Abstracts
The species of Hisonotus from the laguna dos Patos system are reviewed. Two species with wide distributions are redescribed: Hisonotus laevior and H. nigricauda. Six new species are described from that system: H. notopagos from the rio Camaquã drainage; H. carreiro and H. prata, endemic from the headwaters of rio Taquari drainage; H. vireo, widely distributed in the rio Jacuí basin; and H. brunneus and H. heterogaster restricted to tributaries of the rio Jacuí drainage. Hisonotus leptochilus is here considered a junior synonym of H. laevior. Hisonotus armatus, H. charrua, H. leucofrenatus, and H. taimensis are also present in the region and in total there are twelve species of Hisonotus in the laguna dos Patos system. Their distributions are discussed, species are illustrated and a taxonomic key is provided.
Cascudinhos; Endemism; Geographic variation; Neotropical
As espécies de Hisonotus do sistema da laguna dos Patos são revisadas. Duas espécies com amplas distribuições são redescritas: Hisonotus laevior e H. nigricauda. Seis espécies novas são descritas deste sistema: Hisonotus notopagos, da drenagem do rio Camaquã; H. prata e H. carreiro, endêmicas das cabeceiras do rio Taquari; H. vireo, amplamente distribuída na bacia do rio Jacuí; H. brunneus e H. heterogaster dos tributários da bacia do rio Jacuí. Hisonotus leptochilus é considerado sinônimo júnior de H. laevior. Hisonotus armatus, H. charrua, H. leucofrenatus e H. taimensis também são encontradas na região, e ao total doze espécies de Hisonotus estão presentes no sistema da laguna dos Patos sendo suas distribuições discutidas aqui. As espécies são ilustradas e uma chave taxonômica é fornecida.
Taxonomic review of Hisonotus Eigenmann & Eigenmann (Siluriformes: Loricariidae: Hypoptopomatinae) from the laguna dos Patos system, southern Brazil
Tiago Pinto CarvalhoI; Roberto E. ReisII
IUniversity of Louisiana at Lafayette, Department of Biology, P. O. Box 42451, Lafayette, LA 70504, USA. tiagobio2002@yahoo.com.br
IILaboratório de Sistemática de Vertebrados, Pontifícia Universidade Católica do Rio Grande do Sul. P. O. Box 1429, 90619-900 Porto Alegre, RS, Brazil. reis@pucrs.br
ABSTRACT
The species of Hisonotus from the laguna dos Patos system are reviewed. Two species with wide distributions are redescribed: Hisonotus laevior and H. nigricauda. Six new species are described from that system: H. notopagos from the rio Camaquã drainage; H. carreiro and H. prata, endemic from the headwaters of rio Taquari drainage; H. vireo, widely distributed in the rio Jacuí basin; and H. brunneus and H. heterogaster restricted to tributaries of the rio Jacuí drainage. Hisonotus leptochilus is here considered a junior synonym of H. laevior. Hisonotus armatus, H. charrua, H. leucofrenatus, and H. taimensis are also present in the region and in total there are twelve species of Hisonotus in the laguna dos Patos system. Their distributions are discussed, species are illustrated and a taxonomic key is provided.
Key words: Cascudinhos, Endemism, Geographic variation, Neotropical.
RESUMO
As espécies de Hisonotus do sistema da laguna dos Patos são revisadas. Duas espécies com amplas distribuições são redescritas: Hisonotus laevior e H. nigricauda. Seis espécies novas são descritas deste sistema: Hisonotus notopagos, da drenagem do rio Camaquã; H. prata e H. carreiro, endêmicas das cabeceiras do rio Taquari; H. vireo, amplamente distribuída na bacia do rio Jacuí; H. brunneus e H. heterogaster dos tributários da bacia do rio Jacuí. Hisonotus leptochilus é considerado sinônimo júnior de H. laevior. Hisonotus armatus, H. charrua, H. leucofrenatus e H. taimensis também são encontradas na região, e ao total doze espécies de Hisonotus estão presentes no sistema da laguna dos Patos sendo suas distribuições discutidas aqui. As espécies são ilustradas e uma chave taxonômica é fornecida.
Introduction
Hisonotus is part of the Hypoptopomatinae, a group of small loricariids including more than 100 species grouped in 19 genera (Schaefer, 2003; Reis & Carvalho, 2007; Carvalho et al., 2008), distributed in the cis-Andean drainages from Venezuela to northern Argentina. Hisonotus was described by Eigenmann & Eigenmann (1889) based on the following diagnostic characters: belly with large plates, eyes superior, and humeral plate imperforate. The type species, Hisonotus notatus, was collected at Santa Cruz, within urban area of Rio de Janeiro. Regan (1904) conducted the first revision of loricariids, and placed Hisonotus, Parotocinclus Eigenmann & Eigenmann, 1889 and Microlepidogaster Eigenmann & Eigenmann, 1889 under the synonymy of Otocinclus Cope, 1871. Hisonotus was resurrected by Schaefer (1998), being diagnosed by reduced or absent snout plates anterior to the nostril, rostrum with enlarged odontodes, and plates forming the lateral rostral margin thickened (Schaefer, 1998: 387). Those diagnostic features were discussed by Britski & Garavello (2007: 6), being considered variable among species of Hisonotus.
The position of Hisonotus within Hypoptopomatinae diverges to some degree in the phylogenetic analyses of the subfamily using morphological data (Schaefer, 1998; Gauger & Buckup, 2005). In the first analysis (Schaefer, 1998), Hisonotus appears relatively basal within the Otothyrini, more derived than Parotocinclus and "New Taxon 3". In the hypothesis proposed by Gauger & Buckup (2005), the monophyletic status of Otothyrini is not corroborated, and Hisonotus is the sister group to Parotocinclus. More recently, molecular hypothesis supported the polyphyly of Hisonotus (Cramer et al., 2007; Chiachio et al., 2008), species of the genus appearing in at least three different clades. Unfortunately none of these molecular hypotheses included the type species (Hisonotus notatus), which is mandatory for future taxonomic changes and new rearrangements.
The first Hypoptopomatinae described for the laguna dos Patos system was Otocinclus nigricauda. It was described by Boulenger (1891) based on the material collected by Dr. Hermann von Ihering and Herr Sebastian Wolff in the Province (presently State) of Rio Grande do Sul, which had been sent to the British Museum of Natural History. This species was transferred to Hisonotus by Cope (1894) based on the examination of a fish collection from the Rio Jacuhy (rio Jacuí) made by Mr. Herbert Smith. In that work, Cope described several new species including Hisonotus laevior and H. leptochilus, and presented diagnostic comparisons between the four species pertaining to the genus, at that time. After a long period, another species of Hisonotus was described from the laguna dos Patos system, Microlepidogaster taimensis Buckup, 1981 from the marshes of the Taim region. In the last published list of species of laguna dos Patos system (Malabarba, 1989), four species of Hisonotus (that time still Microlepidogaster) were considered valid in this region (H. nigricauda, H. laevior, H. leptochilus, and H. taimensis). Nowadays, Hisonotus consists of 22 nominal species (Schaefer, 2003; Azpelicueta et al., 2007; Britski & Garavello, 2007; Carvalho & Reis, 2009), occurring in the Atlantic coastal drainages of southern and southeastern Brazil, río de La Plata system, laguna dos Patos system, and recently extended to the Amazon basin in the rio Tapajós drainage (Britski & Garavello, 2007).
Buckup (1981: 19) had already mentioned that the difficulties in the study of hypoptopomatines from southern Brazil were in part due to the limitation of the species description and the lack of recent reviews. The objective of this paper is to describe the diversity of Hisonotus in the laguna dos Patos system, in southern Brazil. In this work we are redescribing Hisonotus nigricauda and H. laevior (senior synonym of H. leptochilus), and describing six new species, with an identification key for Hisonotus in this hydrographic system. Although some species in this study do not have some of the features used by Schaefer (1998: 387) to diagnose Hisonotus, they do share apomorphic features with species that can be unambiguously diagnosed as belonging to Hisonotus.
Material and Methods
The studied area is the laguna dos Patos system, an area located in the southeast portion of the Brazilian State of Rio Grande do Sul and a minor portion in northeastern Uruguay. It is one of the Freshwater Ecoregions of the World (FEOW; Abell et al., 2008). The main water bodies that compose the system are the laguna dos Patos (9,280 km2), the laguna Mirim (3,520 km2), and its main tributaries rio Jaguarão, rio Camaquã, and rio Jacuí (Schwarzbold & Schäfer, 1984) (Fig. 1). The entire hydrographical system is connected to the Atlantic Ocean by the channel of Rio Grande. The system is limited by the tributaries of rio Uruguay and rio Pelotas in the north; tributaries of rio Ijuí in the northwest; tributaries of rio Ibicuí in the west; and the rio Negro watershed in the southwest, all part of the rio Uruguay basin. In the eastern portion, the system is limited by the coastal plain, a narrow sandy band (5 to 30 km wide) parallel to the coast line, which has a great number of shallow lakes and lagoons, bordering the rio Tramandaí system in its northeastern limit (Malabarba, 1989). The major tributary river to the laguna dos Patos system is the rio Jacuí, which flows into the lago Guaíba in the northern portion of the laguna dos Patos. The Jacuí basin is formed by several tributaries, including the drainages of rio dos Sinos, rio Caí, and rio Taquari (Fig. 1).
Measurements were made to the nearest 0.1 mm with digital calipers under a stereomicroscope on the left side of specimens. The measurements and their abbreviations follow Carvalho & Reis (2009: 3, fig. 1). Morphometric data are expressed as percents of standard length (SL), except for subunits of the cephalic region that are expressed as percents of head length (HL). Plate counts and nomenclature follow the schemes of serial homology proposed by Schaefer (1997). Vertebral counts include all vertebrae (including the first five vertebrae incorporated into the Weberian apparatus), with the compound caudal centrum (PU1+U1) counted as one element. Accessory patch of teeth is that described by Reis & Schaefer (1992). Cleared and stained specimens (c&s) were prepared according to the method of Taylor & van Dyke (1985). Whenever available, juvenile specimens were also cleared and stained. The term juvenile was used for specimens not totally covered by plates, with an anterior paired crest of odontodes on the parieto-supraoccipital, and also for males without a fleshy flap on the first thickened pelvic-fin ray. Scanning electron microscope pictures were taken from dissected alcohol preserved specimens. Drawings were prepared from c&s specimens using a Zeiss SV8 stereomicroscope with camera lucida attachment. Institutional abbreviations are as follows: Asociación Ictiológica, La Plata (AI); Academy of Natural Sciences of Philadelphia (ANSP); Natural History Museum, London (BMNH); Instituto de Limnología "Dr. Raúl A. Ringuelet", La Plata (ILPLA); Instituto Nacional de Pesquisas da Amazônia, Manaus (INPA), Museo Argentino de Ciências Naturales Bernadino Rivadavia, Buenos Aires, (MACN); Museu Anchieta, Porto Alegre (MAPA); Fundação Zoobotânica do Rio Grande do Sul/ Museu de Ciências Naturais, Porto Alegre (MCN); Museu de Ciências da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre (MCP); Museo de La Plata, La Plata (MLP); Museu Nacional, Rio de Janeiro (MNRJ); Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura, Maringá (NUP); Universidade Federal do Rio Grande do Sul, Porto Alegre (UFRGS); Faculdade de Ciências Universidad de La República, Montevideo (ZVC-P).
