Abstract
Two new Nemesiidae species from Reserva Biológica do Tinguá, Rio de Janeiro, Brazil are described. Acanthogonatus minimussp. nov. differs from the remaining species of the genus by the male palpal bulb, which has very long and twisted embolus, ca. 2/3 the length of the palpal tibia, long and twisted spermathecae, anterior eye row recurved and fovea T-shaped. Chaco tinguasp. nov. differs from the remaining species of the genus by the retrolateral megaspine on tibia I, palpal embolus tip hook-shaped, inferior tarsal claw on all legs, absence of pubescence on the carapace and legs. Both species were collected with Winkler extractors in leaf litter. They are the smallest specimens in their respective genera and also among the world Nemesiidae described to date. The smallest male of A. minimussp. nov. measures 4.22 mm and of C. tinguasp. nov. measures 3.85 mm. Data on the phenology of C. tinguasp. nov. is presented.
Anaminae; Diplothelopsinae; Mata Atlântica; phenology; spider
The Nemesiidae are the second most speciose family of Mygalomorphae, comprising 374 species in 44 genera (Platnick 2014Platnick NI (2014) The world spider catalog. The American Museum of Natural History, version 15.0, available online at: http://research.amnh.org/iz/spiders/catalog [Accessed: 27/IX/2014] doi: 10.5531/db.iz.0001
http://research.amnh.org/iz/spiders/cata...
). The Nemesiidae include medium sized spiders, for example Prorachias bristowei Mello-Leitão, 1924 (length 38.7 mm) and Lycinus caldera Goloboff, 1995 (35.9 mm), and small ones, e.g., Hermacha anomala (Bertkau, 1880) (8.6 mm) and Flamencopsis minima Goloboff, 1995 (10.7 mm) (Bertkau 1880Bertkau P (1880) Verzeichniss der von Prof. Ed. van Beneden auf seiner im Auftrage der Belgischen Regierung unternommen wissenschaftlichen Reise nach Brasilien und La Plata im Jahren 1872-73 gensammelten Arachniden. Mémoires Couronnes de la Academie Royale des Sciences, Lettres et Beaux-Arts de Belgique 43: 1-120., Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189., Lucas et al. 2005Lucas SM, Indicatti RP, Fukami CY (2005) Redescrição de Prorachias bristowei Mello-Leitão 1924 (Araneae, Mygalomorphae, Nemesiidae). Biota Neotropica 5(1a): 1-6. doi: 10.1590/S1676-06032005000200019
https://doi.org/10.1590/S1676-0603200500...
). The Nemesiidae are distributed world-wide and are divided into six subfamilies (Raven 1985Raven RJ (1985) The spider infraorder Mygalomorphae (Araneae): Cladistics and systematics. Bulletin of The American Museum of Natural History 182: 1-180.). Of these, four occur in the Neotropical region: Anaminae, with three genera: Acanthogonatus Karsch, 1880, Longistylus Indicatti & Lucas, 2005 and Hermacha Simon, 1889; Diplothelopsinae, with five genera: Chaco Tullgren, 1905, Chilelopsis Goloboff, 1995, Diplothelopsis Tullgren, 1905, Flamencopsis Goloboff, 1995 and Lycinus Thorell, 1894; Nemesiinae with one genera: Mexentypesa Raven, 1987; and Pycnothelinae with nine genera: Bayana Pérez-Miles et al., 2014, Hermachura Mello-Leitão, 1923, Neostothis Vellard, 1925, Prorachias Mello-Leitão, 1924, Psalistopoides Mello-Leitão, 1934, Pselligmus Simon, 1892, Pycnothele Chamberlin, 1917, Rachias Simon, 1892 and Stenoterommata Holmberg, 1881 (Raven 1985Raven RJ (1985) The spider infraorder Mygalomorphae (Araneae): Cladistics and systematics. Bulletin of The American Museum of Natural History 182: 1-180., 1987Raven RJ (1987) A new mygalomorph spider genus from Mexico (Nemesiinae, Nemesiidae, Arachnida). Jounal of Arachnology 14: 357-362., Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189., Indicatti & Lucas 2005Indicatti RP, Lucas SM (2005) Description of a new genus of Nemesiidae (Araneae, Mygalomorphae) from the Brazilian Cerrado. Zootaxa 1088: 11-16., Lucas & Indicatti 2006Lucas SM, Indicatti RP (2006) On the genus Psalistopoides Mello-Leitão (Araneae, Mygalomorphae, Nemesiidae). Revista Brasileira de Zoologia 23(2): 547-549. doi: 10.1590/S0101-81752006000200030
https://doi.org/10.1590/S0101-8175200600...
, Lucas et al. 2008Lucas SM, Passanha V, Janini CRV, Indicatti RP (2008) On the genus Neostothis Vellard (Araneae, Nemesiidae). Jounal of Arachnology 36: 472-475. doi: 10.1636/CA07-107.1
https://doi.org/10.1636/CA07-107.1...
, Passanha et al. 2014Passanha V, Indicatti RP, Brescovit AD, Lucas SM (2014) Revision of the spider genus Pycnothele Chamberlin, 1917 (Araneae, Nemesiidae). Iheringia 104(2): 228-251. doi: 10.1590/1678-476620141042228251
https://doi.org/10.1590/1678-47662014104...
, Pérez-Miles et al. 2014Pérez-Miles F, Costa FG, Oca LM (2014) Bayana labordai, new genus and species of Nemesiidae (Araneae: Mygalomorphae) from Northern Uruguay and Southern Brazil. Journal of Natural History 48: 1937-1946. doi: 10.1080/00222933.2014.908970
https://doi.org/10.1080/00222933.2014.90...
). In this study we deal with species that belong to Acanthogonatus and Chaco.
Acanthogonatus was established by Karsch (1880), with the type species, A. francki Karsch, 1880, based on a female from Chile. The genus currently includes 28 species, distributed mostly in the Occidental portion of South America, mainly in Chile and Argentina, and includes 25 species (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.). In the Oriental portion, only A. ericae Indicatti et al., 2008 and A. tacuariensis (Pérez-Miles & Capocasale, 1982) are recorded, both in southern Brazil and the second also in Uruguay (Pérez-Miles & Capocasale 1982Pérez-Miles F, Capocasale RM (1982) Arañas del Uruguay, IV. Hallazgo de una tercera especie del genero Pycnothelopsis: Pycnothelopsis tacuariensis sp. nov. (Araneae, Pycnothelidae). Comunicaciones Zoologicas del Museo de Historia Natural de Montevideo 147: 1-7., Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189., Indicatti et al. 2008Indicatti RP, Lucas SM, Ott R, Brescovit AD (2008) Litter dwelling mygalomorph spiders (Araneae: Microstigmatidae, Nemesi idae) from Araucaria forests in Southern Brazil, with the description of five new species. Revista Brasileira de Zoologia 25: 529-546. doi: 10.1590/S0101-81752008000300021
https://doi.org/10.1590/S0101-8175200800...
).
Chaco was established by Tullgren (1905Tullgren A (1905) Aranedia from the Swedish expedition through the Gran Chaco and the Cordilleras. Arkiv for Zoologi 2(19): 1-81.), with the type species, C. obscura Tullgren, 1905, based on a male from Salta, Argentina. The genus currently includes 11 species, six from Argentina, two from Chile, two from Uruguay and one from Brazil (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189., Montes de Oca & Pérez-Miles 2013Montes de Oca L, Pérez-Miles F (2013) Two new species of Chaco Tullgren from the Atlantic coast of Uruguay (Araneae, Mygalomorphae, Nemesiidae). ZooKeys 337: 73-87. doi: 10.3897/zookeys.337.5779
https://doi.org/10.3897/zookeys.337.5779...
, Ferretti 2014Ferretti N (2014) Chaco ansilta new species from Mendoza province, Western Argentina (Araneae: Nemesiidae). Anais da Academia Brasileira de Ciências 2014: 1-12. doi: 10.1095/0001-3765201420140226
https://doi.org/10.1095/0001-37652014201...
).
In an inventory using Winkler extractors in leaf litter in the Reserva Biológica do Tinguá, Rio de Janeiro, Brazil, representatives of two new Nemesiidae species were collected and are herein described: Acanthogonatus minimussp. nov. and Chaco tinguasp. nov. In addition, data on the phenology of C. tinguasp. nov. is provided.
MATERIAL AND METHODS
The material examined is deposited in the following institutions (abbreviation and curator in parentheses): Instituto Butantan, São Paulo (IBSP, A.D. Brescovit), Museu Nacional do Rio de Janeiro, Rio de Janeiro (MNRJ, A.B. Kury), Museu de Zoologia da Universidade de São Paulo, São Paulo (MZSP, R. Pinto da Rocha), and Museu de Ciências Naturais, Fundação Zoobotânica do Rio Grande do Sul, Porto Alegre (MCN, R. Ott). The format of the descriptions follows Lucas & Indicatti (2010Lucas SM, Indicatti RP (2010) Description of two new species of Lycinus (Araneae: Nemesiidae). Zoologia 27(3): 425-430. doi: 10.1590/51984-46702010000300015
https://doi.org/10.1590/51984-4670201000...
