Abstracts
INTRODUCTION: Toxoplasmosis is usually a benign infection, except in the event of ocular, central nervous system (CNS), or congenital disease and particularly when the patient is immunocompromised. Treatment consists of drugs that frequently cause adverse effects; thus, newer, more effective drugs are needed. In this study, the possible activity of artesunate, a drug successfully being used for the treatment of malaria, on Toxoplasma gondii growth in cell culture is evaluated and compared with the action of drugs that are already being used against this parasite. METHODS: LLC-MK2 cells were cultivated in RPMI medium, kept in disposable plastic bottles, and incubated at 36ºC with 5% CO2. Tachyzoites of the RH strain were used. The following drugs were tested: artesunate, cotrimoxazole, pentamidine, pyrimethamine, quinine, and trimethoprim. The effects of these drugs on tachyzoites and LLC-MK2 cells were analyzed using nonlinear regression analysis with Prism 3.0 software. RESULTS: Artesunate showed a mean tachyzoite inhibitory concentration (IC50) of 0.075µM and an LLC MK2 toxicity of 2.003µM. Pyrimethamine was effective at an IC50 of 0.482µM and a toxicity of 11.178µM. Trimethoprim alone was effective against the in vitro parasite. Cotrimoxazole also was effective against the parasite but at higher concentrations than those observed for artesunate and pyrimethamine. Pentamidine and quinine had no inhibitory effect over tachyzoites. CONCLUSIONS: Artesunate is proven in vitro to be a useful alternative for the treatment of toxoplasmosis, implying a subsequent in vivo effect and suggesting the mechanism of this drug against the parasite.
Artesunate; Treatment; Anti-Toxoplasma activity; Toxicity
INTRODUÇÃO: Toxoplasmose é geralmente uma infecção benigna, exceto nos eventos de doença ocular, congênito e do sistema nervoso central, e particularmente quando o paciente é imunocomprometido. O tratamento consiste de drogas que frequentemente causam efeitos adversos, então novas drogas, mais efetivas são necessárias. Neste estudo, a possível atividade de artesunato, uma droga usada com sucesso no tratamento da malária, sobre o crescimento de Toxoplasma gondii em cultura celular é avaliado e comparado à ação de drogas que já estão sendo utilizadas contra este parasita. MÉTODOS: Células LLC-MK2 foram cultivadas em meio RPMI, mantidas em garrafas plásticas descartáveis e incubados a 36ºC com 5% CO2. Taquizoítos da cepa RH foram usados. As seguintes drogas foram testadas: artesunato, cotrimoxazol, pentamidina, pirimetamina, quinino e trimetoprima. Os efeitos dessas drogas sobre taquizoítos foram analisados por análise regressiva não linear com o software Prism 3.0. RESULTADOS: Artesunato mostrou uma concentração inibitória media (IC50) de 0,075µM e uma toxicidade sobre células LLC MK2 de 2,003µM. Pirimetamina foi efetiva a uma IC50 de 0,482µM e uma toxicidade de 11,178µM. Trimetoprima sozinha foi efetiva contra o parasita in vitro. Cotrimoxazol também foi efetivo contra o parasita, mas a concentrações mais altas que aquelas observadas para artesunato e pirimetamina. Pentamidina e quinino não tiveram efeitos inibitórios sobre os taquizoítos. CONCLUSÕES: Provou-se que artesunato in vitro pode ser uma alternativa útil para o tratamento da toxoplasmose, implicando um subsequente efeito in vivo e sugerindo o mecanismo desta droga contra o parasita.