In this paper we are redescribing all the species of Hisonotus originally described from this system, with the exception of Hisonotus armatus and Hisonotus taimensis, which have modern and complete descriptions. Hisonotus charrua and Hisonotus leucofrenatus were not redescribed in this paper because were not originally described from the laguna dos Patos system. The first has a recent and complete description (Almirón et al., 2006), and the second needs a complete review thoroughout it distribution and a neotype designation, which is beyond the scope of this paper.
Principal Component Analysis (PCA) was used to check overall variation among samples including differences in morphometrics among species, being an input to multiple regressions. Analyses were made using all measurements listed above except for the pelvic-fin unbranched ray length (VL), which is strongly correlated with sexual dimorphism. Fin-spine measurements were removed whenever presenting missing entries. PCA on covariances of base 10 logarithmically transformed measurements were obtained using Past version 1.28 2004 (Ryan et al., 1995). The first principal component was partioned out, considering that it mostly accounts for size variation (Strauss, 1985). Factor scores were plotted with Sigma Plot version 6.10 2000 (Brannan et al., 2000). Multiple linear regressions were applied to describe morphometric differences among species or individuals of the same species.
Results
Hisonotus nigricauda (Boulenger, 1891)
Figs. 2, 3, 4a, 5, 6a, 7a, and 8a
Otocinclus nigricauda Boulenger, 1891: 234 [original description, type locality: Rio Grande do Sul, Brazil]. -Regan, 1904: 268-269 [redescription, senior synonym of Hisonotus laevior and Hisonotus leptochilus]. Fowler, 1940: 83 [listed]. -Isbrücker, 1980: 84 [listed]. -Schaefer, 1991: 23 [phylogenetic relationships of Hypoptopomatinae].
Hisonotus nigricauda Cope, 1894: 97 [compared with Hisonotus laevior and H. leptochilus and new generic combination]. -Schaefer, 1998: 383 [transferred to Hisonotus]. -Schaefer, 2003: 232 [listed]. -Reis & Carvalho, 2007: 84 [listed]. -Ferraris, 2007: 248 [listed].
Microlepidogaster nigricauda. -Eigenmann, 1910: 413 [listed]. Miranda Ribeiro, 1911: 88 [listed]. Fowler, 1915: 237 [examined specimen]. -Gosline, 1945: 101 [listed]. -Fowler, 1954: 166-167 [listed and illustrated]. -Malabarba, 1989: 150 [type locality restricted to laguna dos Patos system, probably rio Camaquã]. -Schaefer, 1997: 8 [listed].
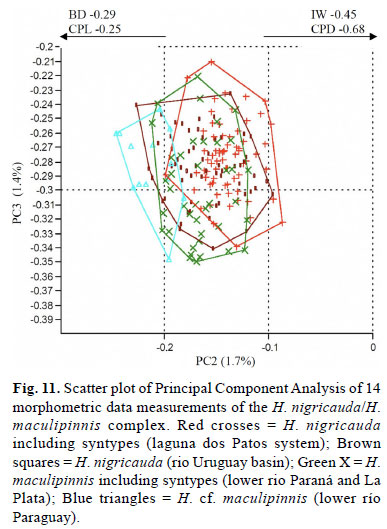
Diagnosis.Hisonotus nigricauda differs from its congeners, except from H. maculipinnis and H. prata, by lacking the posterior portion of the lateral line (Fig. 4a), vs. posterior portion of lateral line present. It differs from H. maculipinnis and H. prata by having an almost completely dark caudal fin, without hyaline areas in the middle portion (Figs. 2-3), vs. caudal fin presenting hyaline areas in the middle portion (see remarks for H. maculipinnis diagnosis).
Description. Morphometrics and meristics in Table 1. Adult size small to medium for members of this genus (less than 40 mm in SL). Body robust, not elongated. Head and body without conspicuous keels. Caudal peduncle round in cross section. Dorsal profile slightly convex from snout tip to dorsalfin origin except slightly concave profile anterior to nares. Straight and posteroventrally sloped at dorsal-fin base, and almost straight from that point to caudal-fin origin. Greatest body depth at dorsal-fin origin. Least body depth at posterior end of caudal peduncle. Posterior profile of caudal-fin margin slightly concave. Head and snout broad, snout rounded to somewhat square in dorsal view, body progressively narrowing posterior to pectoral-fin insertion. Snout region anterior to nares concave, interorbital region slightly convex. Upper margin of orbit somewhat elevate. Eye dorsolaterally positioned. Iris operculum present.
Pectoral fin I,6. Pectoral-fin posterior margin almost straight; when depressed tip extending to middle of pelvic fin. Posterior margin of pectoral-fin spine smooth in adults and juveniles. Pectoral-fin axillary slit present, located below posterior bony margin of cleithral process. Pelvic fin i,5. Tip of depressed pelvic fin not reaching anal-fin origin in females, but extending beyond that point in males. Dorsal fin II,7. Dorsal-fin origin located just posterior of vertical through pelvic-fin origin. Dorsal-fin spinelet somewhat round in shape. Anal fin i,5. First anal-fin pterygiophore exposed anterior to anal fin. Adipose fin absent. Caudal fin i,14,i.
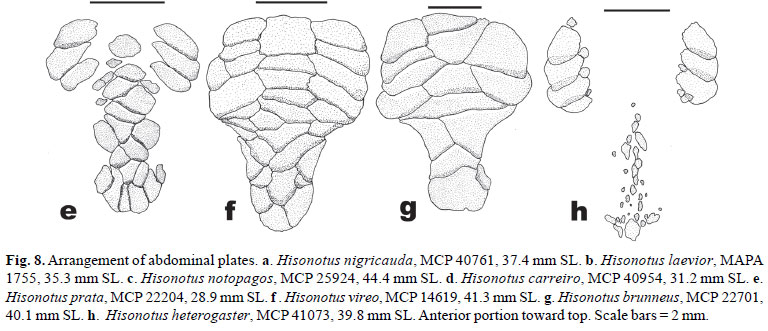
Body almost entirely covered by plates except for region overlying opening of swim bladder capsule, area between pectoral girdle and lower lip, region around anus, and area around base of paired fins. Rostral plate with posterior notch articulation for mesethmoid. Rostral plate thickened, with narrow odontode free band between dorsad and ventrad series of odontodes (Fig. 5), odontode-free area sometimes absent in smaller specimens. Snout plates anterior to nares reduced, small unplated area between rostrum and nares (Fig. 6a). Two or three rows of predorsal plates (modally three; Fig. 7a). Lateral line incomplete, anterior portion short, formed by one to three pored plates. Posterior portion of lateral line absent. Median plate series usually truncated (Fig. 4a). Plates of median abdominal plate series very small, numerous, irregularly arranged. Lateral abdominal plates larger, forming regular series of about four to seven plates on each side (Fig. 8a ). Coracoid and cleithrum exposed and covered by odontodes, except for median region of cleithrum between arrector fossae openings and medial region of coracoids.
Head, fin-spines, and body plates covered with odontodes, these larger on anterior surface of all fin spines, medially directed on pelvic fin. Odontodes on head uniform in size and distribution, except for enlarged odontodes on ventral and dorsal margins of rostrum (Fig. 5). Odontodes on posterior parieto-supraoccipital tip not enlarged, about same size as surrounding areas. Anteroventral margin of compound pterotic with median-to-large size perforations. Infraorbital canal entering infraorbital series via sphenotic. Lips roundish and papillose. Maxillary barbel present.
Premaxillary and dentary teeth slender proximally and flattened distally; bifid, major (medial) cusp large and rounded, minor (lateral) cusp pointed. Accessory patch of teeth absent on dentary and premaxilla.
Compound ventral hypural plate (hypurals 1-2) and compound dorsal hypural plate (hypurals 3-5) not completely fused to each other, median notch on posterior margin of caudal-fin skeleton extending anteriorly. Total vertebrae 2728 (4 c&s), one c&s presenting 25 (apparently anomalous).
Color in alcohol. Ground color of dorsal and lateral surfaces gray to almost black. Dorsal and dorsolateral body surfaces slightly lighter than lateral surface, except for head, which is darker. Ventral surface of body heavily pigmented. Area anterior to nares lighter but not forming conspicuous longitudinal light stripes. Paired, dorsal and anal fins mostly hyaline, except for several transverse dark bands. Caudal fin almost completely dark, except for hyaline area on posterior portion of three uppermost branched rays. Caudal-fin hyaline portion, and the unbranched rays with transverse dark bands.
Sexual dimorphism. Urogenital papilla positioned just behind the anal opening in males. Adult males also possess a developed fleshy flap along the dorsal margin of first thickened pelvic-fin ray, that is absent in females. Flap slightly wider basally and progressively narrowing distally. In adult males, the first and second branched rays of pelvic fin present a fleshy flap at its anteromedial portion. In juvenile males all flaps are smaller or absent. Males have a longer pelvic-fin unbranched ray that extends beyond the anal-fin origin, with the ray never reaching that point in females.
Distribution and habitat.Hisonotus nigricauda is widely distributed in the laguna dos Patos system and in the rio Uruguay basin. In the laguna dos Patos system that species is found mostly in the lower portions of the tributaries near the laguna do Patos, being absent in the upper portions of rio Jacuí basin. In the same manner, H. nigricauda is most commonly collected in the lower portions of the rio Uruguay basin, but being found in the headwaters of rio Negro, rio Quaraí, and rio Ibicuí drainages (Fig. 9). Hisonotus nigricauda is apparently absent in the rio Uruguay basin above the mouth of rio Ibicuí. This species inhabits slow flowing watercourses, of brown waters running over dark organic matter and sandy bottom (Fig. 10c). The individuals were found in between marginal and aquatic vegetation.
Remarks.Hisonotus nigricauda is morphologically very similar to Hisonotus maculipinnis (Regan, 1912) from río de La Plata in Argentina; both nominal species being morphometrically identical (Fig. 11). However, the following features can distinguish them: H. nigricauda has an almost completely dark caudal fin contrasting with a caudal fin with hyaline areas in most specimens of H. maculipinnis (including the type series), especially in juvenile specimens (Fig. 12). Specimens of H. nigricauda usually posses a narrow odontode-free area between dorsal and ventral series of odontodes on the anterior margin of snout, which is absent in H. maculipinnis specimens. These features putatively distinguish the forms, and both seem to be part of a species complex inhabiting the lower portions of the Paraná-Paraguay and laguna dos Patos systems. Further study, however, is necessary to unambiguously demonstrate that these species are not conspecific, which is beyond the purposes of this work.