). The terminology for general structures follows Goloboff (1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.). Spine notation follows Petrunkevitch (1925Petrunkevitch A (1925) Arachnida from Panamá. Transactions of the Connecticut Academy of Arts and Sciences 27: 51-248.). All measurements are in millimeters (mm) and were taken with a stereomicroscope equipped with a millimetric ocular lens. The lengths of leg segments were measured between joints, in dorsal view. Total body length includes the chelicerae but not the pedicel and spinnerets. Spermathecae were dissected and immersed in enzyme (Ultrazyme(r)) during 72 hours for soft tissue digestion to allow observation of internal structures. The material for scanning electronic microscopy (SEM) was cleaned in an ultrasonic cleaner USC 700, Thornton Unique, for six minutes, and was gradually dehydrated through immersion in increasingly concentrated (from 80 to 100%) ethanol for approximately eight hours. After dehydration it was critical-point dried. All the material used in SEM was fixed to stubs with double-faced adhesive copper tape and sputter-coated with gold. Images were taken under high vacuum with a FEI Quanta 250 SEM at the Instituto Butantan. Pictures were taken with a Leica DFC500 digital camera mounted on a Leica MZ16A stereomicroscope, the extended focal range images were composed with Leica Application Suite version 2.5.0. Abbreviations: (AME) anterior median eyes, (ALE) anterior lateral eyes, (PLE) posterior lateral eyes, (PME) posterior median eyes, (PMS) posterior median spinnerets, (PLS) posterior lateral spinnerets, (STC) superior tarsal claws, (ITC) inferior tarsal claw; spines: (d) dorsal, (v) ventral, (p) prolateral, (r) retrolateral, (ap) apical, (VP) ventro-prolateral, (VR) ventro-retrolateral.
Ecological data were obtained at Reserva Biológica do Tinguá, located at Serra do Mar, municipality of Nova Iguaçu, state of Rio de Janeiro (southeastern Brazil). The Reserva has an area of 26,260 ha, and altitudinal gradient from 50-1600 m a.s.l. The climate there is classified as 'Cwb' in the Köppen system, temperate humid highland tropical (IBAMA 2006IBAMA (2006) Plano de Manejo da Reserva Biologica do Tinguá. Brasília, Ministério do Meio Ambiente, 102p. Available online at: http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/rebio_tingua.pdf. [Accessed: 27/VI/2014].
http://www.icmbio.gov.br/portal/images/s...
). This type of climate has a short, poorly defined dry season in July and August, averaging 20°C, and a rainy season in December, January and February averaging 27°C. The annual precipitation is 2,099.3 mm, with the wettest months in December and January (IBAMA 2006IBAMA (2006) Plano de Manejo da Reserva Biologica do Tinguá. Brasília, Ministério do Meio Ambiente, 102p. Available online at: http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/rebio_tingua.pdf. [Accessed: 27/VI/2014].
http://www.icmbio.gov.br/portal/images/s...
). The vegetation is classified as Dense Ombrophylous Forest (Atlantic Rainforest), with large structural variation due to its declivity (IBAMA 2006IBAMA (2006) Plano de Manejo da Reserva Biologica do Tinguá. Brasília, Ministério do Meio Ambiente, 102p. Available online at: http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/rebio_tingua.pdf. [Accessed: 27/VI/2014].
http://www.icmbio.gov.br/portal/images/s...
). The study location, known as Barrelão (22°34'28.9'S, 43°24'57.6'W), close to 400 m a.s.l. altitude is classified as Dense Ombrophylous Submontane Forest (Orsolon-Souza et al. 2011Orsolon-Souza G, Esbérard CEL, Mayhé-Nunes AJ, Vargas AB, Veiga-Ferreira S, Folly-Ramos E (2011) Comparison between Winkler's extractor and pitfall traps to estimate leaf litter ants richness (Formicidae) at a rainforest site in southest Brazil. Brazilian Journal of Biology 71(4): 873-880. doi: http://dx.doi.org/10.1590/S1519-69842011000500008
https://doi.org/10.1590/S1519-6984201100...
: fig. 1 A-C).
Samples were collected in the middle of each of the four seasons of 2002: summer (February 02-04); autumn (May 15-17); winter (June 05-07); spring (October 20-22). During each field excursion 25 points were marked along a 1,200 m transect, and at each point we stretched two perpendicular, 25 m lines; one to the left and the other one to the right. At the end of each line we delimited a 1 m2 plot, totaling 50 plots. Each sample refers to one square meter of leaf litter, which was removed and sieved in a 5 mm mesh and soon after placed into the Winkler extractor where it remained for 48 hours. This procedure was adapted from Delabie et al. (2000Delabie JHC, Agosti D, Nascimejnto IC (2000) Litter ant communities of the Brazilian Atlantic rain forest region, p. 1-17. In: Agosti D, Majer JD, Alonso LT, Schultz TR (Eds). Sampling ground-dwelling ants: case studies from the world's rain forests. Perth, Curtin University School of Environmental Biology, vol. 18.) and Orsolon-Souza et al. (2011Orsolon-Souza G, Esbérard CEL, Mayhé-Nunes AJ, Vargas AB, Veiga-Ferreira S, Folly-Ramos E (2011) Comparison between Winkler's extractor and pitfall traps to estimate leaf litter ants richness (Formicidae) at a rainforest site in southest Brazil. Brazilian Journal of Biology 71(4): 873-880. doi: http://dx.doi.org/10.1590/S1519-69842011000500008
https://doi.org/10.1590/S1519-6984201100...
). Since abundance data did not meet the assumptions of parametric tests, the Kruskal-Wallis (KW) non-parametric analysis of variance was used for detecting between-seasons differences in abundance of males, females and juveniles separately (Zar 1999Zar JH (1999) Biostatistical analysis. Upper Saddle River, Prentice-Hall, 4th, 912p.).
TAXONOMY
Nemesiidae Simon, 1889
Anaminae Simon, 1889
Acanthogonatus Karsch, 1880
Identification key for Acanthogonatus updated from Goloboff (1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.)
Males
Male of A. alegre Goloboff, 1995, A. brunneus (Nicolet, 1849), A. incursus (Chamberlin, 1916), A. juncal Goloboff, 1995, A. mulchen Goloboff, 1995, A. parana Goloboff, 1995, A. peniasco Goloboff, 1995, A. tolhuaca Goloboff, 1995 and A. vilches Goloboff, 1995 are unknown.
-
1. ITC IV absent .................... 2
-
1'. ITC IV present .................... 14
-
2. Apophysis with two apical laminar spines on the same base (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 75-78); palpal tibia with two dorsal sinuous long setae (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 9, 79F, 80E, 89C); bulb with no keels, or with lateral keels .................... 3
-
2'. No tibial apophysis of any kind; ITC I absent; forests in southern Chile and Argentina.A. confususGoloboff, 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.
