and hematological and neurological diseases which are occasionally reported (Hall et al., 1996).
No evidence was found of abnormalities in red blood cell counts, hemoglobin levels, hematocrit, platelet counts, or white blood cell counts (total and specific cell counts). A nine-year-old boy was found among the Mundurukú who was seroreactive to HTLV-II, with a clinical picture of cerebellar ataxia (R. Ishak, W. W. Hall & A. Araújo, unpublished results). Black et al. (1996) reports a previous case of a 40-year-old woman who died with a severe muscular weakness resembling TSP/HAM, although the complete clinical diagnostic criteria were not met.
Some of the Indian communities had been actively searched for signs and symptoms of neurological or hematological disease potentially associated with HTLV-II infection. Although such cases are to be expected, as with HTLV-I infection, the association between clinical disease and HTLV-II among Indians is also a rare and difficult event, not easily described.
Molecular biology and molecular epidemiology of HTLV-II in the Brazilian Amazon Region
The initial subtyping of HTLV-II based on restriction fragment length polymorphism (RFLP) patterns (using the enzyme XhoI) on the amplification products of the env region exhibited two molecular subtypes. Additional phylogenetic analysis combining a standard methodology of nucleic acid amplification, polymorphism patterns using endonucleases, and the nucleotide sequences of env, tax, and 5`LTR (sequences ranging from 541 to 668 nucleotides) has shown the presence of four molecular subtypes, forming four separate phylogenetic groups, namely HTLV-IIa, IIb, IIc, and IId (Figure 3) (Eiraku et al., 1996; Hall et al., 1992; Ishak et al., 1995; Vallinoto et al., 2002; Vandamme et al., 1998).
These molecular subtypes exhibit 3% to 6% of genetic differences. So far, there is no reported clear-cut evidence associating any of the molecular subtypes with pathogenic properties in the infected human host. One of the main molecular differences refers to the length of tax, in that IIb and IIc exhibit an extended 22-25 aa, respectively, while IId is practically the same size as IIa, as shown in Table 3 (Eiraku et al., 1996; Vandamme et al., 1998). No relevant biological difference has been shown thus far in the length of the Tax protein.
In the Americas, HTLV-IIb is largely disseminated among indigenous peoples from North to South with the exception of Brazil where HTLV-IIc is the sole representative of the virus, including the IVDA group (Figure 1) (Eiraku et al., 1996; Ishak et al., 1995).
Molecular characterization of HTLV-II among Brazilian Amazon Indians followed a standard methodology to better describe the molecular epidemiology of the virus. The strains examined so far originated from the Kararaô, Kubenkokre, Gorotire, Tiriyó, Arára Laranjal, and Mundurukú proved to belong to molecular subtype HTLV-IIc (Eiraku et al., 1996; Ishak et al., 1995, 2001; Vallinoto et al., 2002). Furthermore, HTLV-II strains from HIV-1 infected patients in the city of Belém were also subtyped as IIc (Vallinoto et al., 2002).
Despite limited and focal controversy on the uniqueness of subtype HTLV-IIc as a new and characteristic molecular subtype occurring solely in Brazil (Shindo et al., 2002), the nomenclature has been widely accepted and commonly reported in recent papers (Dezzutti et al., in press; Kazanji et al., 2001; Slattery et al., 1999; Vallinoto et al., 2002) and scientific presentations (Kazanji, 2002; Lal, 2002; Merteens, 2002; Vicente, 2002).
Dissemination of HTLV among urban populations of the Brazilian Amazon Region
Apart from Indian communities, the search for past or present HTLV infection has been performed in various urban population groups in the Brazilian Amazon Region. Thus far the search for hematological disease has not resulted in a positive association, but neurological manifestations have already been reported in association with HTLV-I infection (Ishak et al., 2002).
The possibility of using HTLV infection as a marker for human population migration led to a search among non-Indian, epidemiologically isolated human groups such as the quilombos (maroon communities, i.e., Black communities descending from havens of runaway slaves), which still maintain a high rate of intra-group marriage. Three communities, Pacoval and Trombetas (both in Pará) and Curiau (Amapá) were tested, but no seroreactivity was confirmed. The above data differ from findings in the Black community in Salvador, Bahia, which accounts for the high HTLV-I prevalence rates found in that city (Brites et al., 1997; Galvão-Castro, 1994). Slaves in Bahia (located in Northeast Brazil) were traded mainly from the West Coast of Africa, while those in the Amazon or North of Brazil came from the East Coast of Africa, a difference to be considered when discussing the presence or absence of HTLV in groups of African-descendant Brazilians. Furthermore, the main risk factor involved in Salvador is injected drug use, which favors dissemination of the virus, but which is absent in the Amazonian communities examined so far.