Material examined. Brazil, Rio Grande do Sul, laguna dos Patos system: BMNH 91.3.16.53-62, syntypes of Otocinclus nigricauda, 9, 24.8-31.8 mm SL. Rio Jacuí drainage: ANSP 21565, 2, 21.3-22.4 mm SL, rio Jacuí. MCN 16246, 10, 23.7-34.1 mm SL, Porto Alegre, Parque Estadual Delta do Jacuí, Saco da Pólvora, 30º01'S 51º14'W. MCP 19834, 12, 25.6-40.2 mm SL, Amarópolis, creek tributary of rio Jacuí in the sylviculture Santo Amaro, 29º55'S 51º55'W. MCP 20543, 1, 31.1 mm SL, Porto Alegre, rio Jacuí on Saco da Alemoa, 30º00'00"S 51º14'51"W. Rio Caí drainage: MCN 15989, 3, 27.3-31.7 mm SL, Triunfo, mouth of rio Caí, left margin, 29º55'54"S 51º16'29"W. Rio Vacacaí drainage: MCP 19584, 33 + 3 c&s, 22.7-9.1 mm SL, São Gabriel, bridge on road between São Gabriel and Tiaraju, 30º17'29"S 54º20'18"W. MCP 26756, 5, 24.2-29.5 mm SL, São Gabriel, marsh of arroio Piraí, 30º17'07"S 54º20'33"W. Rio Gravataí drainage: MAPA 1737, 1, 26.7 mm SL, Gravataí, arroio Passo dos Ferreiros, tributary of rio Gravataí, 29º56'S 50º58'W. MAPA 1759, 3, 23.5-27.5 mm SL, Canoas, canal south of rio Gravataí. MCP 15059, 3, 29.4-30.6 mm SL, Gravataí, marsh at highway RS-118, 29º58'S 50º56'W. Rio dos Sinos: MCN 11625, 11, 31.3-38.3 mm SL, Sapucaia do Sul, Estação Ecológica do Pesqueiro at Zoológico de Sapucaia do Sul. Lago Guaíba drainage: MAPA 1009, 2, 11.6-23.9 mm SL, Porto Alegre, lago Guaíba at Medianeira. MCN 17416, 10, 22.0-32.3 mm SL, Tapes, arroio Guará tributary of arroio Araçá on road between Barra do Ribeiro and Tapes, 30º29'14"S 51º23'39"W. MCP 21165, 4, 26.8-29.5 mm SL, Eldorado do Sul, creek at margins of highway BR-290, 30º02'36"S 51º20'56"W. MCP 28986, 11, 24.7-34.3 mm SL, Eldorado do Sul, arroio Passo dos Carros, 30º05'S 51º23'W. Laguna dos Patos drainage: MCN 12602, 4, 16.2-24.8 mm SL, Arambaré, arroio do Brejo, 5 km south of Arambaré on road to Santa Rita do Sul, 30º57'00"S 50º45'26"W. MCN 12605, 4, 21.6 mm SL, Arambaré, arroio Santa Rita at Capão do Trago, 31º01'50"S 51º30'27"W. MCP 17677, 6, 19.8-24.7 mm SL, Pelotas, old drainage channel near to Passo do Tabajara, marsh of Pontal da Barra in Laranjal, 31º47'S 52º14'W. MCP 23855, 33, 23.1-34.1 mm SL, Sentinela do Sul, arroio Velhaco on road between Cerro Grande do Sul and Camaquã, 30º41'22"S 51º41'51"W. MCP 23858, 4, 27.2 mm SL, Sentinela do Sul, arroio do Tigre tributary of arroio Velhaco on road from Cerro Grande do Sul to Camaquã, 30º44'30"S 51º46'26"W. Rio Camaquã drainage: MCP 17416, 20 + 3 c&s 25.5-39.1 mm SL, Camaquã, marsh in rio Camaquã at Pacheca, 31º08'S 51º47'W. MCP 19701, 1, 27.2 mm SL, Encruzilhada do Sul, arroio Passo da Miséria on road between Encruzilhada do Sul and Canguçu, 30º57'S 52º26'W. MCP 25881, 51, 15.8-36.7 mm SL, Caçapava do Sul, creek tributary of arroio Seival on road between Lavras do Sul and Capaçava do Sul, 30º44'00"S 53º42'04"W. MCP 25875, 1, 34.2 mm SL, Caçapava do Sul, small creek tributary to arroio Hilário on road between Caçapava do Sul and Lavras do Sul, 30º44'24"S 53º44'51"W. MCP 40761, 10 + 3 c&s, 31.0-38.2 mm SL, Bagé, arroio Banhado Grande on highway BR-153 between Bagé and Caçapava do Sul, 31º14'34"S 53º52'50"W. MCP 44506, 1, 33.2 mm SL, Pinheiro Machado, creek tributary of arroio Boici at Fazenda Chimarrão, 31º14'09"S 53º21'39"W. Rio São Gonçalo drainage: MCP 17415, 32, 27.7-34.9 mm SL, Pelotas, dead canal of rio Pelotas, marginal of highway BR-116, at Retiro, 31º37'S 52º20'W. MCP 17577, 4, 25.1-31.6 mm SL, Pelotas, arroio Totó on road to colônia Z-3, 31º46'S 52º20'W. Laguna Mirim drainage: MCP 11134, 2, 28.2 35.0 mm SL, Arroio Grande, arroio Xasqueiro on highway BR-116 between Pelotas and Arroio Grande, 32º09'S 53º02'W. From rio Uruguay basin: Rio Ibicuí drainage: MCP 9270, 10, 17.0-29.7 mm SL, Mata, creek on road between Santa Maria and Mata, 29º33'S 54º27'W. MCP 9386, 10, 14.5-24.3 mm SL, Cacequi, rio Ibicuí on bridge between São Rafael and Cacequi, 29º41'S 54º41'W. MCP 9473, 9, 20.3-32.5 mm SL, Brazil, São Vicente do Sul, arroio do Salso, road from São Vicente do Sul to Santiago, 29º34'S 54º42'W. MCP 9643, 33, 22.1-34.1 mm SL, Dom Pedrito, rio Santa Maria at km 246 of highway BR-293, between Dom Pedrito and Santana do Livramento, 30º59'S 54º42'W. MCP 14145, 1, 24.5 mm SL, Rosário do Sul, creek on road between Rosário do Sul and Santana do Livramento, 30º18'45"S 54º59'49"W. MCP 14214, 1, 33.2 mm SL, Santana do Livramento, pools at side of rio Santa Maria on road between Dom Pedrito and Santana do Livramento, 30º59'S 54º42'W. MCP 16161, 2, 15.5-28.9 mm SL, Santana do Livramento, lateral pools on road to Campo Seco, 15 km east from highway BR-158, 30º42'S 55º04'W. MCP 19593, 13, 22.3-33.5 mm SL, São Gabriel, bridge over Banhado do Inhatium, highway BR-290, 30º15'43"S 54º31'33"W. MCP 23149, 11, 26.0-31.4 mm SL, São Francisco de Assis, rio Inhacunda at São Francisco de Assis going to Manoel Viana, 29º32'51"S 55º08'11"W. MCP 26865, 88 + 3 c&s, 22.7-38.5 mm SL, Rosário do Sul, arroio do Salso on the highway BR-158, affluent of rio Ibicuí da Armada, 30º22'27"S 55º02'07"W. MCP 27608, 14, 16.2-35.9 mm SL, São Francisco de Assis, arroio Caraí-Passo on road from São Francisco de Assis to Manoel Viana, 29º31'03"S 55º10'49"W. UFRGS 8241, 23, 23.7-34.5 mm SL, Rosário do Sul, creek at the margin of highway BR290, 10 km from Rosário do Sul, 30º12'S 55º03'W. Río Negro drainage: MCP 10000, 34, 15.4-30.1 mm SL, Uruguay, Cerro Largo, lagoon 10 m from río Negro at Arreria, 31º50'S 54º28'W. UFRGS 7183, 17, 29.2-32.5 mm SL, Uruguay, Durazno, arroyo Maestre de Campo, on road to Polanco de Yi, tributary of río Yí, 33º24'55"S 56º12'06"W. UFRGS 9243, 7, 29.3-35.5, Uruguay, Rivera, arroyo Batovi on ruta 27, at km 24, río Tacuarembó drainage. UFRGS 9241, 1, 37.3 mm SL, Uruguay, Rivera, arroyo Cunãpiru on km 12 of ruta 27, río Tacuarémbo drainage, 31º02'21"S 55º29'31"W. UFRGS 9243, 5 + 1 c&s, 30.2-36.5 mm SL, Uruguay, Rivera, lateral pools and arroyo Corrales on ruta 27, tributary of río Tacuarembó, 31º23'26"S 55º15'14"W. Other drainages and rio Uruguay: ILPLA 238, 9, 18.5-25.8 mm SL, Argentina, Corrientes, arroyo Cuay Grande, 28º41'S 56º14'W. MAPA 2493, 15, 23.6-33.6 mm SL, Brazil, Barra do Quarai, arroio Quaraí-Chico, MCP 11568, 4, 23.2-29.1 mm SL, Brazil, Uruguaiana, rio Touro Passo, 29º38'S 56º56'W. MCP 16190, 2, 24.4-26.2 mm SL, Brazil, São Marcos, marginal lagoon of rio Uruguay at praia da Formosa, 29º30'S 56º49'W. MCN 16592, 6, 18.7-33.7 mm SL, Brazil, Itaqui, pool at side of highway BR-472, in marsh drainage canal, Reserva Biológica Estadual de São Donato, 29º00'S 56º10'W. MCN 16639, 1, 28.9 mm SL, Brazil, Maçambará, canal at Reserva Biológica Estadual de São Donato, 29º00'S 56º10'W. MCN 16759, 7, 18.7-33.7 mm SL, Brazil, Itaqui, creek on highway BR-472 between Itaqui and São Borja, Reserva Biológica Estadual de São Donato, 29º00'S 56º10'W.
Hisonotus laevior Cope, 1894
Hisonotus laevior
Cope, 1894: 95 [original description, type locality: Rio Jacuhy, Rio Grande do Sul, Brazil]. -Regan, 1904: 268-269 [junior synonym of
Otocinclus nigricauda
]. -Gosline, 1945: 101 [listed as synonym of
Microlepidogaster nigricauda
]. -Fowler, 1959: 167 [listed as junior synonym of
Microlepidogaster nigricauda
]. -Schaefer, 2003: 322 [listed]. -Reis & Carvalho, 2007: 83 [listed]. -Ferraris, 2007: 248 [listed].