-
3. Bulb with a lateral keel forming a concavity (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 81B, C); central Chile (regions IV, V) .................... A. huaquen Goloboff, 1995
-
3'. Bulb different .................... 4
-
4. Dorsal abdomen yellowish with a chevron (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 98A, 100A); apical article of PLS short, triangular; Argentina (dry regions of Patagonia) and southern Chile .................... 5
-
4'. Dorsal abdomen with pattern formed by numerous mottles (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 84A, 103A, 109B); apical article of PLS longer, digitiform; Argentina (north of Patagonia) .................... 8
-
5. Large spiders (about 20 mm total length, carapace over 9 mm); bulb with two lateral flanges delimiting concave triangular area (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 98C); palpal tibia rather elongate (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 98B) .................... A. patagonicus (Simon, 1905)
-
5'. Medium to small spiders (total length 15 mm or smaller, carapace below 6 mm); bulb variable; palpal tibia shorter .................... 6
-
6. Bulb with low lateral keels (or with single keel) (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 101A, B, 102A, B); metatarsi I with at least 1 or 2 ventral spines .................... 7
-
6'. Bulb with more developed lateral keels (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 100C, D); metatarsi I with no ventral spines .................... A. notatus (Mello-Leitão, 1940)
-
7. Bulb with a single keel (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 102A, B); patella III with 1-1-1; small size (carapace length about 3.5 mm) .................... A. birabeni Goloboff, 1995
-
7'. Bulb with a double keel (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 101A, B); patella III with only 0-0-1; slightly larger (carapace length about 5 mm) .................... A. chilechico Goloboff, 1995
-
8. Dorsal abdomen blackish with yellowishwhitish oblique lines; bulb with widened, flanged tip (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 87B); central-southern Chile (regions IV-VIII) .................... A. pissii (Simon, 1889)
-
8'. Dorsal abdomen with only white dots not forming conti nuous lines, or mottled; bulb with narrower tip, flanged in the base or without flanges .................... 9
-
9. Bulb with no keels (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 89E, F); total length ca. 13 mm; central Argentina (Córdoba, San Luis, San Juan, and Buenos Aires) .................... A. centralis Goloboff, 1995
-
9'. Bulb with serrated, curved and long embolus (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 83B, 85C; Indicatti et al. 2008Indicatti RP, Lucas SM, Ott R, Brescovit AD (2008) Litter dwelling mygalomorph spiders (Araneae: Microstigmatidae, Nemesi idae) from Araucaria forests in Southern Brazil, with the description of five new species. Revista Brasileira de Zoologia 25: 529-546. doi: 10.1590/S0101-81752008000300021
https://doi.org/10.1590/S0101-8175200800... : fig. 17); central Chile, Uruguay and southern Brazil .................... 10 -
10. Metatarsus I slightly curved downward; central Chile .................... A. quilocura Goloboff, 1995
-
10. Metatarsus I straight .................... 11
-
11. Presence of a flange on palpal embolus apex (Indicatti et al. 2008Indicatti RP, Lucas SM, Ott R, Brescovit AD (2008) Litter dwelling mygalomorph spiders (Araneae: Microstigmatidae, Nemesi idae) from Araucaria forests in Southern Brazil, with the description of five new species. Revista Brasileira de Zoologia 25: 529-546. doi: 10.1590/S0101-81752008000300021
https://doi.org/10.1590/S0101-8175200800... : figs. 17, 21); southern Brazil .................... A. ericae -
11'. Absence of a flange on palpal embolus apex (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 85C); Uruguay and southern Brazil .................... A. tacuariensis
-
12. Bulb with three perpendicular flanges along embolus (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 95D, E) .................... A. francki
-
12'. Bulb different .................... 13
-
13. Bulb with a winglike projection (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 96D); PLS apical segment digitiform .................... A. recinto Goloboff, 1995
-
13'. Bulb smooth (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 99D, E); PLS apical segment triangular; Argentina (Patagonia) .................... A. fuegianus (Simon, 1902)
-
14. Anterior tibia with dense prolateral shield of setae (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 103C) .................... 15
-
14'. Anterior tibia without such shield .................... 17
-
15. Palpal tibia widest in basal third, and then uniformly tapering (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 103D); retrolateral thicker setae present along apical 2/3 of article .................... 16
-
15'. Palpal tibia of uniform width along medial half, more abruptly narrowed in the apical third (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 107A); retrolateral thicker setae present only on apical third .................... A. hualpen Goloboff, 1995
-
16. Bulb with its basal portion rounded, abruptly tapered to form embolus base (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 108A); basal portion of the bulb duct strongly sinuous (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 108B) .................... A. patagallina Goloboff, 1995
-
16'. Bulb with its basal portion not rounded, tapering more gradually to form embolus base (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 103F); basal portion of the bulb duct little sinuous (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 103E) .................... A. nahuelbuta Goloboff, 1995
-
17. Tibia I with a strong apical retrolateral megaspine (Fig. 5); palpal bulb with twisted embolus (Figs. 2, 4); total length less than 5 mm; Brazil (Rio de Janeiro) .................... A. minimussp. nov.
-
17'. Tibia I with no retrolateral megaspine; palpal bulb with slightly curved embolus; total length more than 13 mm .................... 18
-
18. No apophysis on anterior tibia .................... A. confusus
-
18'. Low prolateral tibial apophysis, bearing two spines on common base .................... 19
-
19. Palpi long, about three times of the cymbium length (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 79F); embolus ca. half length of palpal bulb (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 79G, H) .................... A. subcalpeianus (Nicolet, 1849)
-
19'. Palpi normal, about two times of the cymbium length; embo lus ca. 2/3 length of palpal bulb (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 92A, B, 93A, B) .................... A. campanae (Legendre & Calderón, 1984)
Females
Female of A. birabeni, A. chilechico and A. patagallina are unknown.
-
1. Claw tufts present; Peru .................... A. incursus
-
1'. Claw tufts absent .................... 2
-
2. ITC IV absent .................... 3
-
2'. ITC IV present .................... 17
-
3. Patella IV with 1-1-1 .................... 4
-
3'. Patella IV with 0-0-1 or (more often) no spines at all .................... 7
-
4. Spermathecae thick, sclerotized (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 97) .................... A. peniasco
-
4'. Spermathecae slightly sclerotized .................... 5
-
5. Spermathecae cactus like (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 89A); Argentina .................... A. centralis
-
5'. Spermathecae different; Chile .................... 6
-
6. Spermathecae with short duct, slightly curved from the internal side (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 95A) .................... A. francki
-
6'. Spermathecae without basal dome, long twisted receptacu lum duct (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 96A) .................... A. recinto
-
7. Apical article of PLS triangular .................... 8
-
7'. Apical article of PLS longer, digitiform .................... 10
-
8. Spermathecae branched (bi- or trifurcated) (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 99A) .................... A. fuegianus
-
8'. Spermathecae with a basal mound or protuberance .................... 9
-
9. Small spiders (total length about 12, carapace length 5-6 mm); carapace slightly patterned, with margins and medial line lighter; lateral stripes of dorsal abdominal chevron conspicuous (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 100A); general coloration yellowish .................... A. notatus
-
9'. Larger spiders (total length well over 20 mm, carapace length 10 mm or more); carapace not appreciably patterned; dorsal abdominal pattern limited mostly to cardiac area (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 98A); general coloration brown with golden hairs .................... A. patagonicus
-
10. Spermathecae with a main branch and a lateral secondary internal branch arising from middle of main branch (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 89A, 9 1B) .................... 11
-
10. Spermathecal duct arising from the inner side of basal mound or protuberance (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 83A, 84B, 86A .................... 12
-
11. Main spermathecal branch widened distally (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 89A); central Argentina .................... A. centralis
-
11'. Main spermathecal branch not widened distally (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 91B); eastern Argentina (Entre Rios) .................... A. parana
-
12. Dorsal abdomen blackish with yellow-white oblique lines; spermathecae with low basal dome (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 86A); central and southern Chile (regions IV-VIII) .................... A. pissii
-
12'. Dorsal abdomen with white dots or spots not forming continuous lines, or yellowish with darker mottles .................... 13
-
13. Small spiders (total length about 10 mm, carapace 4 mm or smaller); spermathecae weakly sclerotized, very short duct (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 84B); patella III with p0-1-1 spines .................... A. juncal
-
13'. Larger (total length 20 mm or more, carapace 8 mm or more); patella III with p1-1-1 spines .................... 14
-
14. Spermathecae with blunt basal mound, and duct strongly curved at the base (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 83A) .................... A. quilocura
-
14'. Spermathecae with basal mound tapering more gradually .................... 15
-
15. Carapace brownish, dorsal abdomen irregularly mottled; with (weak) rastellum; spermathecae with basal dome narrow, duct strong curvate to inner side (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 81A); central Chile .................... A. huaquen
-
15'. Carapace reddish, dorsal abdomen with oblique lines of dots; rastellum absent .................... 16
-
16. Spermathecae with short copulatory ducts, arising from basal dome side (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 85A); Uruguay and southern Brazil .................... A. tacuariensis
-
16'. Spermathecae with short copulatory ducts, arising from the basal dome apex (Indicatti et al. 2008Indicatti RP, Lucas SM, Ott R, Brescovit AD (2008) Litter dwelling mygalomorph spiders (Araneae: Microstigmatidae, Nemesi idae) from Araucaria forests in Southern Brazil, with the description of five new species. Revista Brasileira de Zoologia 25: 529-546. doi: 10.1590/S0101-81752008000300021
https://doi.org/10.1590/S0101-8175200800... : fig. 19); southern Brazil .................... A. ericae -
17. ITC I present .................... 18
-
17'. ITC I absent .................... 25
-
18. Spermathecae very long, twisted (Fig. 6); fovea T-shaped (Fig. 11); Brazil (Rio de Janeiro) .................... A. minimussp. nov.
-
18'. Spermathecae short, not twisted; fovea normal, procurved .................... 19
-
19. Spermathecae two wide (but flat) plates .................... 20
-
19'. Spermathecae different .................... 21
-
20. Sternum long (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 112C); spermathecae fused, without copulatory duct (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 112B), with shallow medial notch .................... A. mulchen
-
20'. Sternum normal (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 112C) .................... 22
-
21. color uniform blackish; patella III with p0-1-1 spines .................... A. tolhuaca
-
21'. Color brownish, with mottled abdomen; patella III with p0-1-1 or p1-1-1 spines .................... A. brunneus
-
22. Spermathecae strongly sclerotized, with a wide cavity, opening through wide passage .................... 23
-
22'. Spermathecae weakly sclerotized, flat and with no appre ciable cavity; duct opening not conspicuous .................... 24
-
23. Spermathecae finger like, strongly curved (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 106A-C) .................... A. nahuelbuta and A. hualpen
-
23'. Spermathecae wider, shaped like two pockets (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 109D) .................... A. vilches
-
24. Carapace patterned, legs ringed; spermathecae with short copulatory ducts, arising from the external side of basal dome (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 79E) .................... A. subcalpeianus
-
24'. Carapace and legs uniform brown; spermathecae single, undivided (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 92C); central Chile (regions IV, V) .................... A. campanae
-
25. Epigastrium posteriorly produced (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189., fig. 94B); spermathecae long (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: 94A); northern Chile (Region II) .................... A. alegre
-
25'. Epigastrium normal; spermathecae shorter (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 80A-C); southern Chile (Regions VIII-X) and Argentina (Neuquén) .................... A. confusus
Acanthogonatus minimus sp. nov. Figs. 1-30
Types. Male holotype from Brazil, Rio de Janeiro: Nova Iguaçu (Reserva Biológica do Tinguá, 22°34'28.9'S, 43°24'57.6'W, 400 m a.s.l.), 2002, E. Folly Ramos et al. coll. (IBSP 166089). Paratypes with the same data as holotype, female (IBSP 166090); one male (IBSP 166091); female (IBSP 166092); female (MNRJ 6803); female (MZSP 67296); female (MCN 52059).