Artesunato; Tratamento; Atividade anti-Toxoplasma; Toxicidade
ARTICLE ARTIGO
In vitro action of antiparasitic drugs, especially artesunate, against Toxoplasma gondii
Ação in vitro de drogas antiparasitárias, especialmente artesunato, contra Toxoplasma gondii
Thaís Cobellis GomesI,II; Heitor Franco de Andrade JúniorII; Susana Angélica Zevallos LescanoI; Vicente Amato-NetoI
ILaboratório de Parasitologia Médica, Instituto de Medicina Tropical de São Paulo, Universidade de São Paulo, São Paulo, SP
IILaboratório de Protozoologia, Instituto de Medicina Tropical de São Paulo, Universidade de São Paulo, São Paulo, SP
Address to Address to: Dra. Thaís Cobellis Gomes Lab. Parasitologia Médica IMTSP/USP Av. Dr. Enéas de Carvalho Aguiar 470 05403-000 São Paulo, SP, Brasil Phone: 55 11 3061-7010; Fax: 55 11 3088-5237 e-mail: thaiscobellis@bol.com.br
ABSTRACT
INTRODUCTION: Toxoplasmosis is usually a benign infection, except in the event of ocular, central nervous system (CNS), or congenital disease and particularly when the patient is immunocompromised. Treatment consists of drugs that frequently cause adverse effects; thus, newer, more effective drugs are needed. In this study, the possible activity of artesunate, a drug successfully being used for the treatment of malaria, on Toxoplasma gondii growth in cell culture is evaluated and compared with the action of drugs that are already being used against this parasite.
METHODS: LLC-MK2 cells were cultivated in RPMI medium, kept in disposable plastic bottles, and incubated at 36ºC with 5% CO2. Tachyzoites of the RH strain were used. The following drugs were tested: artesunate, cotrimoxazole, pentamidine, pyrimethamine, quinine, and trimethoprim. The effects of these drugs on tachyzoites and LLC-MK2 cells were analyzed using nonlinear regression analysis with Prism 3.0 software.
RESULTS: Artesunate showed a mean tachyzoite inhibitory concentration (IC50) of 0.075µM and an LLC MK2 toxicity of 2.003µM. Pyrimethamine was effective at an IC50 of 0.482µM and a toxicity of 11.178µM. Trimethoprim alone was effective against the in vitro parasite. Cotrimoxazole also was effective against the parasite but at higher concentrations than those observed for artesunate and pyrimethamine. Pentamidine and quinine had no inhibitory effect over tachyzoites.
CONCLUSIONS: Artesunate is proven in vitro to be a useful alternative for the treatment of toxoplasmosis, implying a subsequent in vivo effect and suggesting the mechanism of this drug against the parasite.
Keywords: Artesunate. Treatment. Anti-Toxoplasma activity. Toxicity.
RESUMO
INTRODUÇÃO: Toxoplasmose é geralmente uma infecção benigna, exceto nos eventos de doença ocular, congênito e do sistema nervoso central, e particularmente quando o paciente é imunocomprometido. O tratamento consiste de drogas que frequentemente causam efeitos adversos, então novas drogas, mais efetivas são necessárias. Neste estudo, a possível atividade de artesunato, uma droga usada com sucesso no tratamento da malária, sobre o crescimento de Toxoplasma gondii em cultura celular é avaliado e comparado à ação de drogas que já estão sendo utilizadas contra este parasita.
MÉTODOS: Células LLC-MK2 foram cultivadas em meio RPMI, mantidas em garrafas plásticas descartáveis e incubados a 36ºC com 5% CO2. Taquizoítos da cepa RH foram usados. As seguintes drogas foram testadas: artesunato, cotrimoxazol, pentamidina, pirimetamina, quinino e trimetoprima. Os efeitos dessas drogas sobre taquizoítos foram analisados por análise regressiva não linear com o software Prism 3.0.
RESULTADOS: Artesunato mostrou uma concentração inibitória media (IC50) de 0,075µM e uma toxicidade sobre células LLC MK2 de 2,003µM. Pirimetamina foi efetiva a uma IC50 de 0,482µM e uma toxicidade de 11,178µM. Trimetoprima sozinha foi efetiva contra o parasita in vitro. Cotrimoxazol também foi efetivo contra o parasita, mas a concentrações mais altas que aquelas observadas para artesunato e pirimetamina. Pentamidina e quinino não tiveram efeitos inibitórios sobre os taquizoítos.
CONCLUSÕES: Provou-se que artesunato in vitro pode ser uma alternativa útil para o tratamento da toxoplasmose, implicando um subsequente efeito in vivo e sugerindo o mecanismo desta droga contra o parasita.
Palavras-chaves: Artesunato. Tratamento. Atividade anti-Toxoplasma. Toxicidade.
INTRODUCTION
Toxoplasmosis is a highly prevalent cosmopolitan infection, but the disease occurs in only a fraction of infected people, mainly as a nonspecific immune activation syndrome, chronically in ocular forms as chorioretinitis. The main problems are congenital disease and the infection of immunocompromised people, especially those with acquired immunodeficiency syndrome (AIDS) or those undergoing chemotherapy for cancer or transplant rejection1.