Otocinclus laevior. -Isbrücker, 1980: 83 [listed].
Microlepidogaster laevior. -Malabarba, 1989: 150 [listed]. Schaefer, 1997: 8 [listed].
Hisonotus leptochilus
Cope, 1894: 96 new junior synonym [original description, type locality: Rio Jacuhy, Rio Grande do Sul, Brazil]. Regan, 1904: 268-269 [junior synonym of
Otocinclus nigricauda
]. -Gosline, 1945: 101 [listed as junior synonym of
Microlepidogaster nigricauda
]. -Fowler, 1954: 167 [listed as junior synonym of
Microlepidogaster nigricauda
]. -Schaefer, 2003: 322323 [listed]. -Reis & Carvalho, 2007: 83 [listed]. -Ferraris, 2007: 248 [listed].
Otocinclus leptochilus. -Isbrücker, 1980: 83 [listed].
Microlepidogaster leptochilus. -Malabarba, 1989: 150 [listed]. -Schaefer, 1997: 8 [listed].
Hisonotus nigricauda, non (Boulenger, 1891). -Ribeiro et al., 2007: 60 [misidentification, listed and illustrated].
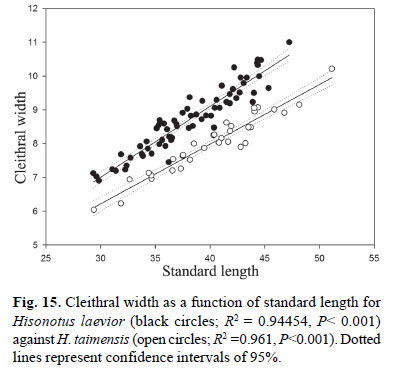
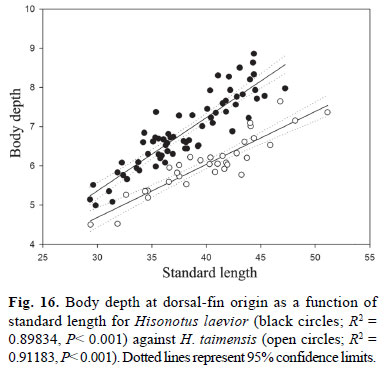
Diagnosis.Hisonotus laevior differs from its congeners, except from H. notopagos and H. taimensis by the higher number of median plate series 25-27 (Table 2), vs. 20-25, by the number of predorsal plates 3-4 (modally 4; Fig. 7b), vs. 23 predorsal plates, and by the vertebral count 30-31, vs. 25-29. Hisonotus laevior is distinguished from H. notopagos by the presence of a posterior notch articulation in the rostral plate, vs. notch articulation in the rostral plate absent; by the possession of a rounded caudal peduncle in cross section, vs. a slight square caudal peduncle in cross section; and by having the area anterior to nares unplated, vs. area anterior nares covered by plates. Hisonotus laevior can be distinguished from H. taimensis by the wider cleithral width 20.6-24.6% SL, mean 22.9%, vs. 18.5-21.3% SL, mean 20.0% (Fig. 15), and by the higher body depth at dorsal-fin origin 16.2-20.8 % SL, mean 18.1%, vs. 13.3-16.3%, mean 15.1% (Fig. 16).
Description. Morphometrics and meristics in Table 3. Adult size medium to large for members of this genus (larger specimen with 47.2 mm in SL). Body robust, somewhat elongated, without conspicuous keels. Caudal peduncle round in cross section. Dorsal profile straight to concave from tip of snout to nares, slightly convex from that point to dorsal-fin origin. Straight and posteroventrally sloped at dorsal-fin base and almost straight from that point to caudal-fin origin. Greatest body depth at dorsal-fin origin. Least body depth at posterior end of caudal peduncle. Posterior profile of caudal fin concave. Head and snout broad, snout rounded to slightly square in dorsal view, body progressively narrowing posterior of pectoral-fin insertion. Snout region anterior of nares concave, interorbital region convex. Upper margin of orbit slightly elevated. Eye dorsolaterally positioned. Iris operculum present.
Pectoral fin I,6. Pectoral fin posterior margin almost straight; its tip extending to middle of pelvic fin when depressed. Posterior margin of pectoral-fin spine smooth. Half portion of spine length serrate in smaller individuals (about or less than 30 mm in SL). Pectoral-fin axillary slit present, located below posterior bony margin of cleithral process. Pelvic fin i,5. Tip of depressed fin not reaching anal-fin origin in females, but extending beyond that point in males. Dorsal fin II,7. Dorsal-fin origin located slightly posterior of vertical through pelvic-fin origin. Dorsalfin spinelet present, laterally extended. Anal fin I,5. First analfin pterygiophore exposed anterior to anal fin or covered by ventral plate series. Adipose fin absent. Caudal fin i,14,i.
Body almost entirely covered by plates except for region overlying opening of swim bladder capsule, area between pectoral girdle and lower lip, region around anus, and area around base of paired fins. Rostral plate with posterior notch articulation for mesethmoid. Rostral plate thickened, with an odontodes-free band between dorsad and ventrad series of odontodes. Snout plates anterior to nares reduced, large paired unplated region between rostrum and nostril. Three or four rows of predorsal plates (modally four; Fig. 7b), smaller specimens sometimes presenting three rows. Lateral line incomplete, with small gap without pores along middle length of body, posterior portion of lateral line present. Median plate series extending to posterior end of caudal peduncle, not truncated. (Fig. 4b). Median abdominal plate series small, irregularly arranged, sometimes presenting a naked area between median and lateral abdominal plate series. Lateral abdominal plates larger, forming a regular series, variable in number (Fig. 8b ). Coracoid and cleithrum exposed and covered by odontodes, except for median region of cleithrum between arrector fossae openings and medial region of coracoids.
Head, fin-spines, and body plates covered with odontodes, these larger on anterior surface of all fin spines, medially directed on pelvic fin. Odontodes on head and trunk of uniform size and distribution, except for enlarged odontodes on ventral and dorsal margin of rostrum. Anterior margin of rostrum presenting an odontode-free area. Odontodes on posterior parieto-supraoccipital tip not enlarged. Anteroventral margin of compound pterotic with median-to-large size perforations. Infraorbital canal entering infraorbital series via sphenotic. Lips roundish and papillose.
Premaxillary and dentary teeth slender proximally and flattened distally; bifid, major (medial) cusp large and rounded, minor (lateral) cusp pointed. Accessory patch of teeth absent on dentary and premaxilla.
Compound ventral hypural plate (hypurals 1-2) and compound dorsal hypural plate (hypurals 3-5) completely fused to each other or with slight median notch on the posterior margin of caudal-fin skeleton, Total vertebrae 3031 (5 c&s).
Color in alcohol. Ground color of dorsal and lateral surfaces of body light to dark brown. Dorsal and dorsolateral surface lighter than lateral surface. Ventral surface of body almost unpigmented except for scattered chromatophores. Dorsolateral surface of head and body with light longitudinal stripes. Stripes narrow, beginning at tip of snout anterior to nares, passing above orbit and reaching posterior end of parieto-supraoccipital, bifurcated and inconspicuous at this point and completely disappearing at vertical above dorsal-fin origin. Paired, dorsal and anal fins mostly hyaline, except for several transverse dark bands with chromatophores forming narrow transverse dark bands; bands most conspicuous on unbranched rays. Caudal fin completely dark brown, except for hyaline posterior portion of uppermost rays. That hyaline portion, and unbranched rays of caudal fin with striped pattern of transverse dark bands. In some specimens caudal fin with inconspicuous light transverse band, formed by rounded clear spots.
Sexual dimorphism. Urogenital papilla positioned just behind the anal opening in males. Adult males possess a fleshy flap along the dorsal margin of first thickened pelvic-fin ray, that is absent in females. Flap slightly wider basally and progressively narrowing distally. Adult males presenting a fleshy flap in the medial portion of first and second branched rays of pelvic fin. In juvenile males flaps are smaller or absent. Males have a longer pelvic-fin unbranched thickened ray that extends beyond the anal-fin origin, with the ray never reaching that point in females.
Distribution and habitat.Hisonotus laevior is widely distributed in the laguna dos Patos system from southernmost tributaries of the laguna Mirim and canal São Gonçalo drainage to the tributaries of the northern rio Jacuí basin (Fig. 17). The species is absent in the headwaters of the rio Jacuí basin. This species inhabits slow to median flowing watercourses, with clear to brown waters running over a sandy bottom, and is found in marginal or submerged aquatic vegetation. Hisonotus laevior is sympatric along its distribution with several species of the genus. Hisonotus laevior occurs in sympatry with H. armatus along almost all its distribution. Hisonotus laevior is collected together with H. nigricauda in some localities of the laguna dos Patos system, mainly in the lower portions of the streams, and occurs in sympatry with H. notopagos in the rio Camaquã drainage. Hisonotus laevior occurs in the banhado do Taim together with Hisonotus taimensis and in coastal plain of the Rio Grande do Sul with H. leucofrenatus.
Remarks.Hisonotus laevior is herein designated as a new senior synonym of Hisonotus leptochilus (Fig. 18). Both species were published in the same paper by Cope (1894). Herbert H. Smith collected these species in 1882, together with a collection of fishes sent to Academy of Natural Sciences of Philadelphia (ANSP). Edward D. Cope studied this material and described several new species including the two species of Hisonotus, collected at Rio Jacuhy (rio Jacuí). According to Papavero (1973) and Malabarba (1989), Herbert H. Smith's itinerary in the State of Rio Grande do Sul included several cities in the laguna dos Patos system and the localities of Caí (São Sebastião do Caí), São João do Montenegro (Montenegro), and Porto Alegre. The later localities mentioned are situated in the lower portion of rio Jacuí and rio Caí drainages, where the species H. laevior is the most abundant taxa of the genus. The descriptions of H. laevior and H. leptochilus were both based on a single specimen each. The differences between these taxa according to Cope (1894: 96) are: "The important characters which distinguish this species [H. leptochilus] from the H. laevior, are the thin and truncate lower lip with feeble tuberculation; the numerous ventral plates; the narrower orbital space, and the greater hispidity, especially of the head". No substantial differences were found in the lower lip tuberculation between all Hisonotus species examinated including the holotypes of H. laevior and H. leptochilus. The holotype of H. leptochilus presents more plates in the abdominal median series than H. laevior (see Cope, 1894 fig. 11b and fig. 12b, respectively). However, this is a polymorphic feature observed within some species of the genus and cannot be used to differentiate H. laevior from H. leptochilus. Another diagnostic feature used by Cope (1894) to differ H. leptochilus from H. laevior was the narrower interorbital space. Although the holotype of H. leptochilus has a narrow interorbital width (39.5% of HL, vs. 41.6% of HL in the holotype of H. laevior), this measurement is within the range of H. laevior (36.9-46.2% of HL; Fig. 19). Finally, no difference in the hispidity of odontodes, in any portion of the body, was found that could suggest the separation of these two species or any Hisonotus in the laguna dos Patos system. For these reasons H. laevior Cope, 1894: 95 is considered senior synonym of H. leptochilus Cope, 1894: 96. Hisonotus leptochilus is here, for the first time, suggested as junior synonym of H. laevior, although, both species had already been erroneously considered as junior synonyms of H. nigricauda before (see synonym list).