A surveillance network was prepared to monitor HTLV infection in urban areas and define the geographic distribution of the virus, resulting in three main findings: (i) the virus was circulating not only in the general population (such as blood donors) but also in specific groups like HIV/HTLV co-infected patients (Ishak et al., 1998; Vallinoto et al., 1998); (ii) for the first time HTLV-IIc was shown to be present in urban areas of the Brazilian Amazon Region; and; (iii) unlike observations elsewhere in Brazil, HTLV-IIc was more common than HTLV-I as a co-infection in HIV-1 patients. The comparison with patients from Salvador displays a difference related to the main risk factor. In Belém, the main risk is multiple partners among homosexuals, while in Salvador the main risk factor is IV drug use.
Final remarks
The origin of this remarkably adapted ancient human retrovirus has received particular attention, both for the HTLV-I and the HTLV-II subtypes (Biggar et al., 1996; Black, 1997; Vallinoto et al., 2002). As for the Amazon Region of Brazil, HTLV-II stands out for its specific natural history of molecular change, and the fact that it became isolated as it reached the new geographic area, brought approximately 10,000 to 15,000 years ago by the human migratory wave that arrived in the North of Brazil (Rothhamer & Silva, 1989). The different migratory pathways taken by Amerindian ancestors could have resulted in the introduction of HTLV-IIc exclusively into the Amazon region, although it is also possible that the virus evolved independently from an ancestral prototype.
The genetic background of the populations inhabiting the Amazon points to a clear tri-hybrid model in which 41% of the contribution is of indigenous origin. In the city of Belém this figure is approximately 35% (Santos & Guerreiro, 1995) and in fact may be greater (41%) when molecular markers including autosomal VNTR, six polymorphic mitochondrial loci, and two STR regions of the Y chromosome are included (Rodrigues, 1999). The presence of HTLV-IIc in urban communities in such areas probably reflects the extensive genetic mixture during colonization of the Americas, particularly in Brazil. Introduction of HTLV-IIc may have been an ancient process occurring over many generations since colonization. However, the lack of evidence of previous contact with HTLV in several Indian communities (Ishak et al., 1995) may be the consequence of founder effect and strongly suggests that the virus was introduced more recently through sexual transmission (Vallinoto et al., 2002).
The genetic similarity of the urban and Indian HTLV-II strains confirms our previous proposals that this virus is being actively transmitted from indigenous to, and subsequently within, urban areas and enlarging its geographic endemicity in this region of Brazil (Eiraku et al., 1996; Ishak et al., 1998; Vallinoto et al., 1998). Further studies are needed to elucidate the modes of transmission of the virus from indigenous communities to urban areas. However, it is anticipated that sexual transmission may play an important role because of the apparent absence of intravenous drug use or active blood donations and/or transfusions within these groups.
The importance of sexual transmission is supported by recent socioeconomic activities (from the 1960s onward), that have permitted contact between the tri-hybrid Brazilians and the native Indian populations harboring the virus. Sexual intercourse, particularly between Indian males and non-Indian Brazilian females, is a common practice, especially among some Kayapó villages (Kubenkokre, Kokraimôro, Aukre, Kararaô, Gorotire, Kikretum) that are closely located to small towns maintained by commercial activities, agriculture, and mining.
HTLV is a valuable marker for tracing human mobility in the Americas that occurred in the past and a probable marker of the social habits of the present human population.
Surprisingly, the other human retrovirus, HIV, is still largely absent from the Amazon region of Brazil. A recent report of HIV-1 infection among the Tiriyó was characterized by heteroduplex mobility assay (Shindo et al., 2002) and by sequencing the gene pro as subtype B (Machado et al., 2003). The event involved extensive participation by the Brazilian Ministry of Health and was covered some two years ago by the mainstream press. The Tiriyó Indians show high mobility among individual members, who commonly travel to urban areas within French Guiana, where it is possible to have sexual contacts with HIV-1 infected persons and bring the virus back to the community.
Due to several restraints such as geographic isolation, but particularly because of the difficulties in obtaining permits to perform studies within Indian communities, few strategic data have been produced regarding HTLV or HIV infection among Amazonian groups. Hepatitis B virus (Santos et al., 1995), Chlamydia trachomatis, C. pneumoniae (Ishak & Ishak, 2001), and Treponema pallidum are some of the other debilitating infectious agents that emphasize the importance of generating strategic data on communicable diseases that compromise the fertility and growth of some epidemiologically closed, small, poorly organized communities with few or no available medical resources and who are prone to contacts with individuals not directly concerned with the perpetuation of the community.