The etiological agent of toxoplasmosis is Toxoplasma gondii, the development of which has many forms. Tachyzoites are found in the acute phase of the disease and are responsible for clinical manifestations. They are susceptible to the immune response of the host and to drug action. Cysts are the resistant form of the parasite, persisting for the host's entire life. Cyst walls are resistant to both drugs and the immune system2.
Felids are the definitive hosts for the parasite, with other mammalians and birds acting as intermediate hosts. Humans can be infected either congenitally or through ingestion of raw or undercooked meat; manipulation of infected meat containing tissue cysts; or consumption of water, fresh vegetables, or other food contaminated by oocysts eliminated in cat feces1,2.
The most effective treatment against toxoplasmosis is a combination of the drugs sulfadiazine and pyrimethamine, which can cause hematological effects that are controlled with the administration of folinic acid. An association of great interest is the one between trimethoprim and sulfamethoxazole. Known as cotrimoxazole, its active compounds act synergistically, inhibiting two consecutive steps of folinic acid biosynthesis in a manner similar to that observed for pyrimethamine-sulfadoxine. Cotrimoxazole is well tolerated and less toxic to hematopoiesis. Human immunodeficiency virusacquired immunodeficiency syndrome (HIV-AIDS) patients taking cotrimoxazole show a high incidence of adverse effects, and its use is discouraged in pregnant women because it crosses the placental barrier3.
Although antifolate compounds, such as pyrimethamine, exhibit good anti-Toxoplasma activity, their toxicity limits widespread use, particularly for extended treatment periods. The discovery of viable low-toxicity compounds capable of preventing and treating T. gondii would represent a great advance in the treatment of infections in immunocompromised patients. Some compounds that are effective against species of Plasmodium could be effective against T. gondii. Those agents were selected for further testing in the present study because malaria and toxoplasmosis are caused by protozoans belonging to the phylum Apicomplexa, and antimicrobial agents that have been effective for the treatment of malaria, such as artemisinin and its derivatives, also have been effective for the treatment of toxoplasmosis. Artemisinin (qinghaosu) is a product extracted from the plant Artemisia annua L. Despite the fact that artemisinin has produced teratogenic effects in laboratory animals, precluding its use in pregnant women, few adverse effects have been observed in humans4,5.
In the present study, the toxicity of artesunate and its effectiveness for the treatment of toxoplasmosis were studied in vitro and compared with the actions of three drugs: pyrimethamine, trimethoprim, and cotrimoxazole, which are currently in use against toxoplasmosis. Pentamidine and quinine, used for the treatment of other protozoans, also were evaluated and compared. Pentamidine is an organic compound and derivative of guanidine that has shown activity against Leishmania sp., African trypanosomiasis, and pneumonia caused by Pneumocystis carinii (jiroveci). Quinine is an alkaloid extracted from species of the genus Cinchona, the application of which is limited to cases of malaria caused by Plasmodium falciparum.
METHODS
Parasites
Tachyzoites of the type I RH strain of T. gondii were routinely maintained by intraperitoneal passage in BALB/c mice.
Drugs
All drugs were obtained from commercial sources (Sigma, USA) or as human use drugs, supplied by the pharmacy of the Hospital das Clínicas da Faculdade de Medicina da Universidade de
São Paulo (HC-FMUSP). Artesunate and quinine dichlorohydrate were supplied by Cipla Medpro (Belville, SA), pentamidine was supplied by Itaca Labs (Rio de Janeiro, BR), and cotrimoxazole was supplied by Ducto (Anapolis, BR).
Cell culture
The epithelial cell line LLC-MK2, derived from rhesus monkey (Macaca mulatta) kidneys, was used. Cells were cultivated in RPMI medium with the addition of 10% inactivated bovine fetal serum and gentamicin. Cultures were kept in disposable plastic bottles and incubated at 36ºC with 5% CO2.