Material examined. All from laguna dos Patos system, Rio Grande do Sul, Brazil. Rio Jacuí drainage: ANSP 21253, holotype of Hisonotus laevior, 39.2 mm SL, rio Jacuí. ANSP 21564, holotype of Hisonotus leptochilus, 40.9 mm SL, rio Jacuí. MCN 5823, 5, 14.8-30.7 mm SL, São Jerônimo, arroio da Porteira at fazenda Capão. MCP 9302, 2, 28.7-30.2 mm SL, Pantano Grande, arroio Dom Marcos, 30º13'32"S 52º37'09"W. MCP 9533, 3, 23.2-27.6 mm SL, Rio Pardo, arroio Francisquinho on highway BR-290 between Butiá and Rio Pardo, 30º09'S 52º08'W. MCP 19835, 12, 25.6-40.2 mm SL, Amarópolis, creek tributary of rio Jacuí in the sylviculture Santo Amaro, 29º55'S 51º55'W. MCP 27329, 2, 26.0-30.2 mm SL, Butiá, arroio Martins upstream of mine Recreio, 30º09'S 51º59'W. MCP 17359, 11, 18.9-31.4 mm SL, Arroio dos Ratos, arroio da Porteira, 29º24'S 51º57'W. UFRGS 2577, 2, 29.7-39.9 mm SL, Triunfo, arroio Gil on road between Barreto and Montenegro, 29º48'S 51º37'W. UFRGS 9153, 4, 33.8-43.2 mm SL, Candelária, riceculture canal near sanga das Oveiras, 29º48'00"S 52º36'58"W. Rio Vacacaí drainage: MCP 23131, 1, 33.5 mm SL, São Sepé, rio São Sepé at bridge lateral to highway BR-153, about 3 km south from São Sepé, 30º11'08"S 53º33'35"W. Rio Caí drainage: MAPA 1741, 17, 17.8-35.9 mm SL, São Sebastião do Caí, creek in Vila Conceição. MAPA 1744, 2, 26.8- 36.0 mm SL, São Sebastião do Caí, arroio Três Mares. MAPA 1755, 24 + 3 c&s, 19.7-43.4 mm SL, São Sebastião do Caí, creek in rio Branco, 29º35'S 51º22'W. MCP 23005, 2, 14.6-39.6 mm SL, Triunfo, arroio Bom Jardim, III Polo Petroquímico near SITEL, 29º50'19"S 51º23'25"W. MCP 26053, 1, 40.3 mm SL, Lindolfo Collor, arroio Feitoria tributary of rio Cadeia, 29º34'54"S 51º14'03"W. UFRGS 8661, 23, 31.6-43.0 mm SL, Butiá, creek on property Cerro Vermelho, 30º08'S 51º59'W. UFRGS 8720, 38, 30.4-46.1 mm SL, Rio Pardo, creek at fazenda Limoeiro, 29º59'S 52º22'W. UFRGS 8771, 8, 35.5-43.8 mm SL, Pantano Grande, creek on the border of fazenda Sanga Funda, 30º08'S 52º23'W. UFRGS 8777, 2, 36.4-48.1 mm SL, Rio Pardo, creek tributary of arroio Iruí at fazenda Limoeiro, 30º02'S 52º22'W. UFRGS 8786, 16, 28.8-43.6 mm SL, Pantano Grande, creek on the border of fazendas Tarumã I and Tarumã II, 30º08'S 52º23'W. Rio dos Sinos dranaige: MCN 18633, 1, 47.2 mm SL, Sapucaia do Sul, pesqueiro at Parque Zoológico of Sapucaia do Sul. Rio Gravataí drainage: MCN 6003, 1, 29.8 mm SL, Santo Antônio da Patrulha, arroio Chicoloma. MAPA 2626, 4, 32.9-38.0 mm SL, Gravataí, arroio Passo dos Ferreiros, 29º56'S 50º58'W. MAPA 2354, 1, 33.4 mm SL, Santo Antônio da Patrulha, arroio Ramos between Santo Antônio da Patrulha and highway BR-290. MAPA 2628, 2, 35.5-36.5 mm SL, Canoas, canal at south of rio Gravataí. MCN 6062, 1, 39.7 mm SL, Gravataí, Banhado Grande at fazenda Quatro Irmãos. MCP 14652, 5, 33.7-41.3 mm SL, Morungava, rio Morungava, 29º50'S 50º54'W. MCP 41574, 2, 36.2-40.8 mm SL, Gravataí, marsh at highway RS-118, BR-290, 29º58'S 50º56'W. Lago Guaíba drainage: MAPA 1734, 2, 28.5-27.5 mm SL, Guaíba, creek at fazenda Juncal km 39 of BR-290. MAPA 1848, 5, 32.5-43.8 mm SL, Porto Alegre, arroio Dilúvio between PUCRS and UFRGS câmpus. MCN 14795, 1, 39.1 mm SL, Mariana Pimentel, arroio Ribeiro Pequeno on road RS-711, 29º23'53"S 50º44'37"W. MCN 17567, 2, 32.3-33.8 mm SL, Porto Alegre, arroio Lami on road of Quirinas. MCP 16034, 12, 20.6-31.4 mm SL, Viamão, lago Guaíba at praia de Itapuã near to mouth of riacho Itapuã, 30º15'00"S 51º02'20"W. MCP 23466, 1, 40.9 mm SL, Viamão, arroio at praia da Pedreira, Parque Estadual de Itapuã, 30º21'30"S 51º02'48"W. MCP 28116 27.1-42.8 mm SL, Viamão, arroio Sandu, 30º14'S 51º00'W. MNRJ 25597, 1, 31.5 mm SL, Barra do Ribeiro, creek on road between Barra do Ribeiro and Guaíba about 8 km from Barra do Ribeiro. UFRGS 1239, 1, 38.1 mm SL, Viamão, arroio Alexandrina. UFRGS 2346, 7, 19.2-37.8 mm SL, Eldorado do Sul, lago Guaíba at Vila Sans Soucy. Laguna dos Patos drainage: MAPA 1753, 7, 24.1-42.3 mm SL, Tapes, arroio Teixeira on BR-116. MAPA 1757, 2, 28.5-33.3 mm SL, Palmares do Sul, canal 16 km north of sanga Pangaré on road between Capivari and Mostardas. MCN 2622, 1, 44.4 mm SL, Rio Grande, arroio Vieira, 32º06'S 52º09'W. MCN 12682, 7, 32.0-46.7 mm SL, São Lourenço do Sul, arroio Inhuquipá (Turuçu) near Formosa, 31º28'S 52º05'W. MCN 17632, 3, 18.5-40.0 mm SL, Palmares do Sul, channel of lagoa dos Gateados at fazenda São Sebastião do Fundo, 30º27'S 50º39'W. MCN 17549, 1, 38.7 mm SL, Palmares do Sul, channel of lagoa dos Gateados at fazenda São Sebastião do Fundo, 30º27'S 50º39'W. MCN 18138, 2, 32.5-32.6 mm SL, Palmares do Sul, south margin of lagoa do Casamento, 30º29'S 50º36'W. MCP 16016, 6, 14.7-34.8 mm SL, Viamão, creek on Varzinha at Itapuã, 30º19'S 50º56'W. MCP 19068, 1, 34.2 mm SL, Rio Grande, arroio Bolacha, 32º09'S 52º11'W. MCP 21375, 6, 25.6-40.5 mm SL, São José do Norte, laguna dos Patos at Barra Falsa upstream dam, 31º34'19"S 51º27'35"W. MCP 23856, 10, 31.6-43.8 mm SL, Sentinela do Sul, arroio Faxinal tributary of arroio Velhaco on road from Cerro Grande do Sul to Camaquã, 30º43'48"S 51º45'24"W. MCP 23859, 2, 27.1-41.8 mm SL, Sentinela do Sul, arroio Capivaras on road between Sentinela do Sul and Vila Aurora. 30º39'27"S 51º36'12"W. MCP 23860, 23, 27.6-46.2 mm SL, Tapes, arroio Texeira on highway BR-116, 30º37'26"S 51º32'45"W. MCP 31157, 4, 31.7-33.2 mm SL, Rio Grande, Saco da Mangueira, 32º05'S 52º09'W. MCP 41575, 23, 25.1-42.7 mm SL, Sentinela do Sul, arroio do Tigre tributary of arroio Velhaco on road from Cerro Grande do Sul to Camaquã, 30º44'30"S 51º46'26"W. MCP 41576, 2, 31.4-31.9 mm SL, Sentinela do Sul, arroio Velhaco on road from Cerro Grande do Sul to Camaquã, 30º41'22"S 51º41'51"W. UFRGS 4220, 3, 22.2-35.9 mm SL, Tapes, arroio Velhaco between Tapes and Camaquã, about 24 km from mouth in laguna dos Patos, 30º45'S 51º38'W. UFRGS 6698, 10, 13.8-38.3 mm SL, Viamão, lagoa Negra, 30º21'35"S 50º58'34"W. Rio Camaquã drainage: MCP 23854, 12, 34.8-44.7 mm SL, Cristal, creek tributary to arroio Santa Isabel on highway BR-116, about 17 km south from Cristal, 30º08'55"S 52º01'50"W. MCP 23857, 21, 27.8-38.9 mm SL, Camaquã, arroio Duro on road between Vila Aurora and Dom Feliciano, 30º45'34"S 51º51'57"W. MCP 25754, 4, 25.0-37.2 mm SL, Camaquã, arroio Maria Ulghim on road between Camaquã and Vila Aurora, 30º49'S 50º49'W. MCP 26042, 1, 42.3 mm SL, Caçapava do Sul, arroio do Banhado highway BR-153 about 8 km northwest from Minas do Camaquã, 30º51'48"S 53º29'50"W. MCP 41607, 1, 36.7 mm SL, Caçapava do Sul, small creek tributary to arroio Hilário on road between Caçapava do Sul and Lavras do Sul, 30º44'24"S 53º44'51"W. UFRGS 8239, 4, 32.5-41.3 mm SL, Canguçu, creek tributary to rio Camaquã on highway RS-471, 31º23'S 52º40'W. UFRGS 8758, 13, 23.9-40.5 mm SL, Amaral Ferrador, creek at fazenda Ferraria, 30º52'S 52º15'W. MCP 44501, 2, 26.0-32.1, Pinheiro Machado, creek tributary to arroio Boici at fazenda Chimarrão, 31º13'44"S 53º22'18"W. Rio São Gonçalo drainage: MCP 20821, 6, 37.8-39.4 mm SL, Pedro Osório, arroio Reduzino, 31º54'S 52º55'W. MCP 21424, 13, 33.4-48.9 mm SL, Herval, small creek tributary to arroio Arambaré, between Pedro Osório and Airosa Galvão, 31º58'19"S 53º05'39"W. MCP 21144, 4, 31.5-37.3 mm SL, Piratini, arroio Piratinizinho on vicinal road to highway BR-293, 31º43'02"S 52º59'34"W. MCP 25153, 19, 33.1-46.3 mm SL, Pedro Osório, arroio Arambaré on road between Pedro Osório and Basílio, tributary of rio Piratini, 31º54'35"S 53º01'40"W. MCP 34778, 1, 37.6 mm SL, Herval, arroio Arambaré on road between Pedro Osório and Herval, 31º58'37"S 53º06'15"W. MCP 34779, 8, 33.3-48.8 mm SL, Pedro Osório, arroio Mata Olho on road between Pedro Osório and Basílio, 31º54'56"S 53º00'16"W. MCP 37683, 4, 31.7-41.7 mm SL, Pedro Osório, creek tributary to arroio Arambaré near Carvalho de Freitas, about 35 km from Pedro Osório on the railroad bridge, 31º57'52"S 53º06'17"W. Laguna Mirim drainage. MCP 26074, 2, 38.9-41.22 mm SL, Rio Grande, canal do arroio Taim, Estação Ecológica do Taim, 32º37'S 52º34'W.