Acknowledgements
We wish to thank the Indian communities, as well as our collaborators W. W. Hall, W. Harrington Jr., S. E. B. Santos, J. F. Guerreiro, A. K. C. R. Santos, E. Santos, V. Monteiro, E. S. Abrahim, and S. Caniceiro. The present study was funded by grants from the Brazilian National Research Council (CNPq), the Pará State Fund for Science and Technology of the State Executive Secretariat for Science, Technology, and the Environment, the National Institutes of Health (NIH, United States), the Brazilian National Coordinating Body for STD/ AIDS of the Ministry of Health, and the Federal University in Pará.
Submitted on March 10, 2003
Approved on March 19, 2003
- ANDRADA-SERPA, M. J.; TOSWILL, J.; SCHOR, D.; LINHARES, D.; DOBBIN, J. & PEREIRA, M. S., 1989. Seroepidemiologic survey for antibodies to human retroviruses in human and non-human primates in Brazil. International Journal of Cancer, 44:389-393.
- ARAUJO, A. Q. C.; AFONSO, C. R.; SCHOR, D. & ANDRADA-SERPA, M. J., 1993. Spastic paraparesis of obscure origin. A case-control study of HTLV-I positive and negative patients from Rio de Janeiro, Brazil. Journal of the Neurological Sciences, 116:165-169.
- BIGGAR, R. J.; TAYLOR, M. E.; NEEL, J. V.; HJELLE, B.; LEVINE, P. H.; BLACK, F. L.; SHAW, G. M.; SHARP, P. M. & HAHN, B. H., 1996. Genetic variants of T-lymphotropic virus type II in American Indian Groups. Virology, 216:165-173.
- BIGLIONE, M.; GESSAIN, A.; QUIRUELAS, S.; FAY, O.; TABORDA, M. A.; FERNANDEZ, E.; LUPO, S.; PANZITA, A. & DE THÉ, G., 1993. Endemic HTLV-II infection among Tobas and Mataco Amerindians from North Argentina. Journal of Acquired Immune Deficiency Syndromes, 6:631-633.
- BLACK, F. L., 1997. Tracing prehistoric migrations by the viruses they carry: Human T-cell lymphotropic viruses as markers of ethnic relationships. Human Biology, 69:467-482.
- BLACK, F. L.; BIGGAR, R. J.; LAL, R. B.; GABBAI, A. A. & VIEIRA FILHO, J. P. B., 1996. Twenty-five years of HTLV type II follow-up with a possible case of tropical spastic paraparesis in the Kayapo, a Brazilian Indian Tribe. AIDS Research and Human Retroviruses, 12:1623-1627.
- BRITES, C.; HARRINGTON Jr., W.; PEDROSO, C.; NETTO, E. M. & BADARÓ, R., 1997. Epidemiological characteristics of HTLV-I and II coinfection in Brazilian subjects infected by HIV-1. Brazilian Journal of Infectious Diseases, 1:42-47.
- CASTRO-COSTA, C. M.; SALGUEIRO, M. R.; CARTON, H.; VALE, O. C. & ARRUDA, A. M., 1989. Tropical spastic paraparesis in northeastern Brazil. Arquivos de Neuro-Psiquiatria, 47:134-138.
- COFFIN, J. M., 1996. Retroviridae. In: Fundamental Virology (B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman & S. E. Straus, ed.), pp. 763-843, Philadelphia: Lippincott Raven.
- DEZZUTTI, C. S.; COWAN, E. P. & LAL, R. B., 2003. Human T-cell lymphotropic virus types I and II. In: Manuals of Clinical Microbiology (P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller & R. H. Yolken, ed.), v. 2, pp. 1282-1290, 8th Ed., Washington, DC: American Society for Microbiology Press.
- DUENAS-BARAJAS, E.; BERNAL, J. E.; VAUGTH, D. R.; NERURKAR, V. R.; SARMIENTO, P.; YNAGIHARA, R. & GAJDUSEK, D. C., 1993. Human retroviruses in Amerindians of Colombia: High prevalence of human T-cell lymphotropic virus type II infection among the Tunebo Indians. American Journal of Tropical Medicine and Hygiene, 49:657-663.
- ECHEVERRIA-DE PEREZ, G.; LEON-PONTE, M.; NOYA, O.; BOTTO, C.; GALLO, D. & BIANCO, N., 1993. First description of endemic HTLV-II infection among Venezuelan Amerindians. Journal of Acquired Immune Deficiency Syndromes, 6:1368-1372.
- EGAN, J. F.; O'LEARY, B.; LEWIS, M.; MULCAHY, F.; SHEEHY, N.; HASEGAWA, H.; FITZPATRICK, F.; O'CONNOR, J. J.; O'RIONDAN, J. & HALL, W. W., 1999. High rate of human T-lymphotropic virus type IIa infection in HIV type 1-infected intravenous drug abusers in Ireland. AIDS Research and Human Retroviruses, 15:699-705.