Diagnosis. Acanthogonatus minimussp. nov. differs from remaining species of the genus by the male palpal bulb with very long and twisted embolus ca. of 2/3 length of palpal tibia (Figs. 1-4, 13) and spermatheca with long and twisted duct (Fig. 6), anterior eyes row recurved and T-shaped fovea (Figs. 7, 11).
Description. Male (holotype). Carapace dark yellow, with light pubescence, legs light yellow (Fig. 7). Abdomen dorsally light yellow with two groups of anterior stains, with brown chevron and ventrally light yellow, with light pubescence (Fig. 7). Total length 4.22. Carapace 1.85 long, 1.45 wide, with very short 0.10 and slightly procurved fovea. Abdomen 1.95 long, 1.10 wide. Thoracic region flat. Clypeus absent. Eye tubercle weakly elevated, wider than long (Fig. 7). Eye group trapezoidal. Anterior and posterior eye rows recurved (Fig. 7). Eye sizes: AME 0.10, ALE 0.14, PME 0.09 and PLE 0.12. Chelicerae with seven teeth in prolateral row, with ca. 20 basal smaller teeth (Figs. 9, 16) and rastellum weak, formed by thin setae (Figs. 8, 9, 16). Intercheliceral tumescence very small, pale yellow, covered by ca. nine thin, short, sparse setae (Figs. 9, 16, 17). Labium 0.14 long, 0.30 wide, with no cuspules (Fig. 8). Maxilla subquadrate with 18 blunt cuspules on internal basal angle (Figs. 8, 19, 20). Serrula poorly developed, with ca. 15 teeth (Fig. 18). Sternum slightly circular 1.04 long, 0.85 wide, slightly domed. Six sternal sigilla small, oval, very shallow, inconspicuous (Fig. 8). Palp: measurements: femur 0.85/patella 0.55/tibia 0.70/cymbium 0.40/total 2.50; spination: femur d0-1-1-1-1-0, tibia r0-0-0-1p-1p. Legs: measurements: I: femur 1.35/patella 0.80/tibia 1.00/metatarsus 0.90/tarsus 0.60/total 4.55; II: 1.05/0.75/0.75/0.70/0.50/3.75; III: 1.05/0.60/1.10/0.95/0.55/4.25; IV: 1.35/0.75/1.05/1.20/0.57/4.92; spination: I: femur d1-1-1-1-0, tibia v1r megaspine (Fig. 5), metatarsus v0-1r-0-2ap; II: femur d1-1-1-1, tibia v0-1-0, metatarsus v0-1r0-2ap; III: femur d1-1-1-2, patella p0-1-1, r0-1-0, tibia d0-0-1-0, v0-1r-0, p0-1-0, r0-1-0, metatarsus d1r-1p-0-2-0, v2-0-1r-1p-0-3ap, p0-1-0-1-0, r0-1-0-0; IV: femur d1-1-1-0, patella r0-1-0, tibia p0-1-0-1-0, rp0-1-0-1-0, metatarsus d2-1p-0-2, v1p-1p-0-3ap, p0-1-0-1-0, r0-1-0-0-0. Slightly thickened femora III (Fig. 7). Metatarsal preening combs: III: 4 VR, 5 VP; IV: 4 VR, 3 VP. Tarsi I-IV not flexible. Scopula on tarsi I-II and 1/4 of metatarsi I-II. Scopula on tarsi I-II undivided, very light (Fig. 24) and symmetric. Scopula on tarsi III-IV absent. STC large with double row of teeth. Teeth on tarsal claws: I-II: 13, 10, 10, 9; III: 5, 5, 4, 5; IV: 6, 4, 5, 6. ITC on all legs (Figs. 5, 24, 25). Trichobothria with rounded, broad, elevated base and 14-16 developed ridges (Figs. 22, 23). Tarsal organ rounded, slightly elevated with two concentric ridges (Fig. 21). Book-lung openings with long narrow slit. Four spinnerets, PLS: basal segment 0.35, median segment 0.45, apical segment domed 0.50 long (Figs. 7, 8, 10). PMS with articulate spigots (Figs. 10, 26) and PLS with sparse and enlarged pumpkiniform spigots along inner edge of spinning field and elsewhere covered with articulate spigots (Figs. 10, 27, 28). Cymbium with thin setae, resembling tarsal scopula setae (Figs. 29, 30). Palpal tibia short, narrow (Fig. 1) and with shallow ventral excavation. Palpal bulb almost piriform (Figs. 1-4) with rounded ridges (Figs. 13-15).
Female (paratype IBSP 112980). Coloration as in male (Figs. 11, 12). Total length 5.40. Carapace 2.35 long, 1.75 wide, with very short 0.22 and slightly procurved fovea. Abdomen 2.50 long, 1.55 wide. Thoracic region as in male. Clypeus absent (Fig. 11). Eye tubercle weakly elevated, wider than long. Eye group trapezoidal. Anterior and posterior eye rows recurved (Fig. 11). Eye sizes: AME 0.10, ALE 0.18, PME 0.10 and PLE 0.15. Chelicerae with nine teeth in prolateral row, with ca. 15 basal smaller teeth and rastellum weak, formed by thin setae. Labium 0.24 long, 0.56 wide, without cuspule. Maxilla subquadrate with 30 cuspules on internal basal angle. Serrula as in male. Sternum slightly oval 1.30 long, 1.10 wide. Six sternal sigilla small, oval, very shallow, inconspicuous (Fig. 12). Palp: measurements: femur 1.00/patella 0.60/tibia 0.70/tarsi 0.70/total 3.00; spination: femur d1-1-1-1-1, tibia v3ap. Legs: measurements: I: femur 1.50/patella 1.10/tibia 1.05/metatarsus 0.85/tarsus 0.60/total 5.10; II: 1.30/0.90/0.80/0.85/0.60/4.45; III: 1.15/0.75/0.70/1.00/0.65/4.25; IV: 1.45/0.90/1.15/1.40/0.70/5.60; spination: I: femur d1-1-1-1, metatarsus v0-1r-0-2ap; II: femur d1-1-1-1-1, metatarsus v0-2-0-3ap; III: femur d1-1-1-1-1, patella p0-0-1-1, r0-1-0, tibia d0-0-1, v0-1r-0-2ap, p0-1-0-1-0, r0-1-0, metatarsus d1r-2-0-0-2, v1r-0-2-0-3ap, p0-1-0-1-0; IV: femur d1-1-1-1, tibia v2ap, p0-1-0-1-0, r0-1-0-1-0 metatarsus d2-0-1r-0-2-0, v20-2-0-3ap, p0-1-0-1-0-0-1, r0-1-0-0. Slightly thickened femora III. Metatarsal preening combs: III: 4 VR, 3 VP; IV: 3 VR, 2 VP. Tarsi I-IV not flexible. Scopula on tarsi I-II undivided, very light and symmetric. Scopula on distal half of metatarsi I. Scopula on tarsi III-IV absent. STC large with double row of teeth. Teeth on tarsal claws: I-II: 10, 10, 10, 10; III: 6, 6, 5, 6; IV: 5, 5, 5, 5. ITC on all legs. Trichobothria and tarsal organ as in male. Book-lung openings as in male. Four spinnerets, PLS: basal segment 0.30, median segment 0.30, apical segment domed 0.55 long. Spigots as in male.
Variation. Males (n = 3): total length 4.22-4.40; carapace 1.85-1.90 long; maxillae with 15-18 cuspules. Females (n = 11): total length 4.00-5.40; carapace 1.80-2.35 long; maxillae with 25-30 cuspules.
Additional material examined. Brazil, Rio de Janeiro: Nova Iguaçu (Reserva Biológica do Tinguá, 22°34'28.9'S; 43°24'57.6'W, 400 m a.s.l.), 2002, E. Folly Ramos et al. coll., 1 female, 5 juveniles (IBSP 166093); male (IBSP utilized in SEM); 5 females (IBSP 166094-166098); 2 females (MNRJ 6804).
Distribution. Known only from the type locality.
Etymology. The specific name is a Latin adjective (= the smallest) referring to the small size of this species.
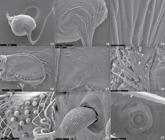
Acanthogonatus minimussp. nov.: (1-5) male: (2-5) holotype: (1-4) left palp: (1) maxilla, tibia and palpal bulb, prolateral view; (2) prolateral view; (3) ventral view; (4) retrolateral view; (5) leg I, retrolateral view; (6) spermathecae paratype (IBSP 112980), dorsal view. Scale bars: 1 = 1 mm; 2-6 = 0,5 mm.
Acanthogonatus minimussp. nov., male: (7, 8, 10) holotype: (7) body, dorsal view; (8) body, ventral view; (9) left chelicera, arrow shows intercheliceral tumescence, prolateral view; (10) spinnerets, ventral view; (11-12) female paratype (IBSP 112980): (11) body, dorsal view; (12) body, ventral view. Scale bars: 7-8, 11-12 = 1.00 mm; 9 = 0.25 mm, 10 = 0.50 mm.