Hisonotus leucofrenatus (Miranda Ribeiro, 1908)
Otocinclus leucofrenatus Miranda Ribeiro, 1908: 2 [original description, type locality: Rio das Pedras, Ribeira de Iguape River basin, Brazil].
Microlepidogaster laevior non (Cope, 1884). -Gomes, 1947: 30-32 [misidentified redescription].
Hisonotus leucofrenatus. -Schaefer, 2003: 323 [listed] -Andreata et al., 2006 [karyological description]. -Oyakawa et al., 2007: 94 [comments on ecology and illustration]. -Reis & Carvalho, 2007: 84 [listed]. -Ferraris, 2007: 248 [listed]. -Menezes et al., 2007: 214 [illustrated].
Diagnosis.Hisonotus leucofrenatus differs from all congeners except H. armatus, H. leucophrys, and H. notatus by having the combination of anterior margin of the snout completely covered by odontodes, vs. anterior margin of the snout with a narrow or wide odontode-free band; and large plates in the abdominal median series, usually comprising one series of plates between the lateral abdominal plates, vs. abdominal median series of plates small, with several plate series irregularly arranged between the lateral abdominal plates. Hisonotus leucofrenatus differs from H. armatus, H. leucophrys, and H. notatus by an almost completely dark caudal fin (Fig. 21a) or presence of a faint series of light hyaline spots, forming a vertical bar in the midventral portion (Fig. 20a), vs. a rounded hyaline blotch in the midventral portion in H. notatus (Fig. 21b), and one or more series of conspicuous light vertical bars in the ventral portion in H. armatus (Fig. 21c-d) and H. leucophrys. Hisonotus leucofrenatus further differs from H. armatus and H. leucophyrs by the color pattern of dorsal surface of the head, which is plain dark to light brown, vs. dorsal surface of the head covered by vermiculate and ovoid white and dark spots. Hisonotus leucofrenatus can be also distinguished from H. leucophyrs by the absence of a conspicuous tuft of odontodes on parieto-supraoccipital tip, vs. presence of a conspicuous tuft of odontodes on parietosupraoccipital tip and by the presence of comparatively narrower light horizontal stripes on posterodorsal portion of head, vs. broader light horizontal stripes on the posterodorsal surface of the head.
Description. A redescription of Hisonotus leucofrenatus is beyond the scope of this paper.
Distribution.Hisonotus leucofrenatus is distributed along the eastern coastal plain of laguna dos Patos in Rio Grande do Sul (Fig. 22) and is sympatric with H. laevior in this region. Outside the boundaries of the laguna dos Patos system, H. leucofrenatus is distributed in the Brazilian Atlantic coastal drainages of São Paulo, Paraná, Santa Catarina, and Rio Grande do Sul States; from the rio Ribeira de Iguape basin (type locality) to the rio Tramandaí system.
Remarks.Hisonotus leucofrenatus is found in the rio Tramandaí system, where it has been misidentified by Gomes (1947) as Hisonotus laevior in the resurrection and redescription of the latter. Although the three specimens used in the redescription of Gomes (1947) were not examined, extensive material from the same river (rio Maquiné) and other localities in the rio Tramandaí system were examined. Hisonotus laevior is absent in that system, being H. leucofrenatus the only species of Hisonotus in that region. Also noteworthy is the presence of H. leucofrenatus in the laguna dos Patos system. That species has a patchy distribution in that region contrasted with its wider distributions in neighboring basins. Hisonotus leucofrenatus was described from the rio Ribeira de Iguape basin, a Brazilian Atlantic coastal drainage in southern São Paulo State. No difference was found between examined populations from rio Ribeira de Iguape and rio Tramandaí and all can be diagnosed as above. Recently, a karyologycal characterization of H. leucofrenatus by Andreata et al. (2006), revealed no differences between H. leucofrenatus populations along the Atlantic coastal drainages from rio Ribeira de Iguape to rio Itapocú drainage at northeast Santa Catarina, indicating not only the morphological similarity mentioned here, but karyotype homogeneity. Considering the wide range of this species, an accurate review of H. leucofrenatus populations from Brazilian southeastern coastal drainages should be done to determine with certainty whether these populations are a single species.
Material examined. All from Brazil. Laguna dos Patos system: MCN 4640, 1, 29.1 mm SL, Viamão, lagoa Negra at Parque Estadual de Itapuã. MCN 17790, 1, 33.2 mm SL, lagoa do Casamento at southeast coast of Ilha Grande, Palmares do Sul, 30º24'11"S 50º36'56"W. MCN 18137, 5, 25.4-28.9 mm SL, Palmares do Sul, lagoa do Casamento at southern margin, 30º29'S 50º35'W. MCP 21373, 1, 36.4 mm SL, São José do Norte, laguna dos Patos at Barra Falsa, downstream dam, 31º34'19"S 51º27'35"W. MCP 41630, 7, 26.3-39.0 mm SL, São José do Norte, laguna dos Patos at Barra Falsa, upstream dam, 31º34'19"S 51º27'35"W. Outside of laguna dos Patos system. INPA 28608, 10, 33.6-44.0 mm SL, São Paulo, rio Tejuco on road to Itapirapuã Paulista. MCP 11540, 60 + 3 c&s, Santa Catarina, rio Cubatão (norte). MCP 13645, 12, Rio Grande do Sul, Capão da Canoa, Canal at Vila Cornélios between lagoa Itapeva and lagoa dos Quadros. MCP 14834, 8, Santa Catarina, Praia Grande, rio Canoas 8 km from Praia Grande on the road to Mãe dos Homens. MCP 20722, 3, Rio Grande do Sul, creek tributary to rio Maquiné near Maquiné. MCP 21342, 7, Rio Grande do Sul, canal between lagoa Emboaba and lagoa Emboabinha. MCP 29298, 2, Rio Grande do Sul, rio Três Forquilhas on Vila Itati. MCP 32217, 31, Santa Catarina, Jaraguá do Sul, rio Alma tributary of rio Jaraguá. MCP 39094, 4, 38.3-47.6 mm SL, Paraná, Morretes, rio Marumbi tributary of rio Nhundiaquara, 25º29'S 48º49'W. MZUSP 36565, 20, São Paulo, small creek on road from Eldorado to Sete Barras. MZUSP 60220, 11, São Paulo, córrego Fria. UFRGS 4986, 4, Rio Grande do Sul, arroio Água Parada tributary to rio Maquiné. UFRGS 11526, 19, 22.2-30 mm SL, Rio Grande do Sul, Mostardas, lagoa do Bacupari, 30º31'S 50º25'W.
Hisonotus taimensis (Buckup, 1981)
Microlepidogaster taimensis Buckup, 1981: 22 (original description, type locality: novo canal do arroio Taim, Estação Ecológica do Taim, Rio Grande, Rio Grande do Sul, Brasil). -Buckup & Malabarba, 1983 [listed]. -Malabarba, 1989: 150 [listed]. -Grosser et al., 1994 [listed].
Hisonotus taimensis. -Schaefer, 2003: 323 [listed, new combination]. -Bemvenuti & Moresco, 2005: 55 [listed and illustrated]. -Reis & Carvalho, 2007: 84 [listed]. -Ferraris, 2007: 248 [listed].
Diagnosis.Hisonotus taimensis differs from its congeners, except from H. notopagos and H. laevior by the higher number of lateral plates 26-29 (Table 2), vs. 20-25, by the number of predorsal plates 3-4 (usually 4), vs. 2-3, and by vertebral count 31-32, vs. 25-29 vertebrae. Hisonotus taimensis differs from H. notopagos by the presence of a posterior notch articulation in the rostral plate, vs. absence of a notch articulation in the rostral plate, by having a round caudal peduncle in cross section, vs. a slightly square caudal peduncle in cross section, and by having the area anterior to nares unplated, vs. area anterior to nares covered by dermal plates. Hisonotus taimensis can be distinguished from H. laevior by the narrow cleithral width 18.5-21.3% SL, mean 20.0%, vs. 20.6-24.6% SL, mean 22.9% (Fig. 15), and by the lower body depth at dorsalfin origin 13.3-16.3% SL, mean 15.1%, vs. 16.2-20.8% SL, mean 18.1% (Fig. 16).
Description. Morphometrics and meristics in Table 4. This species was described by Buckup (1981) and will not be redescribed here.
Distribution and habitat.Hisonotus taimensis is known from localities nearby the laguna Mirim (Fig. 22). This species inhabits, at type locality, slow flowing watercourses with brown waters of muddy and sandy bottom. The individuals are found in between aquatic vegetation of the genus Eichhornia, in grasses or similar kind of submersed vegetation. According to Buckup (1981) the enlarged rostral and pelvic-fin odontodes contribute to them cling the stems and roots of Eichhornia. See Grosser et al. (1994) for detailed description of the species habitat and distribution in the Taim region.