- EIRAKU, N.; NOVOA, P.; FERREIRA, M. C.; MONKEN, C.; ISHAK, R.; FERREIRA, O. C.; ZHU, S. W.; LORENCO, R.; ISHAK, M.; AZEVEDO, V.; GUERREIRO, J. F.; POMBO-DE-OLIVEIRA, M.; LOUREIRO, P.; HAMMERSCHLAK, N.; IJICHI, S. & HALL, W. W., 1996. Identification and characterization of a new and distinct molecular subtype of human T-cell lymphotropic virus type 2. Journal of Virology, 70:1481-1492.
- FARIAS-DE-CARVALHO, S. M.; POMBO-DE-OLIVEIRA, M. S.; THULER, L. C.; RIOS, M.; COELHO, R. C.; RUBIM, L. C.; SILVA, E. M.; REIS, A. M. & CATOVSKY, D., 1997. HTLV-I and HTLV-II infections in hematologic disorder patients, cancer patients, and healthy individuals from Rio de Janeiro, Brazil. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 15:238-242.
- FEIGAL, E.; MURPHY, E.; VRANIZAN, K.; BACCHETTI, P.; CHAISSON, R.; DRUMMOND, J. E.; BLATTNER, W.; McGRATH, M.; GREENSPAN, J. & MOSS, A., 1991. Human T-cell lymphotropic virus types I and II in intravenous drug users in San Francisco: Risk factors associated with seropositivity. Journal of Infectious Diseases, 164:36-42.
- FERREIRA Jr., O. C.; VAZ, R. S.; CARVALHO, M. B.; GUERRA, C.; FABRON, A. L.; ROSEMBLIT, J. & HAMERSCHLAK, N., 1995. Human T-lymphotropic virus type I and type II infections and correlation with risk factors in blood donors from São Paulo, Brazil. Transfusion, 35:258-263.
- FERRER, J. F.; DEL PINO, N.; ESTEBAN, E.; SHERMAN, M. P.; DUBE, S.; DUBE, D. K.; BASOMBRIO, M. A.; PIMENTEL, E.; SEGOVIA, A.; QUIRULAS, S. & POIESZ, B. J., 1993. High rate infection with the human T-leukemia retrovirus type II in four Indian populations of Argentina. Virology, 19:576-584.
- FERRER, J. F.; ESTEBAN, E.; DUBE, S.; BASOMBRIO, M. A.; SEGOVIA, A.; PERALTA-RAMOS, M.; DUBE, D. K.; SAYRE, K.; AGUAYO, N.; HENGST, J. & POIESZ, B. J., 1996. Endemic infection with human T-cell leukemia/lymphoma virus type IIb in Argentinean and Paraguayan Indians: Epidemiology and molecular characterization. Journal of Infectious Diseases, 174:944-953.
- FUJIYOSHI, T.; LI, H. C.; LOU, H.; YASHIKI, S.; KARINO, S.; ZANINOVIC, V.; ONEEGLLO, S. G.; CAMACHO, M.; ANDRADE, R.; HURTADO, L. V.; GOMES, L. H.; DAMIANI, E.; CARTIER, L.; DIPIERRI, J. E.; HAYAMI, M.; SONODA, S. & TAJIMA, K., 1999. Characteristic distribution of HTLV type I and HTLV type II carriers among native ethnic groups in South America. AIDS Research and Human Retroviruses, 15:1235-1239.
- FUKUSHIMA, Y.; LEWIS, M. J.; MONKEN, C.; KOMURO, K.; KUSAGAWA, S.; SATO, H.; TAKEBE, Y.; YAMAZAKI, S.; NGUYEN, T. H.; HOANG, A.; HOANG, T. L.; HONDA, M. & HALL, W. W., 1998. Identification and molecular characterization of human T-cell lymphotropic virus type II infections in intravenous drug abusers in the former South Vietnam. AIDS Research and Human Retroviruses, 14:537-540.
- GALVÃO-CASTRO, B., 1994. Investigação laboratorial da infecção pelo HTLV-I/II e freqüência da co-infecção com o HIV. Revista da Sociedade Brasileira de Medicina Tropical, 27:472-477.
- GESSAIN, A.; BARIN, F.; VERNAT, J. C.; GOUT, O.; MAURS, L.; CALENDER, A. & DE THÉ, G., 1985. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet, 2:407-410.
- GESSAIN, A. & DE THÉ, G., 1996. What is the situation of human T-cell lymphotropic virus type II (HTLV-II) in Africa? Origin and dissemination of genomic subtypes. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 13(Sup. 1): S228-S235.