Acanthogonatus minimussp. nov., male: (13-15) left palpal bulb, retro-ventral view: (14-15) detail of rounded ridges; (16-17) left chelicera, prolateral view: (17) detail of intercheliceral tumescence; (18) serrula on maxilla, dorsal view; (19-20) cuspules on maxilla: (20) detail of a cuspule, lateral view; (21) tarsal organ, leg IV, dorsal view.
Acanthogonatus minimussp. nov., male: (22-30) left tarsi I: (22, 23) trichobothria; (22) dorso-retrolateral view; (23) retrolateral view; (24-25) tarsi I: (24) retrolateral view; (25) detail of tarsal claws and scopula; (26) spigots in the apex of right posterior median spinneret, ventral view; (27, 28) right posterior lateral spinneret, ventral view: (28) pumpkiniform spigot in the inner edge of apical article; (29, 30) cymbial left palp, prolateral view; (30) setae on the cymbium, prolateral view.
Diplothelopsinae Schiapelli & Gerschman, 1967
Chaco Tullgren, 1905
Identification key for Chaco updated from Goloboff (1995)Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189. and Ferretti (2014)Ferretti N (2014) Chaco ansilta new species from Mendoza province, Western Argentina (Araneae: Nemesiidae). Anais da Academia Brasileira de Ciências 2014: 1-12. doi: 10.1095/0001-3765201420140226
https://doi.org/10.1095/0001-37652014201...
Males
Male of C. patagonica Goloboff, 1995 and C. tecka Goloboff, 1995 are unknown.
-
1. ITC IV present; Brazil (Rio de Janeiro) .................... C. tinguasp. nov.
-
1'. ITC IV absent .................... 2
-
2. Tibial apophysis with five or more spines .................... 3
-
2'. Tibial apophysis with less than five spines .................... 5
-
3. Embolus extremely long, bulb in lateral view, abruptly constricted to form embolus (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 146D); northern Argentina (Salta and Jujuy) .................... C. obscura Tullgren, 1905
-
3'. Embolus shorter, bulb more gradually tappering .................... 4
-
4. Embolus as about half longer of total bulb length having short keels on base (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 148E); northern Argentina (Tucumán and Catamarca) .................... C. tucumana Goloboff, 1995
-
4'. Embolus as about one third or less longer of total bulb length having longer keels on base (Montes de Oca & Pérez-Miles 2013Montes de Oca L, Pérez-Miles F (2013) Two new species of Chaco Tullgren from the Atlantic coast of Uruguay (Araneae, Mygalomorphae, Nemesiidae). ZooKeys 337: 73-87. doi: 10.3897/zookeys.337.5779
https://doi.org/10.3897/zookeys.337.5779... : fig. 4C); Uruguay (atlantic coast) .................... C. costai Montes de Oca & Pérez-Miles, 2013 -
5. Tibial apophysis with four spines .................... 6
-
5'. Tibial apophysis with less than four spines .................... 8
-
6. Palpal bulb with a strongly sinuous spermatic duct (Montes de Oca & Pérez-Miles 2013Montes de Oca L, Pérez-Miles F (2013) Two new species of Chaco Tullgren from the Atlantic coast of Uruguay (Araneae, Mygalomorphae, Nemesiidae). ZooKeys 337: 73-87. doi: 10.3897/zookeys.337.5779
https://doi.org/10.3897/zookeys.337.5779... : fig. 3A); Uruguay (atlantic coast) .................... C. castanea Montes de Oca & Pérez-Miles, 2013 -
6'. Palpal bulb with straighter spermatic duct; Chile .................... 7
-
7. Maxillary cuspules less than 20 (usually 13). Construct beveled doors (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 142, 143), grayish coloration; Chile (Region IV) .................... C. socos Goloboff, 1995
-
7'. Maxillary cuspules more than 20 (usually 33). Construct thin trap-doors (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: figs. 151, 152), brownish coloration; Chile (Region V) .................... C. tigre Goloboff, 1995
-
8. Tibial apophysis with three spines; bulb gradually tapering and embolus bent (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 154D, E); northwestern Argentina (San Juan) .................... C. sanjuanina Goloboff, 1995
-
8'. Tibial apophysis with two spines; bulb more abruptly tapering and embolus straight (Ferretti 2014Ferretti N (2014) Chaco ansilta new species from Mendoza province, Western Argentina (Araneae: Nemesiidae). Anais da Academia Brasileira de Ciências 2014: 1-12. doi: 10.1095/0001-3765201420140226
https://doi.org/10.1095/0001-37652014201... : fig. 2B); central western Argentina (Mendoza) .................... C. ansilta Ferretti, 2014
Females
-
1. ITC IV present; Brazil (Rio de Janeiro) .................... C. tinguasp. nov.
-
1'. ITC IV absent) .................... 2
-
2. Spermathecae a single undivided tube (sometimes spiraled); northwestern Argentina and Uruguay .................... 3
-
2'. Spermathecae a single tube with a basal protuberance .................... 6
-
3. Spermathecae very long (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 147E, F); northern Argentina (Salta and Jujuy) .................... C. obscura
-
3'. Spermathecae short .................... 4
-
4. Spermathecae with reniform fundus (Montes de Oca & Pérez-Miles 2013Montes de Oca L, Pérez-Miles F (2013) Two new species of Chaco Tullgren from the Atlantic coast of Uruguay (Araneae, Mygalomorphae, Nemesiidae). ZooKeys 337: 73-87. doi: 10.3897/zookeys.337.5779
https://doi.org/10.3897/zookeys.337.5779... : fig. 3E); Uruguay (atlantic coast) .................... C. castanea -
4'. Spermathecae with subspherical fundus .................... 5
-
5. Spermathecae with a sinuous neck (Montes de Oca & Pérez-Miles 2013Montes de Oca L, Pérez-Miles F (2013) Two new species of Chaco Tullgren from the Atlantic coast of Uruguay (Araneae, Mygalomorphae, Nemesiidae). ZooKeys 337: 73-87. doi: 10.3897/zookeys.337.5779
https://doi.org/10.3897/zookeys.337.5779... : fig. 4E); Uruguay (atlantic coast) .................... C. costai -
5'. Spermathecae with a straight neck (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 148A, B); northern Argentina (Tucumán and Catamarca) .................... C. tucumana
-
6. Pseudo preening combs present; metatarsus IV with nume rous strong spines on superioanterior face; southern Argen tina (Chubut) .................... C. tecka
-
6'. No pseudo preening combs; metatarsus IV with few spines on superoanterior face .................... 7
-
7. Sternal sigilla almost inconspicuous; color yellowish light; Argentina .................... 8
-
7'. Sternal sigilla normal, maxilla with medium number of cuspules (15-50), color brownish or ash gray; Chile .................... 10
-
8. Maxillae with few cuspules (6-10), labium with few cuspules irregularly arranged, very light pubsescence .................... 9
-
8'. Maxillae with medium number of cuspules (about 12); labium with 8 cuspules in transverse line; northern Argen tina (San Juan) .................... C. sanjuanina
-
9. Femora normal, spermathecae with sinuous neck and sub spherical fundus (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 156C); southern Argentina (Chubut) .................... C. patagonica
-
9'. Femora incrassate, spermathecae with straight and very long neck and fundus not well differentiate (Ferretti 2014Ferretti N (2014) Chaco ansilta new species from Mendoza province, Western Argentina (Araneae: Nemesiidae). Anais da Academia Brasileira de Ciências 2014: 1-12. doi: 10.1095/0001-3765201420140226
https://doi.org/10.1095/0001-37652014201... : fig. 2D); central western Argentina (Mendoza) .................... C. ansilta -
10. Spermathecae with very short and thin neck and well developed subspherical fundus (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 150B), construct thin trap-doors, brownish coloration; Chile (Region V) .................... C. tigre
-
10'. Spermathecae with long neck and less developed fundus (Goloboff 1995Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.: fig. 153B), construct beveled doors, grayish coloration; Chile (Region IV) .................... C. socos
Chaco tingua sp. nov. Figs. 31-62
Types. Male holotype from Brazil, Rio de Janeiro: Nova Iguaçu (Reserva Biológica do Tinguá, 22°34'28.9'S; 43°24'57.6'W, 400 m a.s.l.), 2002, E. Folly Ramos et al. leg. (IBSP 166099). Paratypes with the same data as holotype, female (IBSP 166100); female (IBSP 166101); male (IBSP 166102); male and female (MNRJ 6805); male and female (MZSP 67297); male and female (MCN 52060).
Diagnosis. Chaco tinguasp. nov. differs from remaining species of the genus by the retrolateral megaspine on tibia I (Figs. 35, 53), palpal embolus tip hook-shaped (Fig. 34), ITC on all legs, absence of pubescence on the carapace and legs (Figs. 38, 40).