In vitro assays of drug effectiveness and toxicity
The assays were conducted in four stages, the first three on consecutive days. On the first day, LLC-MK2 cells were extracted from a plastic bottle with ATV enzyme and counted in a Neubauer counting chamber. Cells were diluted in RPMI medium containing 10% inactivated bovine fetal serum until a concentration of 1 × 104 was obtained. One hundred microliters of the mixture was added to each well of a 96-well plate and placed inside the CO2 incubator. On the second day, the supernatant from all the wells was aspirated. Tachyzoites from the peritoneal fluid of BALB/c mice were extracted with a syringe and diluted in complete RPMI medium containing 10% bovine fetal serum until a concentration of 1 × 104 was obtained. One hundred microliters of the mixture was added to the wells in rows A to E and columns 1 to 12 of the plate and put into a CO2 incubator. The remaining wells were filled with complete RPMI containing 10% bovine fetal serum. On the third day, the plate was washed with complete RPMI medium containing 10% inactivated bovine fetal serum. A mother solution of each drug was prepared by diluting each one in a solution of complete RPMI medium containing 10% inactivated bovine fetal serum until a 200µL/mL concentration was obtained. Two-fold serial dilutions of all compounds were performed, starting with initial concentrations of 100µg/mL. Therefore, the concentrations used were as follows: 100, 50, 25, 12.5, 6.25, 3.1, 1.6, 0.8, 0.4, 0.2, and 0.1µg/mL. For pyrimethamine and trimethoprim, the first dilution was made in dimethyl sulfoxide, and the remaining dilutions were made in RPMI because of the low solubility of these compounds in water. On another 96-well plate, 200µL of each drug solution was pipetted into wells in column 1, whereas 100µL of RPMI medium containing 10% inactivated bovine fetal serum solution was pipetted into the wells in columns 2 to 12. A two-fold serial dilution was performed by transferring 100µL from column 1 to those in column 2 and repeating this procedure until column 11. The contents of the second plate were transferred to the first plate and placed inside the CO2 incubator. The reaction was interrupted when the tachyzoites had destroyed all the cells from the positive control wells. After that, the supernatant was moved, and the plate was washed with PBS, fixed with methanol, and stained with 1% aqueous crystal violet solution. After being washed, the plate was dried, and 200µL of methanol was added to dissolve the stain. The A620 was measured with an ELISA microplate reader. Adherent live cells were stained. The A620 is proportional to the number of viable cells. This allowed both the measurement of T. gondii cell destruction, or cell toxicity, and detection of infected or non-infected cell layers.
Statistical analysis
Drug effects on both tachyzoites in vitro and LLC-MK2 cells were analyzed using nonlinear regression analysis with Prism 3.0 software, yielding mean inhibitory concentrations (IC50) for the studied compounds.
RESULTS
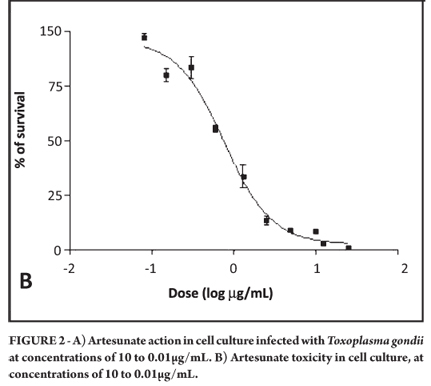
Artesunate at a concentration of 100µg/mL killed all cells in the culture. Therefore, the experiment was performed again with a maximum concentration of 10µg/mL. It was observed that artesunate was effective against tachyzoites at an IC50 of 0.075µM, resulting in preservation of the cell line (Figure 1A and 1B). The mean toxicity of the drug was 2.003µM (Figure 2A and 2B).
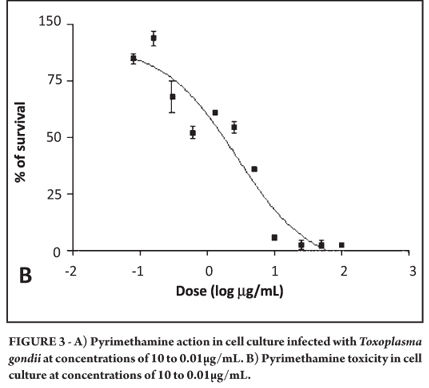
Cell death also was observed with pyrimethamine at 100µg/mL in the cell culture so that experiment also was performed again with a maximum concentration of 10µg/mL. It was observed that pyrimethamine was effective against tachyzoites at an IC50 of 0.482µM, resulting in the preservation of the cell line. The mean toxicity of the drug was 11.178µM (Figure 3A and 3B).