Material examined. All from laguna dos Patos system, Rio Grande do Sul, Brazil. Paratypes of M. taimensis: MAPA 1054, 1, 36.3 mm SL, Santa Vitória do Palmar, channel at km 114 on highway BR-471, Estação Ecológica do Taim. MAPA 1055, 10591061, 4, 37.5-51.1 mm SL, Rio Grande, channel at east of lagoa do Jacaré, Estação Ecológica do Taim. MAPA 1070, 1, 26.1 mm SL, Santa Vitória do Palmar, old course of arroio Taim, Estação Ecológica do Taim. MAPA 1013, 1014, 1016, 1018, 1019, 1024, 1026-1028, 1063-1065, 1067-1069, 15, 30.1-47.4 mm SL, Rio Grande, new channel of arroio Taim, Estação Ecológica do Taim. MCN 4835-4844, 10, 34.7-45.9 mm SL, Rio Grande, new channel of arroio Taim, Estação Ecológica do Taim. MCN 7660, 2, 23.9-50.0 mm SL, Rio Grande, north channel of lagoa do Jacaré, Estação Ecológica do Taim. UFRGS 352-353, 2, 40.4-43.5 mm SL, Rio Grande, new channel of arroio Taim, Estação Ecológica do Taim. UFRGS 396, 1, 37.3 mm SL. Rio Grande, new channel of arroio Taim near the highway BR-471. Non-types: MCP 14467, 2 + 3 c&s, 21.8-42.5 mm SL, Rio Grande, new channel of arroio Taim, Estação Ecológica do Taim. MCP 17417, 27 + 3 c&s, 24.6-41.8 mm SL, Rio Grande, new channel of arroio Taim. UFRGS 697, 2, 32.8-37.4 mm SL, Rio Grande, Estação Ecológica do Taim. UFRGS 2462, 5, 21.1-42.3 mm SL, Rio Grande, Estação Ecológica do Taim, curve in the new channel of arroio Taim on highway BR-471. UFRGS 2583, 1, 36.2 mm SL, Rio Grande, Estação Ecológica do Taim, southeast margin of lagoa das Flores. MCN 6474, 1, 46.2 mm SL, Chuí, Barra do Chuí, 33º44'S 53º22'W. CIMC 2341, 2, 28.0-30.7 mm SL, Rio Grande, canal near Estação Ecológica do Taim. CIMC 2405, 67, 20.6-37.3 mm SL, Capão do Leão, canal São Gonçalo near sanga das Traíras. INPA 791, 1, 47.5 mm SL, Rio Grande, arroio Bolacha. INPA 790, 2, 25.1-44.1 mm SL, Rio Grande, arroio Senandes.
Hisonotus charrua Almirón, Azpelicueta, Casciotta & Litz, 2006
Hisonotus charrua Almirón et al., 2006: 88 [original description, type locality: Uruguay, Departamento Tacuarembó, río Uruguay basin, Canãda de Los Penã]. -Reis & Carvalho, 2007: 83 [listed]. Reis & Carvalho, 2009: 35 [included in key].
Hisonotus sp. 2. Cramer et al., 2007: 54 [included in molecular phylogeny of Loricariidae].
Diagnosis.Hisonotus charrua differs from its congeners, except from H. armatus, H. leucophrys, H. leucofrenatus, H. laevior, H. notopagos, and H. taimensis by the color pattern of the caudal fin, which has a conspicuous hyaline transversal band composed by clear spots (a second posterior band in larger individuals) in a dark background, vs. hyaline areas not forming a clearly defined transversal bands in the middle portion of caudal fin. Hisonotus charrua is distinguished from H. armatus, H. leucophrys, and H. leucofrenatus by having a narrow naked band without odontodes on the anterior margin of snout, vs. snout completely covered by odontodes; and from H. laevior, H. notopagos, and H. taimensis by the number of lateral plates 23-25 (Table 2), vs. 25-28 plates; by the number of predorsal plates, 3, vs. 3-4 (modally 4) predorsal plates; and by the number of vertebrae 28-29, vs. 30-32 vertebrae.
Description. This species was recently described by Almirón et al. (2006) and will not be redescribed here.
Distribution and habitat.Hisonotus charrua is widely distributed in the middle and lower portions of the rio Uruguay basin from the northern rio Ijuí drainage in the Rio Grande do Sul State, Brazil, to the southern río Negro drainage in Uruguay. Besides the limits of the rio Uruguay basin, this species is known from the coastal streams of Uruguay, and from the headwaters of Jaguarão and São Gonçalo drainages in the laguna dos Patos system (Fig. 22). The species is sympatric with H. ringueleti Aquino, Miquelarena & Schaefer along its distribution in the middle rio Uruguay basin (Carvalho & Reis, 2009). Hisonotus charrua is collected together with H. nigricauda in several streams tributaries to rio Ibicuí and río Negro drainages. This species inhabits a variety of habitats, usually small to medium size creeks with loose stones, and gravel bottom, with median to rapid-flowing water. Collected associated to marginal vegetation composed by grasses or Echinodorus uruguayensis (Almirón et al., 2006)
Remarks. No morphological differences were found between populations of Hisonotus charrua from rio Uruguay basin and the headwaters of rio Jaguarão and rio São Gonçalo drainages in the laguna dos Patos system (Table 5). Cramer et al. (2007) in a molecular phylogeny of Hypoptopomatinae + Neoplecostominae, using a mitochondrial gene (cytochrome c oxidase I), included a sample from the laguna dos Patos system population of H. charrua (Hisonotus sp. 2 - MCP 25139). According to these authors, the H. charrua haplotype from the laguna dos Patos system is sister to H. laevior, while the H. charrua haplotype from rio Uruguay has a more basal position in a clade composed also of H. taimensis and H. armatus. However, there is low resolution in this clade and it seems necessary the inclusion of more samples/genes to unambiguously determine the phyletic status of the H. charrua morphospecies.
Material examined. From laguna dos Patos system: Rio São Gonçalo drainage: MCP 25139, 15 + 2 c&s, 32.4-46.4 mm SL, Brazil, Pinheiro Machado, arroio dos Pires on bridge of railroad at Passo dos Pires, 31º38'S 53º27'W. Rio Jaguarão drainage: MCP 44500, 10, 16.9-45.4 mm SL, Brazil, Pedras Altas, creek tributary to arroio Jaguarão at Fazenda Sao Francisco II, 31º53'09"S 53º36'45"W. From rio Uruguay basin: Río Negro drainage: ZVC-P 5639, holotype, 49.2 mm SL, Uruguay, Tacuarembó, Cañada de Los Peña, 31º39'05"S 56º12'19"W. AI 165, paratypes, 5, 37.1-48.7 mm SL, collected with the holotype. MCP 9648, 1, 34.2 mm SL, Brazil, Bagé, rio Negro on bridge of highway BR-293 between Bagé and Aceguá, 31º21'S 54º03'W. MCP 16177, 3, 27.1-36.1 mm SL, Brazil, Bagé, rio Piraizinho on road from Bagé to Dom Pedrito, 31º17'S 54º09'W. MCP 40256, 4 + 1 c&s, 32.8-50.3 mm SL, same locality of holotype. UFRGS 9242, 15, 26.6-38.3 mm SL, Uruguay, Durazno, arroyo Maestre de Campo on road to Polanco de Yí, tributary of río Yí, 33º24'55"S 56º12'06"W. UFRGS 7184, 9 + 2 c&s, 18.0-46.2 mm SL, Uruguay, Rivera, lateral pools and arroyo Corrales on ruta 27, tributary of río Tacuarembó, 31º23'26"S 55º15'14"W. UFRGS 7185, 12, 15.9-39.8 mm SL, Uruguay, Rivera, arroyo Batovi at km 24 of ruta 27, tributary of río Tacuarembó drainage. UFRGS 7186, 3, 15.8-20.6 mm SL, Uruguay, Tacuarembó, arroyo Batovi on km 365 of ruta 5, about 20 km from Tacuarembó, 31º43'S 55º46'W. UFRGS 7187, 3, 16.1-28.3 mm SL, Uruguay, Rivera, arroyo Cunãpiru on km 12 of ruta 27, río Tacuarembó drainage, 31º02'21"S 55º29'31"W. Other drainages of rio Uruguay: MACN 7593, 1, 40.5 mm SL, Argentina, Entre Ríos, Parque Nacional El Palmar. UFRGS 7977, 2, 32.7-38.5 mm SL, Artigas, Uruguay, arroyo Guaviyú on ruta 3, 30º38'00"S 57º41'16"W. UFRGS 9195, 8, 25.1-40.9 mm SL, Uruguay, Artigas, arroyo Mandiyú on ruta 3, 30º51'55"S 57º39'57"W. AI 176, paratype, 1, 35.9 mm SL, Uruguay, Salto, arroyo Aspinillar at Constitución. Uruguay coastal drainages: MAPA 1969, 15, 14.7-47.2 mm SL, Uruguay, Maldonado, San Carlos, arroyo Maldonado. MCP 40257, 3, 37.3-40.3 mm SL, Uruguay, Canelones, arroyo Tropa Vieja, 34º44'59"S 55º50'46"W. MCP 40255, 38.8 mm SL, Uruguay, Canelones, arroyo Sauce on km 38 of ruta 7, 34º38'48"S 55º58'27"W. Rio Quaraí drainage: AI 186, paratype, 1, 40.5 mm SL, Uruguay, Artigas, arroyo Catalán Grande, 30º50'40"S 56º12'19"W. MCP 11351, 3, 12.8-18.4 mm SL, Brazil, Quaraí, small creek affluent of arroio Garupá, 30º09'S 56º13'W. MCP 35303, 3, 33.8-41.5 mm SL, Brazil, Quaraí, arroio Garupá on road from Quaraí to Harmonia, about 33 km northeast of Quaraí, 30º09'45"S 56º14'08"W. MCP 40904, 23 + 4 c&s, 17.6-45.6 mm SL, Brazil, Quaraí, arroio Quaraí-Mirim on road between Uruguaiana and Quaraí, 30º18'S 56º19'W. UFRGS 7926, 1, 21.1 mm SL, Uruguay, Artigas, arroyo Yucutujá on ruta 3. Rio Ibicuí drainage: MCP 9630, 2, 23.4-33.4 mm SL, Brazil, Dom Pedrito, rio Santa Maria at km 246 of highway BR-293, between Dom Pedrito and Santana do Livramento, 30º59'S 54º42'W. MCP 23090, 1, 44.4 mm SL, Brazil, São Francisco de Assis, arroio Sanga Funda about 15 km southeast from São Francisco de Assis, 29º39'02"S 55º00'06"W. MCP 27539, 4 + 2 c&s, 28.8-46.6 mm SL, Brazil, Jaguari, arroio do Tigre on highway BR-453/Ijucapirama, about 2,5 km northeast from BR-453, 29º28'18"S 54º40'19"W. MCP 27567, 1, 28.6 mm SL, Brazil, Jaguari, creek affluent of rio Tunas on highway BR-453/Ijucapirama, tributary to rio Jaguari, 29º26'27"S 54º35'35"W. MCP 27604, 7, 15.2-35.3 mm SL, Brazil, Jaguari, arroio Capivari on highway BR-453/Jari, tributary to rio Jaguari, 29º21'10"S 54º28'32"W. MCP 27611, 15, 16.5-40.7 mm SL, Brazil, São Francisco de Assis, arroio Caraí-Passo on road from São Francisco de Assis to Manuel Viana, 29º31'03"S 55º10'49"W. MCP 27625, 3, 29.8-37.0 mm SL, Brazil, Tupanciretã, rio Santana near Jari, tributary to rio Jaguari, 29º14'33"S 54º16'47"W. MCP 41631, 5, 34.4-39.74 mm SL, Brazil, São Francisco de Assis, rio Inhacunda at São Francisco de Assis going to Manuel Viana, 29º32'51"S 55º08'11"W. MCP 41634, 6, 19.1-37.2 mm SL, Brazil, São Francisco de Assis, creek affluent to rio Inhacunda at São Francisco de Assis about 300 m from potery. UFRGS 2576, 1, 34.5 mm SL, Brazil, Santa Maria, arroio Taquara at km 10 on highway RS-453 between Santa Maria and Santiago. UFRGS 8332, 3, 36.8-39.7 mm SL, Brazil, Bagé, rio Santa Maria on highway BR-293 between Bagé and Dom Pedrito, 31º08'S 54º22'W. UFRGS 8333, 1, 40.4 mm SL, Brazil, Bagé, arroio Santa Maria Chico affluent of rio Santa Maria on highway BR-293, 31º04'S 54º29'W. Rio Ijuí drainage: MCP 9616, 1, 32.5 mm SL, Brazil, Panambi, riacho Raiz Mana at Condor on road from Palmeira das Missões to Panambi, 28º13'S 53º29'W. MCP 16751, 1, 34.5 mm SL, Brazil, Jóia, creek affluent of rio Ijuizinho near Jóia, on road from Eugênio de Castro to Jóia, 28º39'S 54º07'W. MCP 34968, 2 c&s, 19.3-34.9 mm SL, Brazil, Roque Gonzales, arroio Portão, 28º06'21"S 54º58'33"W. MCP 37232, 2 + 2 c&s, 36.1-38.4 mm SL, Brazil, São Pedro do Butiá, arroio Albino, 28º08'10"S 54º55'28"W. MCP 37270, 4, 23.1-33.6 mm SL, Brazil, Dezesseis de Novembro, lageado Araçá, 28º12'23"S 54º56'58"W. UFRGS 4329, 3, 37.8-45.6 mm SL, Brazil, Panambi, rio Caxambu, at km 275 of highway BR-285, 28º19'S 53º39'W.