Description. Male (holotype). Carapace brown, legs brownish yellow, with no pubescence (Fig. 38). Abdomen dorsally brown with one anterior stain and five median-posterior yellow bands and ventrally yellow, with light pubescence (Fig. 38). Total length 4.20. Carapace 2.00 long, 1.60 wide, with short 0.11 and procurved fovea (Fig. 38). Abdomen 1.90 long, 1.20 wide. Thoracic region slightly raised. Clypeus absent. Eye tubercle weakly elevated, wider than long (Fig. 38). Eye group trapezoidal. Anterior and posterior eye rows recurved (Fig. 38). Eye sizes: AME 0.16, ALE 0.16, PME 0.11 and PLE 0.10. Chelicerae with five teeth in prolateral row (Figs. 36, 49), with ca. 12 basal smaller teeth and strong rastellum, formed by four very thick short setae, without on raised mound (Figs. 36, 39, 49). Intercheliceral tumescence small, pale yellow, covered by ca. 10 thin, long, sparse setae (Figs. 36, 49, 50). Labium 0.08 long, 0.16 wide, with no cuspules (Fig. 39). Maxilla subquadrate with ca. 20 pointed and elongated cuspules on internal basal angle. Serrula very poorly developed, with ca. five teeth (Fig. 48). Sternum circular 0.48 long, 0.42 wide, domed. Six sternal sigilla small, oval, very shallow, inconspicuous (Fig. 39). Palp: measurements: femur 0.85/patella 0.45/tibia 0.55/cymbium 0.30/total 2.15; spination: femur d0-0-0-0-1-1, tibia r0-0-0-1p-0. Legs: measurements: I: femur 1.55/patella 0.90/tibia 1.15/metatarsus 0.90/tarsus 0.65/total 5.15; II: 1.35/0.85/0.90/1.00/0.65/4.75; III: 1.30/0.75/0.90/1.10/0.50/4.55; IV: 1.80/0.75/1.50/1.75/0.90/6.70; spination: I: femur d0-1-1-1, patella v1ap, tibia v1r-0-2-0-2-(1r megaspine, Figs. 35, 53), metatarsus v2ap; II: femur d0-1-1-1, patella 0, tibia v0-1r-0-2ap, p0-1-0-1-0, metatarsus v2ap; III: femur d0-1p-2-1r, patella p0-1-1-1, tibia d0-1-0, v2ap, p1-1-0, metatarsus d1r-1p-1r-1p-0-2, v3ap, p0-1-0-0; IV: femur d0-1-1-1-0, patella 0, tibia v3ap, p1-0-1-0, r0-1-0-1, metatarsus d1r-1r-0-1p-0-0-2, v0-0-1r-0-3ap, r1-0-1-0-0-0. Thickened femora III (Fig. 38). Group of ca. 10 thickened setae on apical prolatero-dorsal femur IV. Metatarsal preening combs absent. Tarsi I-IV not flexible (Figs. 35, 38-41, 51, 54). Scopula on tarsi I-II and 2/3 of metatarsi I-II (Figs. 35, 51). Scopula on tarsi I-II undivided, very light and symmetric. Scopula on tarsi III-IV absent (Figs. 38, 54). STC large with double row of teeth (Fig. 52). Teeth on tarsal claws: I and II: 7, 4, 4, 6; III: 6, 4, 4, 6; IV: 6, 4, 5, 6. ITC on all legs (Figs. 52, 55). Trichobothria with oval, broad, elevated base and 10-13 developed ridges (Figs. 51, 55, 57). Tarsal organ oval, slightly elevated with two concentric ridges (Fig. 56). Book-lung openings with long narrow slit. Four spinnerets, PLS: basal segment 0.10, median segment 0.11, apical segment domed 0.05 long (Figs. 39, 58). PMS with 3-4 articulate spigots only on apex. PLS covered with sparse articulate spigots, one enlarged on apex of medial article (Fig. 58). Cymbium with thin setae, resembling tarsal scopula setae (Figs. 45, 59). Palpal tibia short, wide (Fig. 31) and with lightly deep ventral excavation (Figs. 31, 45). Palpal tibia excavation and basal region of tegulum with grooves, evidencing a possible stridulatory apparatus (Fig. 45). Palpal bulb piriform with six keels and short and curved embolus (Figs. 32-34, 45-47).
Female (paratype IBSP 166100). Coloration as in male (Figs. 40-41). Total length 5.80. Carapace 2.50 long, 2.00 wide, with short 0.20 and procurved fovea (Fig. 40). Abdomen 2.65 long, 1.90 wide. Thoracic region as in male. Clypeus absent. Eye tubercle weakly elevated, wider than long (Fig. 40). Eye group trapezoidal. Anterior and posterior eye rows recurved (Fig. 40). Eye sizes: AME 0.11, ALE 0.16, PME 0.10 and PLE 0.11. Chelicerae with seven teeth in prolateral row, with ca. 12 basal smaller teeth and strong rastellum formed by five very thick short setae, without on raised mound (Figs. 41, 42, 60). Labium 0.5 long, 0.6 wide, without cuspule. Maxilla with 28 blunt cuspules on internal basal angle (Figs. 41, 42, 61). Serrula absent. Sternum slightly oval 1.5 long, 1.25 wide. Six sternal sigilla small, oval, very shallow, inconspicuous (Fig. 41). Palp: measurements: femur 1.00/patella 0.62/tibia 0.65/tarsi 0.6/total 2.87; spination: tibia v3ap. Legs: measurements: I: femur 1.25/patella 0.85/tibia 0.95/metatarsus 0.70/tarsus 0.55/total 4.3; II: 1.25/0.80/0.75/0.65/0.55/4.00; III: 1.25/0.80/0.80/0.80/0.6/4.25; IV: 1.75/1.00/1.25/1.40/0.80/6.2; spination: I: metatarsus v2ap; II: femur metatarsus v0-1-0-2ap; III: patella p0-1-1-1, tibia d0-1-0, p0-1-0-0, metatarsus d1r-2-1p-0-2, v3ap, p0-1-0-0; IV: tibia p0-1-0, metatarsus d1r-1p-0-2, v4ap, p0-1-0-0. Thickened femora III (Fig. 40). Group of ca. 10 thickened setae on apical prolatero-dorsal femur IV (Figs. 40, 43). Metatarsal preening combs absent. Tarsi I-IV not flexible (Fig. 41). Scopula on tarsi I-II undivided, very light and symmetric. Scopula on distal half of metatarsi I. Scopula on tarsi II-IV absent. STC large with double row of teeth (Fig. 62). Teeth on tarsal claws: I: 9, 5, 5, 9; II: 6, 5, 5, 7; III: 6, 4, 4, 6; IV: 5, 3, 4, 5. ITC on all legs (Fig. 62). Trichobothria and tarsal organ as in male. Book-lung openings as in male. Four spinnerets, PLS: basal segment 0.20, median segment 0.16, apical segment domed 0.04 long (Figs. 41, 44). Spigots as in male. Spermathe ca with a single receptacula arising from prolateral border of the long basal dome (Fig. 37).
Variation. Males (n = 10): total length 3.85-4.20; carapace 1.60-2.00 long; maxillae with 18-20 cuspules. Females (n = 20): total length 4.50-5.80; carapace 1.00-2.50 long; maxillae with 22-28 cuspules.
Additional material examined. Brazil, Rio de Janeiro: Nova Iguaçu (Reserva Biológica do Tinguá, 22°34'28.9'S; 43°24'57.6'W, 400 m), 2002, E. Folly-Ramos et al. coll., 5 males (IBSP 166103-166107); 54 females, 7 juveniles (IBSP 166108); 6 females, pitfall traps (IBSP 166109-166112); 1 male and 4 females (MNRJ 6806); 2 females, pitfall traps (MZSP 67298); 2 females, pitfall traps (MCN 52061).
Distribution. Known only from the type locality.
Natural history. Two females were observed in captivity. They built a horizontal silk-lined burrow on surface of soil with depth of ca. 12 mm and closed by a thin trap-door.
Etymology. The specific name is a noun in apposition taken from the type locality.
Chaco tinguasp. nov.: (31-36) male: (31-35) holotype: (31-34) left palp: (31) palpal tibia and cymbium, prolateral view; (32) prolateral view; (33) ventral view; (34) retrolateral view; (35) leg I, retrolateral view; (36) right chelicera, arrow shows intercheliceral tumescence, prolateral view; (37) spermathecae paratype (IBSP. 03 out 2002), dorsal view. Scale bars: 31-36 = 0.5 mm; 36 = 0.25 mm.
Chaco tinguasp. nov.: (38-39) male holotype: (38) body, dorsal view; (39) body, ventral view; (40-44) female paratype (IBSP. 03 out 2002): (40) body, dorsal view; (41) body, ventral view; (42) chelicerae and maxillae, ventral view; (43) legs III-IV, arrow shows the group of thickened setae on femur IV; (44) spinnerets, ventral view. Scale bars: 40, 41, 43 = 1 mm; 42, 44 = 0.5 mm.
Chaco tinguasp. nov., male: (45-47) left palpal bulb, ventral view: (46) keels; (47) keels detail; (48) serrula on maxilla, arrows shows teeth, dorsal view; (49, 50) right chelicera, prolateral view: (50) detail of intercheliceral tumescence; (51-53) left leg I, retrolateral view: (51) tarsi I; (52) detail of claws and scopula; (53) detail of megaspine.