- GESSAIN, A. & GOUT, O., 1992. Chronic myelopathy associated with human T-lymphotropic virus type I (HTLV-I). Annals of International Medicine, 117:933-946.
- GONGORA-BIACHI, R. A.; LAL, R. B.; RUDOLPH, D. L.; CASTRO-SANSORES, C.; GONZALEZ-MARTINEZ, P. & PAVIA-RUZ, N., 1997. Low prevalence of HTLV-II in Mayan Indians in the Yucatan Peninsula, Mexico. In: VIII International Conference on Human Retrovirology: HTLV, Annals, Abstract ED02, Rio de Janeiro: International Retrovirology Association HTLV and Related Viruses.
- GOUBAU, P.; LIU, H.; LANGE, G. G.; VANDAMME, A. M. & DESMYTER, J., 1993. HTLV-II seroprevalence in Pygmies across Africa since 1970. AIDS Research and Human Retroviruses, 9:709-712.
- HALL, W. W.; ISHAK, R.; ZHU, S. W.; NOVOA, P.; EIRAKU, N.; TAKAHASHI, H.; FERREIRA, M. C.; AZEVEDO, V. N.; ISHAK, M. O. G.; FERREIRA, O. C.; MONKEN, C. & KURATA, T., 1996. Human T-lymphotropic virus type II (HTLV-II): Epidemiology, molecular properties, and clinical features of infection. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 13(Sup. 1): S204-S214.
- HALL, W. W.; KAPLAN, M. H.; SALAHUDDIN, S. Z.; OYAIZU, N.; GURGO, C.; CORONESI, M.; NAGASHIMA, K. & GALLO, R., 1990. Concomitant infections with human T-cell leukemia viruses (HTLVs) and human immunodeficiency virus (HIV): Identification of HTLV-II infection in intravenous drug abuse (IVDAs). In: Human Retrovirology (W. A. Blattner, ed.), pp. 115-127, New York: Raven Press.
- HALL, W. W.; KUBO, T.; IJICHI, S.; TAKAHASHI, H. & ZHU, S. W., 1994. Human T-cell leukemia virus type II (HTLV-II): Emergence of an important newly recognized pathogen. Seminars in Virology, 5:165-178.
- HALL, W. W.; TAKAHASHI, H.; LIU, C.; KAPLAN, M. H.; SHEEWIND, O.; IJICHI, S.; NAGASHIMA, K. & GALLO, R. C., 1992. Multiple isolates and characteristics of human T-cell leukemia virus type II. Journal of Virology, 66:2456-2463.
- HENEINE, W.; KAPLAN, J.; GRACIA, F.; LAI, R.; ROBERTS, B.; LEVINE, P. H. & REEVES, W. C., 1991. HTLV-II endemicity among Guaymi Indians in Panama. New England Journal of Medicine, 324: 565.
- HJELLE, B.; ZHU, S. W.; IGICHI, S.; TAKAHASHI, H. & HALL, W. W., 1993. Endemic HTLV-II infection in Southwestern U.S. Indians involves two prototype variants of virus. Journal of Infectious Diseases, 168:737-740.
- IJICHI, S.; ZANINOVIC, V.; LEON, F. E.; KATAHIRA, Y.; SONODA, S.; MIURA, T.; HAYAMI, M. & HALL, W. W., 1993. Identification of human T-cell leukemia virus type IIb in the Wayu, an aboriginal population of Colombia. Japanese Journal of Cancer Research (Gann), 84:1-4.
- ISHAK, M. O. G. & ISHAK, R., 2001. O impacto da infecção por Chlamydia em populações indígenas da Amazônia brasileira. Cadernos de Saúde Pública, 17:385-396.
- ISHAK, R.; CAVALCANTE, F.; VALLINOTO, A. C. R.; AZEVEDO, V. N. & ISHAK, M. O. G., 2002. HTLV-I associated myelopathy in the Northern region of Brazil (Belém-Pará): Serological and clinical features of three cases. Revista da Sociedade Brasileira de Medicina Tropical, 35:243-246.
- ISHAK, R.; HARRINGTON Jr., W. J.; AZEVEDO, V. N.; EIRAKU, N.; ISHAK, M. O. G.; GUERREIRO, J. F.; SANTOS, S. E. B.; KUBO, T.; MONKE, C.; ALEXANDER, S. & HALL, W. W., 1995. Identification of human T-cell lymphotropic virus type IIa infection in the Kayapo, an indigenous population of Brazil. AIDS Research and Human Retroviruses, 11:813-821.
- ISHAK, R.; ISHAK, M. O. G.; AZEVEDO, V. N.; SANTOS, D. E. M.; VALLINOTO, A. C. R.; SARAIVA, J. C. P.; CRESCENTE, J. A. & HALL, W. W., 1998. Detection of HTLV-IIa in blood donors in an urban area of the Amazon Region of Brazil (Belém, Pará). Revista da Sociedade Brasileira de Medicina Tropical, 31:193-197.