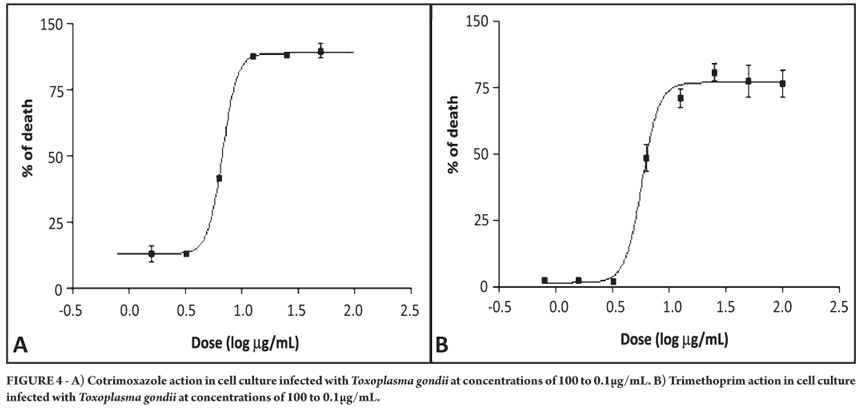
Cotrimoxazole proved to be effective against tachyzoites at an IC50 of 11.884µM, whereas trimethoprim was effective against Toxoplasma gondii at an IC50 of 20.116µM. Neither drug was toxic to the cell culture at the tested concentrations (Figure 4A and 4B).
Pentamidine had no effect on tachyzoites at the tested concentrations, resulting in destruction of the cell line. The same results were observed for quinine. The mean toxicity of pentamidine was 1,316µM, and for quinine, it was 91.030µM.
The selectivity index (SI) could be calculated only for artesunate and pyrimethamine. The SI for the first one was 26.707, and that for the latter was 23.191.
In the present study, artesunate showed the highest efficacy, followed by pyrimethamine. Moreover, artesunate presented the highest toxicity among the studied compounds, again followed by pyrimethamine.
An absence of cotrimoxazole and trimethoprim was observed, yet each showed a weaker effect than artesunate and pyrimethamine. Pentamidine and quinine had no inhibitory effects over T. gondii in this study.
The mean inhibitory concentrations and toxicities, 95% confidence intervals, and r2 values for all drugs used in the study are shown in Table 1. The selectivity index for two of the six drugs could be calculated and also is shown. As can be seen, the most effective drugs against T. gondii were pyrimethamine and artesunate.
DISCUSSION
An in vitro effect of artesunate against T. gondii was found. Previous reports had demonstrated that this compound showed action against other parasites, such as Plasmodium6 and Fasciola7. Although inhibitory concentrations were higher than those used against the parasites that cause malaria, artemisinin and many of its derivatives were effective against T. gondii5. In this experiment, artesunate exhibited a strong effect against T. gondii tachyzoites in in vitro assays. This work represents an initial step toward future studies of the in vivo action of this compound and its effect on the cystic form of the parasite. Artesunate could be an alternative to the standard pyrimethamine-sulfonamide treatment.
Effective action of artesunate against tachyzoites was observed, and it was the highest among all studied compounds. Several studies demonstrating the action of artemisinin derivatives have been conducted, including Ke Ou-Yang et al.8, D'Angelo et al.9, and Sarciron et al.10. El Zawary, in 2008, studied the in vitro action of artesunate against RH strain Toxoplasma and observed a significant reduction in the viability and effect of tachyzoites exposed to drugs compared with a no-treatment control. Although the efficacy of artesunate was demonstrated both here and in El Zawary, the inhibitory concentration values for artesunate found by El Zawary were higher than those observed in the present work. However, because there is no standard methodology for drug testing in cell lines, different outcomes could result11.
Clark et al.12,13 observed that artesunate is toxic to the embryos of mice, rabbits, and nonhuman primates, causing cardiovascular and skeletal problems, even death, when given at higher doses and over longer periods than recommended for the treatment of malaria12,13. At this time, no adverse effects related to the drug have been reported in pregnant women treated with artemisinin, including artesunate. Although the number of pregnant women exposed to artemisinin during the first trimester is considered too small to demonstrate safety, the absence of any adverse effects to the babies in these limited published clinical studies is encouraging.