Chaco tinguasp. nov.: (54-59) male: (54-57) left tarsi IV: (54-55) prolateral view; (56) tarsal organ, dorsal view; (57) trichobothria, dorso-prolateral view; (58) articulate spigots on apical article of posterior lateral spinnerets; (59) left palp, cymbial setae, prolateral view; (60-62) female: (60) left chelicerae, rastellum, ventral view; (61) cuspules on maxillae, ventral view; (62) claws of tarsi IV, retrolateral view.
Ecological data
A total of 1,613 spiders (1,163 Araneomorphae and 450 Mygalomorphae) were collected in leaf litter with Winkler extractor over the course of 12 months, during four field expeditions at the Reserva Biológica do Tinguá. Of these, 436 spiders (15 males, 84 females, 351 juveniles) belong to two new Nemesiidae species, Acanthogonatus minimussp. nov. and Chaco tinguasp. nov.
Were recorded 22 individuals of A. minimussp. nov., three males (registered in summer), 11 females (seven in the spring collections) and eight juveniles. Since we only collected a few specimens of A. minimussp. nov. it is not possible to draw conclusions on the fenology of the species, but that is sufficient data to compare it with what we know about other species of the genus. We observed that male and female specimens of Acanthogonatus ericae Indicatti et al., 2008 were collected with pitfall traps in the winter and the spring of southern Brazil (Indicatti et al. 2008Indicatti RP, Lucas SM, Ott R, Brescovit AD (2008) Litter dwelling mygalomorph spiders (Araneae: Microstigmatidae, Nemesi idae) from Araucaria forests in Southern Brazil, with the description of five new species. Revista Brasileira de Zoologia 25: 529-546. doi: 10.1590/S0101-81752008000300021
https://doi.org/10.1590/S0101-8175200800...
). In another study using pitfall traps (Ferretti et al. 2012Ferretti N, Pompozzi G, Copperi S, Pérez-Miles F, Gonzalez A (2012) Mygalomorph spider community of a natural reserve in a hilly system in central Argentina. Journal of Insect Science 12(31): 1-16. doi: 10.1673/031.012.3101
https://doi.org/10.1673/031.012.3101...
), males of Acanthogonatus centralis Goloboff, 1995 were recorded when temperatures were medium or low (autumn, end of winter and spring), while females and juveniles were collected when temperatures were high or low (summer and winter).
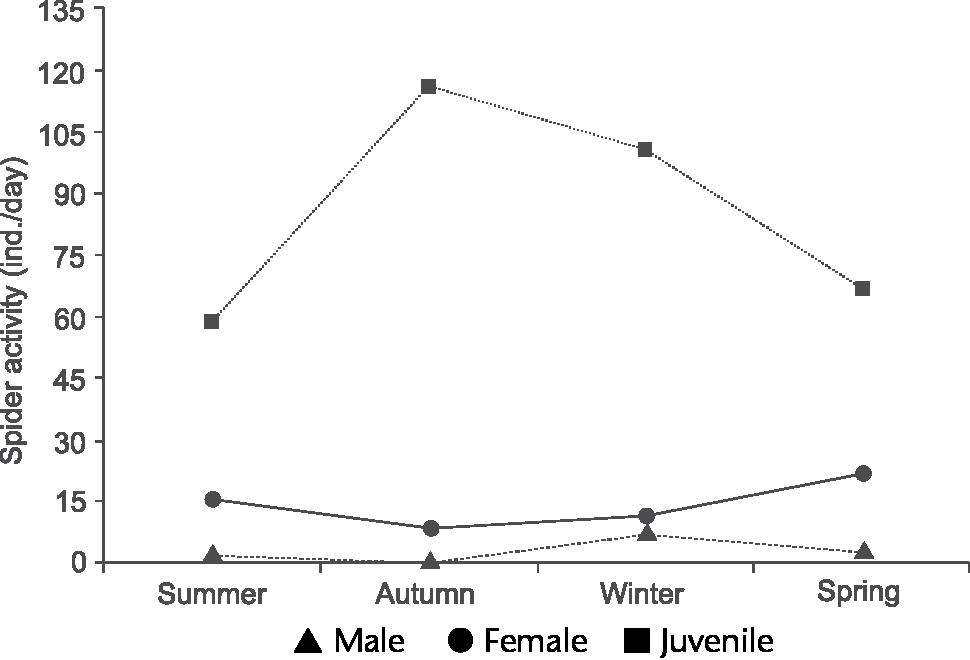
Contrasting with A. minimus, C. tinguasp. nov. was abundant in the samples, totaling 12 adult males (2.89%), 59 females (14.2%) and 343 juveniles (83.5%). The phenology of C. tinguasp. nov. is illustrated in Fig. 63, which shows that the main activity period of males was during the winter (seven individuals), decreasing in the spring (three individuals). These values are statistically significant when the seasons are compared (KW = 9.173, GL = 3, p = 0.0270). However, the data obtained on male abundance are are not conclusive since the sample size is small. During the winter the lowest mean temperature and precipitation were recorded during the sampling period (Fig. 64), showing that the winter is probably the mating period of C. tinguasp. nov. If the same parameter is observed among females, an increase in the abundance curve is detected, but without clear activity peaks. A slight increase in activity was observed in the spring, when 22 females were collected, and during the summer, when 16 specimens were collected; these differences, however, are not statistically significance. Our dataset does not show clear activity peaks for females of C. tinguasp. nov., but there was a small and yet noticeable increase in activity in the spring and summer, when temperatures and precipitation were higher (Fig. 64).
Chaco tinguasp. nov. Phenology based on specimen activity (individuals/day) in Reserva Biológica do Tinguá, Rio de Janeiro using Winkler extractor.
During field captures in the autumn we observed a lack of males and a smaller number of females (N = 6) of C. tinguasp. nov. Juvenile specimens were numerous in samples from the four seasons, with significance values (KW = 10.902, GL = 3, p = 0.0122). We suggest that the autumn is the growing period, because newly born Nemesiidae spiders were found forming assemblages in the litter, making their detection easier using the Winkler extractor.
Another observation we have made while looking at the samples obtained with the Winkler extractor is the massive presence of females, five times greater than the number of males. This pattern could be explained by the selectivity of the Winkler trap compared with pitfall traps. The first favors the capture of spiders at an area of a square meter, whereas soil traps such as pitfall traps capture wandering spiders (Costa & Pérez-Miles 2002Costa FG, Pérez-Miles F (2002) Reproductive biology of Uruguayan theraphosids (Araneae, Theraphosidae). Journal of Arach nology 30: 571-587. doi: 10.1636/0161-8202(2002)030[0571:RBOUTA]2.0.CO;2
https://doi.org/10.1636/0161-8202(2002)0...
, Candiani et al. 2005Candiani DF, Indicatti RP, Brescovit AD (2005) Composição e diversidade da araneofauna (Araneae) de serapilheira em três florestas urbanas na cidade de São Paulo, São Paulo, Brasil. São Paulo, Brasil. Biota Neotropica 5(1a): 1-13. doi: 10.1590/S1676-06032005000200010
https://doi.org/10.1590/S1676-0603200500...
, Indicatti et al. 2005Indicatti RP, Candiani DF, Brescovit AD, Japyassú HF (2005) Diversidade de aranhas (Arachnida, Araneae) de solo na bacia do Reservatório do Guarapiranga, São Paulo, São Paulo, Brasil. Biota Neotropica 5(1a): 1-12. doi: 10.1590/S1676-06032005000200013
https://doi.org/10.1590/S1676-0603200500...
). In addition it is known that female mygalomorphs are more sedentary, whereas males wander a whole lot more (Costa & Pérez-Miles 2002Costa FG, Pérez-Miles F (2002) Reproductive biology of Uruguayan theraphosids (Araneae, Theraphosidae). Journal of Arach nology 30: 571-587. doi: 10.1636/0161-8202(2002)030[0571:RBOUTA]2.0.CO;2
https://doi.org/10.1636/0161-8202(2002)0...
, Gonzalez-Filho et al. 2012Gonzalez-Filho HMO, Lucas SM, Paula FS, Indicatti RP, Brescovit AD (2012) On the taxonomy of Acanthoscurria Ausserer from Southeastern Brazil with data on the natural history of A. gomesiana Mello-Leitão (Araneae, Mygalomorphae, Theraphosidae). International Journal of Zoology 2012: 1-11., Ferretti et al. 2012Ferretti N, Pompozzi G, Copperi S, Pérez-Miles F, Gonzalez A (2012) Mygalomorph spider community of a natural reserve in a hilly system in central Argentina. Journal of Insect Science 12(31): 1-16. doi: 10.1673/031.012.3101
https://doi.org/10.1673/031.012.3101...
, Paula et al. 2014Paula FS, Gabriel R, Indicatti RP, Brescovit AD, Lucas SM (2014) On the Brazilian Amazonian species of Acanthoscurria (Araneae, Theraphosidae). Zoologia 31(1): 63-80. doi: 10.1590/S1984-46702014000100008
https://doi.org/10.1590/S1984-4670201400...
).
ACKNOWLEDGMENTS
We wish to thank Alexander Bernhart for correcting the English in an earlier version of this manuscript; Ronaldo B. Francini Filho for the suggestions on the statistical analyses; three anonymous referees and the editor for their critical comments, which helped us to improve this manuscript; Instituto Chico Mendes de Conservação da Biodiversidade for the permits and the support with field work; Beatriz Maurício for her help with SEM images. This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (grants 2012/18287-1 to RPI; 2011/50689-0 to SML and ADB) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (grants 2009/12017-0 to ADB and 479377/2012-0 to RPI).