- ISHAK, R.; VALLINOTO, A. C. R.; AZEVEDO, V. N.; LEWIS, M.; HALL, W. W. & ISHAK, M. O. G., 2001. Molecular evidence of mother-to-child transmission of HTLV-IIc in the Kararaô Village (Kayapo) in the Amazon region of Brazil. Revista da Sociedade Brasileira de Medicina Tropical, 34:519-525.
- JOHNSON, J. M.; HARROD, R. & FRANCHINI, G., 2001. Molecular biology and pathogenesis of the human T-cell leukemia/lymphotropic virus type 1 (HTLV-1). Journal of Experimental Pathology, 82:135-147.
- KALYANARAMAN, V. S.; SARNGADHARAN, M. G.; ROBERT-GUROFF, M.; MIYOSHI, I.; BLAYNEY, D.; GOLDE, D. & GALLO, R. C., 1982. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science, 218:571-573.
- KAZANJI, M., 2002. Molecular Characterization and phylogenetic analysis of HTLV-I/II from French Guiana, Surinam and Guiana. In: VII International Symposium on HTLV in Brazil, Annals, Abstract 11, p. 6, Belém: International Retrovirology Association HTLV and Related Viruses.
- KAZANJI, M.; BENOIT, B.; MEDDEB, M.; MEERTENS, L.; MARTY, C.; GESSAIN, A. & TALARMIN, A., 2001. Molecular characterization and phylogenetic analysis of a human T cell leukemia virus type 2 strain from French Guiana. AIDS Research and Human Retroviruses, 17: 563-568.
- LAL, R. B., 2002. Diagnostic strategies for HTLV: Where do we stand. In: VII International Symposium on HTLV in Brazil, Annals, Abstract 26, p. 8, Belém: International Retrovirology Association HTLV and Related Viruses.
- LAL, R. B.; GONGORA-BIACHI, R. A.; PARDI, D.; SWITZER, W. M.; GOLDMAN, I. & LAL, A. A., 1993. Evidence for mother-to-child transmission of human T-cell lymphotropic virus type II. Journal of Infectious Diseases, 168:586-591.
- LESSA, I.; MORAES, D.; MOURA, L. & MELO, A., 1993. HTLV-I and myelopathy in Salvador (Northeastern Brazil). Arquivos de Neuro-Psiquiatria, 51:447-451.
- LEVINE, P. H.; JACOBSON, S.; ELLIOT, R.; CAVALLERO, A.; COLCLOUGH, G.; DORRY, C.; KNIGGE, R. M.; DRUMMOND, J.; NISHIMURA, M.; TAYLOR, M. E.; WIKTOR, S. & SHAW, G. M., 1993. HTLV-II infection in Florida Indians. AIDS Research and Human Retroviruses, 9:123-127.
- MACHADO, L. F. A.; SOUZA, M. I.; SILVA, R. F. P.; FERNADES, L. M.; AZEVEDO, V. N.; LOBATO, L.; VALLINOTO, A. C. R.; ISHAK, M. O. G. & ISHAK, R., 2003. Evidência sorológica e molecular da infecção pelo vírus da imunodeficiência humana tipo 1 (HIV-1) na tribo Tiriyó, Brasil. Revista da Sociedade Brasileira de Medicina Tropical, 36(Sup. 1):521.
- MALONEY, E. M.; BIGGAR, R. J.; NEEL, J. V.; TAYLOR, M. E.; HAHN, B. H.; SHAW, G. M. & BLATTNER, W. A., 1992. Endemic human T-cell lymphotropic virus type II infection among isolated Brazilian Amerindians. Journal of Infectious Diseases, 166: 100-107.
- MEDEOT, S.; NATES, S.; RECALDE, A.; GALLEGO, S.; MATURANO, E.; GIORDANO, M.; SERRA, H.; REATEGUI, J. & CABEZAS, C., 1999. Prevalence of antibody to human T-cell lymphotropic virus types 1/2 among aboriginal groups inhabiting northern Argentina and the Amazon region of Peru. American Journal of Tropical Medicine and Hygiene, 60:623-629.
- MERTEENS, L., 2002. Widespread distribution of novel highly divergent STLV-3 strains among different African monkey species. In: VII International Symposium on HTLV in Brazil, Annals, Abstract 12, p. 6, Belém: International Retrovirology Association HTLV and Related Viruses.