In this work, cotrimoxazole was effective against T. gondii and not toxic to the cell culture at tested concentrations. The synergistic in vitro effect between trimethoprim and sulfamethoxazole was demonstrated many decades ago, by Grossman and Remington14 and Derouin and Chastang18. More recent studies such as those by Dumas et al.15 and Soheilian et al.16 support the use of this combination to prevent cerebral and ocular toxoplasmosis14-16.
Lindsay et al.17 examined the ability of pentamidine and nine of its analogs to inhibit the replication of RH strain T. gondii in Vero cell cultures. In that study, pentamidine at 25 and 10µg/mL was shown to have significant effects over tachyzoite replication. Lindsay et al.17 obtained a different result from that in the present work, where no anti-Toxoplasma activity of pentamidine was observed at tested concentrations. The conflicting results between these studies may be due to the different cell lines used or differences in methodology because there is no standard model among the authors who perform assays with drugs17.
Pyrimethamine is the main drug of choice for the treatment of toxoplasmosis. It is well known that this drug exhibits in vitro activity against the parasite, as demonstrated by studies such as Derouin and Chastang18, Cantin and Chamberland19, Ven et al.20, and Meneceur et al.4. The findings of the present study were very similar to those previously mentioned, all of them showing effective action at concentrations between 0.05 and 0.24µg/mL on RH strain. When compared with artesunate, pyrimethamine has been shown to be less effective but also less toxic. The selectivity index obtained for these drugs were similar; therefore, artesunate was shown to be a promising option for the treatment of toxoplasmosis18-20.
No anti-Toxoplasma activity was observed for quinine at tested concentrations. These results are compatible with the in vitro experiments from Holfels, which tested quinine sulfate on RH strain T. gondii at 2, 10, and 20µg/mL and observed no inhibitory effects on intracellular tachyzoites.
Since the 1970s, several works have demonstrated the effectiveness of trimethoprim against T. gondii in vitro, among them, Grossman and Remington, Derouin and Chastang, Ven et al.20, and D'Angelo et al.9. Most of these works used RH strain, and all of them found IC50 values between 2 and 10µg/mL. The authors of these studies point out that a murine model is inadequate to evaluate trimethoprim's efficacy because of the difference in the drug's half-life in human and rat sera with a mean toxicity of 60µg/mL, and more human in vivo studies are therefore needed. Because of the short half-life of trimethoprim, a significant inhibitory concentration may not be sustained in human sera, which could explain the poor efficacy of this agent alone. Therefore, besides its low toxicity, trimethoprim on its own is not considered an alternative to pyrimethamine for the treatment of toxoplasmosis9,14,18,20.
In this study, artesunate showed the highest efficacy among the compounds studied, followed by pyrimethamine. Along with their higher efficacy, however, these drugs showed higher toxicity in cell culture. Trimethoprim has shown both efficacy and low toxicity, but treatment with this drug alone is not effective. It is combined with sulfamethoxazole to form cotrimoxazole, which also has been tested and shown to be effective and nontoxic at administered concentrations, lending support to the use of this drug as an alternative treatment to toxoplasmosis.
The data obtained in the present study suggest that artesunate could be a useful alternative to antifolates in the treatment of toxoplasmosis. Further study of artesunate is still required, specifically into its action against T. gondii in vivo and its efficacy against tissue cysts.
The possible toxic effects of artesunate on pregnant women who are being treated for malaria should continue to be investigated, keeping in mind that the dose necessary to kill T. gondii is higher than that for Plasmodium sp.
ACKNOWLEDGMENTS
We gratefully thank Roselaine A.P. Cardoso for technical assistance.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
FINANCIAL SUPPORT
Thais Cobellis Gomes was a fellow of Fundação do Desenvolvimento Administrativo (FUNDAP), and this work was performed during her program in Advanced Techniques in Clinical Pathology of Escola de Educação Permanente do HCFMUSP. This work was supported by Laboratório de Investigação Médica do HCFMUSP, Parasitology Lab. and Protozoology Lab.
Received in 18/05/2011
Accepted in 10/01/2012
- 1. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363:1965-1976.
- 2. Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect 2002; 8:634-640.
- 3. Masters PA, O'Bryan TA, Zurlo J, Miller DQ, Joshi N. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med 2003; 163:402-410.
- 4. Meneceur P, Bouldouyre M-A, Aubert D, Villena I, Menotti J, Sauvage V, et al. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob Agents Chemother 2008; 52:1269-1277.