- Bertkau P (1880) Verzeichniss der von Prof. Ed. van Beneden auf seiner im Auftrage der Belgischen Regierung unternommen wissenschaftlichen Reise nach Brasilien und La Plata im Jahren 1872-73 gensammelten Arachniden. Mémoires Couronnes de la Academie Royale des Sciences, Lettres et Beaux-Arts de Belgique 43: 1-120.
- Candiani DF, Indicatti RP, Brescovit AD (2005) Composição e diversidade da araneofauna (Araneae) de serapilheira em três florestas urbanas na cidade de São Paulo, São Paulo, Brasil. São Paulo, Brasil. Biota Neotropica 5(1a): 1-13. doi: 10.1590/S1676-06032005000200010
» https://doi.org/10.1590/S1676-06032005000200010 - Costa FG, Pérez-Miles F (2002) Reproductive biology of Uruguayan theraphosids (Araneae, Theraphosidae). Journal of Arach nology 30: 571-587. doi: 10.1636/0161-8202(2002)030[0571:RBOUTA]2.0.CO;2
» https://doi.org/10.1636/0161-8202(2002)030[0571:RBOUTA]2.0.CO;2 - Delabie JHC, Agosti D, Nascimejnto IC (2000) Litter ant communities of the Brazilian Atlantic rain forest region, p. 1-17. In: Agosti D, Majer JD, Alonso LT, Schultz TR (Eds). Sampling ground-dwelling ants: case studies from the world's rain forests. Perth, Curtin University School of Environmental Biology, vol. 18.
- Ferretti N (2014) Chaco ansilta new species from Mendoza province, Western Argentina (Araneae: Nemesiidae). Anais da Academia Brasileira de Ciências 2014: 1-12. doi: 10.1095/0001-3765201420140226
» https://doi.org/10.1095/0001-3765201420140226 - Ferretti N, Pompozzi G, Copperi S, Pérez-Miles F, Gonzalez A (2012) Mygalomorph spider community of a natural reserve in a hilly system in central Argentina. Journal of Insect Science 12(31): 1-16. doi: 10.1673/031.012.3101
» https://doi.org/10.1673/031.012.3101 - Goloboff PA (1995) A revision of the South American spiders of the family Nemesiidae (Araneae, Mygalomorphae). Part I: species from Peru, Chile, Argentina, and Uruguay. Bulletin of the American Museum of Natural History 224: 1-189.
- Gonzalez-Filho HMO, Lucas SM, Paula FS, Indicatti RP, Brescovit AD (2012) On the taxonomy of Acanthoscurria Ausserer from Southeastern Brazil with data on the natural history of A. gomesiana Mello-Leitão (Araneae, Mygalomorphae, Theraphosidae). International Journal of Zoology 2012: 1-11.
- IBAMA (2006) Plano de Manejo da Reserva Biologica do Tinguá. Brasília, Ministério do Meio Ambiente, 102p. Available online at: http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/rebio_tingua.pdf. [Accessed: 27/VI/2014].
» http://www.icmbio.gov.br/portal/images/stories/imgs-unidades-coservacao/rebio_tingua.pdf - Indicatti RP, Candiani DF, Brescovit AD, Japyassú HF (2005) Diversidade de aranhas (Arachnida, Araneae) de solo na bacia do Reservatório do Guarapiranga, São Paulo, São Paulo, Brasil. Biota Neotropica 5(1a): 1-12. doi: 10.1590/S1676-06032005000200013
» https://doi.org/10.1590/S1676-06032005000200013 - Indicatti RP, Lucas SM (2005) Description of a new genus of Nemesiidae (Araneae, Mygalomorphae) from the Brazilian Cerrado. Zootaxa 1088: 11-16.
- Indicatti RP, Lucas SM, Ott R, Brescovit AD (2008) Litter dwelling mygalomorph spiders (Araneae: Microstigmatidae, Nemesi idae) from Araucaria forests in Southern Brazil, with the description of five new species. Revista Brasileira de Zoologia 25: 529-546. doi: 10.1590/S0101-81752008000300021
» https://doi.org/10.1590/S0101-81752008000300021 - Lucas SM, Indicatti RP (2006) On the genus Psalistopoides Mello-Leitão (Araneae, Mygalomorphae, Nemesiidae). Revista Brasileira de Zoologia 23(2): 547-549. doi: 10.1590/S0101-81752006000200030
» https://doi.org/10.1590/S0101-81752006000200030 - Lucas SM, Indicatti RP (2010) Description of two new species of Lycinus (Araneae: Nemesiidae). Zoologia 27(3): 425-430. doi: 10.1590/51984-46702010000300015
» https://doi.org/10.1590/51984-46702010000300015 - Lucas SM, Indicatti RP, Fukami CY (2005) Redescrição de Prorachias bristowei Mello-Leitão 1924 (Araneae, Mygalomorphae, Nemesiidae). Biota Neotropica 5(1a): 1-6. doi: 10.1590/S1676-06032005000200019
» https://doi.org/10.1590/S1676-06032005000200019 - Lucas SM, Passanha V, Janini CRV, Indicatti RP (2008) On the genus Neostothis Vellard (Araneae, Nemesiidae). Jounal of Arachnology 36: 472-475. doi: 10.1636/CA07-107.1
» https://doi.org/10.1636/CA07-107.1 - Montes de Oca L, Pérez-Miles F (2013) Two new species of Chaco Tullgren from the Atlantic coast of Uruguay (Araneae, Mygalomorphae, Nemesiidae). ZooKeys 337: 73-87. doi: 10.3897/zookeys.337.5779
» https://doi.org/10.3897/zookeys.337.5779 - Orsolon-Souza G, Esbérard CEL, Mayhé-Nunes AJ, Vargas AB, Veiga-Ferreira S, Folly-Ramos E (2011) Comparison between Winkler's extractor and pitfall traps to estimate leaf litter ants richness (Formicidae) at a rainforest site in southest Brazil. Brazilian Journal of Biology 71(4): 873-880. doi: http://dx.doi.org/10.1590/S1519-69842011000500008
» https://doi.org/10.1590/S1519-69842011000500008 - Passanha V, Indicatti RP, Brescovit AD, Lucas SM (2014) Revision of the spider genus Pycnothele Chamberlin, 1917 (Araneae, Nemesiidae). Iheringia 104(2): 228-251. doi: 10.1590/1678-476620141042228251
» https://doi.org/10.1590/1678-476620141042228251 - Paula FS, Gabriel R, Indicatti RP, Brescovit AD, Lucas SM (2014) On the Brazilian Amazonian species of Acanthoscurria (Araneae, Theraphosidae). Zoologia 31(1): 63-80. doi: 10.1590/S1984-46702014000100008
» https://doi.org/10.1590/S1984-46702014000100008 - Pérez-Miles F, Capocasale RM (1982) Arañas del Uruguay, IV. Hallazgo de una tercera especie del genero Pycnothelopsis: Pycnothelopsis tacuariensis sp. nov. (Araneae, Pycnothelidae). Comunicaciones Zoologicas del Museo de Historia Natural de Montevideo 147: 1-7.
- Pérez-Miles F, Costa FG, Oca LM (2014) Bayana labordai, new genus and species of Nemesiidae (Araneae: Mygalomorphae) from Northern Uruguay and Southern Brazil. Journal of Natural History 48: 1937-1946. doi: 10.1080/00222933.2014.908970
» https://doi.org/10.1080/00222933.2014.908970 - Petrunkevitch A (1925) Arachnida from Panamá. Transactions of the Connecticut Academy of Arts and Sciences 27: 51-248.
- Platnick NI (2014) The world spider catalog. The American Museum of Natural History, version 15.0, available online at: http://research.amnh.org/iz/spiders/catalog [Accessed: 27/IX/2014] doi: 10.5531/db.iz.0001
» https://doi.org/10.5531/db.iz.0001» http://research.amnh.org/iz/spiders/catalog - Raven RJ (1985) The spider infraorder Mygalomorphae (Araneae): Cladistics and systematics. Bulletin of The American Museum of Natural History 182: 1-180.
- Raven RJ (1987) A new mygalomorph spider genus from Mexico (Nemesiinae, Nemesiidae, Arachnida). Jounal of Arachnology 14: 357-362.
- Tullgren A (1905) Aranedia from the Swedish expedition through the Gran Chaco and the Cordilleras. Arkiv for Zoologi 2(19): 1-81.
- Zar JH (1999) Biostatistical analysis. Upper Saddle River, Prentice-Hall, 4th, 912p.
-
Errata
Page 127, caption of the Figures 1-6, line 2:Where read: (2) prolateral view; (3) ventral view; (4) retrolateral view;Should read: (2) retrolateral view; (3) ventral view; (4) prolateral view;Page 132, caption of the Figures 31-37, line 2:Where read: (33) ventral view; (34) retrolateral view;Should read: (33) retrolateral view; (34) ventral view;
Publication Dates
-
Publication in this collection
Mar-Apr 2015
History
-
Received
10 Sept 2014 -
Reviewed
31 Jan 2015 -
Accepted
05 Feb 2015