- MIURA, T.; FUKUNAGA, T.; IGARASHI, T.; YAMASHITA, M.; IDO, E.; FUNAHASHI, S.; ISHIDA, T.; WASHIO, K.; UEDA, S.; HASHIMOTO, K.; YOSHIDA, M.; OSAME, M.; SINGHAL, B.; ZANINOVIC, V.; CARTIER, L.; SONODA, S.; TAJIMA, K.; INA, Y.; GOJOBORI, T. & HAYAMI, M., 1994. Phylogenetic subtypes of human T-lymphotropic virus type I and their relations to anthropological background. Proceedings of the National Academy of Sciences of the United States of America, 91:1124-1127.
- MIYOSHI, I.; KUBONISHI, I.; YOSHIMOTO, S.; AGAKI, T.; OHTSHUKI, Y.; SHIRAISHI, Y.; NAGATA, K. & HINUMA, Y., 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature, 294:770-771.
- MOREIRA Jr., E. D.; RIBEIRO, T. T.; SWANSON, P.; SAMPAIO, C.; MEIO, A.; BRITES, C.; BADARO, R.; TOEDTER, G.; LEC, H. & HARRINGTON Jr., W., 1993. Seroepidemiology of Human T-cell lymphotropic virus type I/II in Northeastern Brazil. Journal of Acquired Immune Deficiency Syndromes, 6:959-963.
- MUELLER, N.; OKAYAMA, A.; STUVER, S. & TACHIBANA, N., 1996. Findings from Miyazaki cohort study. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 13(Sup. 1):S2-S7.
- PARDI, D.; SWITZER, W. M.; HADLOCK, K. G.; KAPLAN, J. E.; LAL, R. B. & FOLKS, T. M., 1993. Complete nucleotide sequence of an Amerindian human T-cell lymphotropic virus type II (HTLV-II) isolate: Identification of a variant HTLV-IIb subtype from a Guaymi Indian. Journal of Virology, 67:4659-4664.
- PETERS, A. A.; COULTHART, M. B.; OGER, J. J.; WATERS, D. J.; CRANDALL, K. A.; BAUMGARTNER, A. A.; WARD, R. H. & DEKABAN, G. A., 2000. HTLV type I/II in British Columbia Amerindians: A seroprevalence study and sequence characterization of an HTLV type IIa isolate. AIDS Research and Human Retroviruses, 16:883-892.
- POIESZ, B. J.; RUSCETTI, F. W.; GADZAR, A. F.; BUNN, P. A.; MINNA, J. D. & GALLO, R. C., 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proceedings of the National Academy of Sciences of the United States of America, 77:7415.
- POIESZ, B. J.; RUSCETTI, F. W.; REITZ, M. S.; KALYANARAMAN, V. S. & GALLO, R. C., 1981. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sezary T cell leukemia. Nature, 294:268-271.
- POMBO-DE-OLIVEIRA, M. S.; MATUTES, E.; FANADAS, L. C.; SCHULZ, T. F.; CALABRO, M. L.; NUCCI, M.; ANDRADA-SERPA, M. J.; TEDDER, R. S.; WEISS, R. A. & CATOWSKY, D., 1990. Adult T-cell leukaemia/lymphoma in Brazil and its relation to HTLV-I. Lancet, 336:987.
- RODRIGUES, J. D., 1999. Análise da Estrutura Genética da População de Belém Utilizando Polimorfismos de DNA Nuclear, Mitocondrial e do Cromossomo Y. Dissertação de Mestrado, Belém: Universidade Federal do Pará.
- ROSENBLATT, J. D.; GIORGI, J. V.; GOLDE, D. W.; EZRA, J. B.; WU, A.; WINGER, C. D.; GLASPY, J.; WACHSMAN, W. & CHEN, I. S. Y., 1988. Integrated human T-cell leukemia virus II genome in CD8+ T cells from a patient with "atypical" hairy cell leukemia: Evidence for distinct T and B cell lymphoproliferative disorders. Blood, 71:363-369.
- ROTHHAMER, F. & SILVA, C., 1989. Peopling of Andean South America. American Journal of Physical Anthropology, 78:403-410.
- ROUS, P., 1991. A transmissible avian neoplasm (sarcoma of the common fowl). Journal of Experimental Medicine, 12:696.
- SALEMI, M.; VANDAMME, A. M.; GRADOZZI, C.; van LAETHEM, K.; CATTANEO, E.; TAYLOR, G.; CASOLI, C.; GOUBAU, P.; DESMYTER, J. & BERTAZZONI, U., 1998. Evolutionary rate and genetic heterogeneity of human T-cell lymphotropic virus type II (HTLV-II) using isolates from European injecting drugs users. Journal of Molecular Evolution, 46:602-611.