- 5. Holfels E, McCauley J, Mack D, Milhous WK, McLeod R. In vitro effects of artemisinin ether, cycloguanil, hydrochloride (alone and in combination of sulfadiazine), quinine sulfate, mefloquine, primaquine phosphate, trifluoperazine hydrochloride, and verapamil on Toxoplasma gondii Antimicrob Agents Chemother 1994; 38:1392-1396.
- 6. Chotivanich K, Udomsangpetch R, Chierakul W, Newton P, Ruangveerayuth R, Pukrittayakamee S, et al. In vitro efficacy of antimalarial drugs against Plasmodium vivax on the western border of Thailand. Am J Trop Med Hyg 2004; 70:395-397.
- 7. Keiser J, Shu-hua X, Tanner M, Utzinger J. Artesunate and artemether are effective fasciolicides in the rat model and in vitro J Antimicrob Chemother 2006; 57:1139-1145.
- 8. Ke O-Y, Krug EC, Marr JJ, Berens RL. Inhibition of growth of Toxoplasma gondii by Qinghaosu and derivatives. Antimicrob Agents Chemother. 1990; 34:1961-1965.
- 9. D'Angelo JG, Bordón C, Posner GH, Yolken R, Jones-Brando L. Artemisinin derivatives inhibit Toxoplasma gondii in vitro at multiple steps in the lytic cycle. J Antimicrob Chemother 2009; 63:146-150.
- 10. Sarciron ME, Saccharin C, Petavy AF, Peyron P. Effects of artesunate, dihydroartemisinin and an artesunate-dihydroartemisinin combination against Toxoplasma gondii Am J Trop Med Hyg 2000; 62:73-76.
- 11. El Zawawy L. Effect of artesunate on Toxoplasma gondii: in vitro and in vivo studies. J Egypt Soc Parasitol 2008; 38:185-201.
- 12. Clark RL, Arima A, Makori N, Nakata Y, Bernard F, Gristwood W. et al. Artesunate: developmental toxicity and toxicokinetics in monkeys. Birth Defects Res B Dev Reprod Toxicol 2008; 83:418-434.
- 13. Clark RL, White TE, A Clode S, Gaunt I, Winstanley P, Ward SA. Developmental toxicity of Artesunate and an Artesunate combination in the rat and rabbit. Birth Defects Res B Dev Reprod Toxicol 2004; 71:380-394.
- 14. Grossman PL, Remington JS. The effect of trimethoprim and sulfamethoxazole on Toxoplasma gondii in vitro and in vivo Am J Trop Med Hyg 1979; 28:445-455.
- 15. Dumas J, Pizzolato G, Pechère J. Evaluation of trimethoprim and sulfamethoxazole as monotherapy or in combination in the management of toxoplasmosis in murine models. Int J Antimicrob Agents. 1999; 13:35-39.
- 16. Soheilian M, Sadoughi M-M, Ghajamia M, Dehghan MH, Yazdani S, Behboudi H, et al. Prospective Randomized Trial of Trimethoprim/Sulfamethoxazole versus Pyrimethamine and Sulfadiazine in the Treatment of Ocular Toxoplasmosis. Ophthalmology 2005; 112:1876-1882.
- 17. Lindsay DS, Blagburn BL, Hall JE, Tidwell RR. Activity of Pentamidine and Pentamidine analogs against Toxoplasma gondii in cell cultures. Antimicrob Agents Chemother 1991; 35:1914-1916.
- 18. Derouin F, Chastang C. In vitro effects of folate inhibitors on Toxoplasma gondii Antimicrob Agents Chemother 1989; 33:1753-1759.
- 19. Cantin L, Chamberland S. In vitro evaluation of the activities of Azithromycin alone and combined with Pyrimethamine against Toxoplasma gondii Antimicrob Agents Chemother 1993; 37:1993-1996.
- 20. Ven A, Ven E, Camps W, Melchers W, Koopmans P, Meer J, et al. Anti-Toxoplasma effects of pyrimethamine, trimethoprim and sulphonamides alone and in combination: implications for therapy. J Ant Chemother 1996; 38:75-80.
Publication Dates
-
Publication in this collection
23 Aug 2012 -
Date of issue
Aug 2012
History
-
Received
18 May 2011 -
Accepted
10 Jan 2012