- SANTOS, A. K. C. R.; ISHAK, M. O. G.; SANTOS, S. E. B.; GUERREIRO, J. F. & ISHAK, R., 1995. A possible correlation between the host genetic background in the epidemiology of hepatitis B virus in the Amazon Region of Brazil. Memórias do Instituto Oswaldo Cruz, 90:435-441.
- SANTOS, S. E. B. & GUERREIRO, J. F., 1995. The indigenous contribution to the formation of the population of the Brazilian Amazon Region. Revista Brasileira de Genética, 18:311-315.
- SHIMOTOHNO, K.; TAKAHASHI, Y.; SHIMIZU, N.; GOJOBORI, T.; GOLDE, D. W. & CHEN, I. S. Y., 1985. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: An open reading frame for the protease gene. Proceedings of the National Academy of Sciences of the United States of America, 82:3101-3105.
- SHINDO, N.; ALCANTARA, L. C.; van DOOREN, S.; SALEMI, M.; COSTA, M. C.; KASHIMA, S.; COVAS, D. T.; TEVA, A.; PELLEGRINI, M.; BRITO, I.; VANDAMME, A. M. & GALVÃO-CASTRO, B., 2002. Human retroviruses (HIV and HTLV) in Brazilian Indians: Seroepidemiological study and molecular epidemiology of HTLV type 2 isolates. AIDS Research and Human Retroviruses, 18:71-77.
- SLATTERY, J. P.; FRANCHINI, G. & GESSAIN, A., 1999. Genomic evolution, patterns of global dissemination, and interspecies transmission of human and simian T-cell leukemia/lymphotropic viruses. Genome Research, 9:525-540.
- TALARMIN, A.; VION, B.; URETA-VIDAL, A.; DU FOU, G.; MARTY, C. & KAZANJI, M., 1999. First seroepidemiological study and phylogenetic characterization of human T-cell lymphotropic virus type I and II infection among Amerindians in French Guiana. Journal of General Virology, 80:3083-3088.
- TAYLOR, G. P., 1996. The epidemiology of HTLV-I in Europe. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 13(Sup. 1): S8-S14.
- TREJO, S. R. & RATNER, L., 2000. The HTLV receptor is a widely expressed protein. Virology, 268:41-48.
- URETA-VIDAL, A.; GESSAIN, A.; YOSHIDA, M.; TEKAIA, F.; GARIN, B.; GUILLEMAIN, B.; SCHULZ, T.; FARID, R. & DE THÉ, G., 1994. Phylogenetic classification of human T cell leukemia/lymphoma virus I genotypes in five major molecular and geographical subtypes. Journal of General Virology, 75:3655-3666.
- VALLINOTO, A. C. R.; AZEVEDO, V. N.; SANTOS, D. E. M.; CANICEIRO, S.; MESQUITA, F. C. L.; HALL, W. W.; ISHAK, M. O. G. & ISHAK, R., 1998. Serological evidence of HTLV-I and HTLV-II coinfections in HIV-1 positive patients in Belém, State of Pará, Brazil. Memórias do Instituto Oswaldo Cruz, 93: 407-409.
- VALLINOTO, A. C. R.; ISHAK, M. O. G.; AZEVEDO, V. N.; VICENTE, A. C. P.; KOKO, O.; HALL, W. W. & ISHAK, R., 2002. Molecular epidemiology of Human T-lymphotropic vírus type II infection in Amerindian and urban populations of the Amazon region of Brazil. Human Biology, 74:633-644.
- van DOOREN, S.; SALEMI, M. & VANDAMME, A. M., 2001. Dating the origin of the human T-cell lymphotropic virus type-I (HTLV-I) subtypes. Molecular Biology and Evolution, 18:661-671.
- VANDAMME, A. M.; SALEMI, M.; van BRUSSEL, M.; LIU, H.-F.; LAETHEM, K. V.; RANST, M. V.; MICHELS, L.; DESMYTER, J. & GOUBAU, P., 1998. African origin of human T-lymphotropic virus type 2 (HTLV-2) supported by a potential new HTLV-2d subtype in Congolese Bambuti Efe Pygmies. Journal of Virology, 72:4327-4340.
- VICENTE, A. C. P., 2002. HTLV-II in Brazil: Epidemiological aspects and evolution. In: VII International Symposium on HTLV in Brazil, Annals, Abstract 14, p. 6, Belém: International Retrovirology Association HTLV and Related Viruses.
- VITELS, C. R.; GRACIA, F. I. & GIUSTI, R. A., 1995. Evidence for sexual and mother to child transmission of human T lymphotropic virus type II among Guaymi Indians, Panama. Journal of Infectious Diseases, 171:1022-1026.
Publication Dates
-
Publication in this collection
05 Sept 2003 -
Date of issue
Aug 2003
History
-
Received
10 Mar 2003 -
Accepted
19 Mar 2003