Hisonotus armatus Carvalho, Lehmann, Pereira & Reis, 2008 Figs.
21c-d, 23 , and 27d
Hisonotus armatus Carvalho et al., 2008: 510 [original description, type locality: Brazil, Rio Grande do Sul, Pedro Osório, arroio Arambaré, about 5 km south of Vila Basílio on the road to Pedro Osório].
Hisonotus nigricauda non (Boulenger, 1981). -Lucena et al., 1994 [listed].
Hisonotus sp. 3. -Cramer et al., 2007: 54 [molecular phylogeny of Loricariidae].
Hisonotus sp. 5. -Reis & Carvalho, 2007: 84 [listed]. -Cramer et al., 2007: 54 [molecular phylogeny of Loricariidae].
Diagnosis.Hisonotus armatus differs from all congeners except H. leucofrenatus, H. leucophrys, and Hisonotus notatus by the combination of having the anterior margin of the snout completely covered by odontodes, vs. anterior margin of the snout with a narrow or wide odontode-free band; and having large plates in the abdominal median series, usually comprising of one series of plates between the lateral abdominal plates, vs. abdominal median series of plates small, with several plate series irregularly arranged between the lateral abdominal plates. Hisonotus armatus differs from H. leucofrenatus and H. notatus by the presence on caudal fin of a series of light hyaline spots, forming one or more vertical light bands (Fig. 21c-d), vs. a rounded hyaline blotch or no hyaline area in the midventral portion of caudal fin (Fig. 21a-b). Further differs from H. leucofrenatus and H. notatus by the color pattern of dorsal surface of the head, which is covered by vermiculate and ovoid white and dark spots, vs. dorsal surface of the head plain dark to light brown. Hisonotus armatus differs from H. leucophyrs by the absence of a conspicuous tuft of odontodes on parieto-supraoccipital tip, vs. presence of a conspicuous tuft of odontodes on parietosupraoccipital tip, and by the presence of comparatively narrower light stripes on posterodorsal portion of head, vs. broader light stripes on the posterodorsal surface of the head.
Description. Morphometrics and meristics in Table 6. This species was recently described by Carvalho et al. (2008) and will not be redescribed here.
Distribution and habitat. Hisonotus armatus is widely distributed in the laguna dos Patos system (Fig. 24). This species inhabits slow to median flowing watercourses, with clear to brown waters over sandy bottom and is found in marginal or submerged aquatic vegetation. Hisonotus armatus is sympatric through its distribution with H. laevior. It was also collected together with H. nigricauda in some localities of the lago Guaíba drainage, with H. vireo in the rio Jacuí drainage, and with H. notopagos in the upper rio Camaquã drainage.
Material examined. In addition to the material listed in Carvalho et al. (2008) the following specimens were examined: All from laguna dos Patos system, Rio Grande do Sul, Brazil. MAPA 2624, 10, 21.9-38.0 mm SL, Gravataí, arroio Passo dos Ferreiros. MNRJ 25583, 22, 11.2-37.8 mm SL, Barra do Ribeiro, rio Guaíba at mouth of arroio Ribeiro. MNRJ 25611, 16, 20.8-28.5 mm SL, Porto Alegre, rio Guaíba north of Ponta dos Quatis. MCN 6456, 1, 39.4 mm SL, Três Coroas, arroio José Velho between Canastra Baixa and linha Café Alta. MCN 14236, 8, 14.6-21.8 mm SL, Canela, rio Paranhana at Larangeiro Dam, 29º23'54"S 50º45'26"W. MCN 14795, 1, 33.5, Canela, arroio Casca northeast of UHE Canastra, 29º23'53"S 50º44'37"W. MAPA 1752, 6, 15.0-42.0 mm SL, São Sebastião do Caí, left margin of rio Caí. MCP 41549, 4, 12.8-19.0 mm SL, Cotiporã, rio Carreiro on road between Dois Lageados and Cotiporã, 28º59'43"S 51º45'16"W. MCP 43107, 3, 28.3-30 mm SL, Nova Roma do Sul, rio das Antas downstream to hydroeletric plant UHE Castro Alves. MCP 43549, 1, 29.2 mm SL, Faria Lemos, córrego Pedrinho near Alcântara, 29º05'31"S 51º37'23"W. MCP 44023, 1, 35.2 mm SL, Bento Gonçalves, rio das Antas near to hydroeletric plant UHE 14 de Julho, 29º04'S 51º40'W. UFRGS 9974, 4, 28.6-40.3 mm SL, Veranópolis, rio da Prata downstream to hydroeletric plant PCH Jararaca, 28º56'S 51º26'W. MCN 8071, 1, 19.0 mm SL, Triunfo, rio Jacuí at south margin of Ilha das Cabras.
Hisonotus notopagos, new species
Figs. 4c, 6c, 8c , 25 , 26 , and 27c
Hisonotus
sp. 6. -Reis & Carvalho, 2007: 84 [listed].
Holotype. MCP 44517, 45.0 mm SL, female, Brazil, Rio Grande do Sul, Lavras do Sul, small creek tributary of arroio das Lavras on road from Lavras do Sul to Bagé, rio Camaquã drainage, 30º50'18"S 53º55'43"W, 31 Jul 2006, T. P. Carvalho, A. R. Cardoso & J. M. Wingert.
Paratypes. All from laguna dos Patos system, Rio Grande do Sul, Brazil. Rio Camaquã drainage: MCP 40757, 4, 35.4-41.6 mm SL, collected with the holotype. ANSP 188701, 3, 39.0-44.7 mm SL, Pinheiro Machado, creek tributary to arroio Boici, at fazenda Chimarrão, 31º13'54"S 53º21'45"W, 25 Jan 2006. L. E. Lanés, M. V. Volcan, A. C. Gonçalves & M. Burns. MCP 25803, 5, 31.8-34.9 mm SL, Bagé, arroio do Tigre, on secondary road between Bagé and Lavras do Sul, 27 Apr 2000, C. A. S. Lucena, J. F. P. Silva & V. A. Bertaco, 31º04'47"S 53º54'03"W. MCP 25924, 1 + 2 c&s, 34.8-44.4 mm SL, Lavras do Sul, arroio da Mantiqueira on secondary road between Bagé and Lavras do Sul, 30º54'24"S 53º58'06"W, 26 Apr 2000, C. A. S. Lucena, J. F. P. Silva & V. A. Bertaco. MCP 40762, 3, 31.6-41.8 mm SL, Lavras do Sul, arroio da Mantiqueira on secondary road between Bagé and Lavras do Sul, 30º54'24"S 53º58'06"W, 31 Jul 2006, T. P. Carvalho, A. R. Cardoso & J. M. Wingert. MCP 40749, 2, 35.5-39.0 mm SL, Bagé, arroio das Traíras on highway BR-153, 31º05'29"S 53º43'33"W, 1 Aug 2006, T. P. Carvalho, A. R. Cardoso & J. M. Wingert. MCP 40763, 1, 51.7 mm SL, Lavras do Sul, small creek tributary to arroio das Lavras, about 2 km from Lavras on road to Bagé, 30º50'02"S 53º53'52"W, 31 Jul 2006, T. P. Carvalho, A. R. Cardoso & J. M. Wingert. MCP 44504, 9, 24.9-39.7 mm SL, Encruzilhada do Sul, arroio Maria Santa, 30º40'36"S 52º32'57"W, 16 Jun 2007, L. E. Lanés & A. C. Gonçalves. MCP 44507, 7, 37.2-45.3 mm SL, Pinheiro Machado, creek tributary to arroio Boici, at fazenda Chimarrão, 31º14'09"S 53º21'39"W. MZUSP 104943, 4, 34.6-36.9 mm SL, Pinheiro Machado, creek tributary to arroio Boici, at fazenda Chimarrão, 31º13'44"S 53º22'18"W, 25 Jan 2006. L. E. Lanés, M. V. Volcan, A. C. Gonçalves & M. Burns. UFRGS 8966, 43 + 3 c&s 17.4-39.2 mm SL, Brazil, Rio Grande do Sul, Encruzilhada do Sul, small creek in Encruzilhada do Sul tributary to rio Camaquã drainage, 30º35'17"S 52º33'54"W, 14 Dec 2006, J. Anza & R. Hirano.
Diagnosis.Hisonotus notopagos differs from its congeners by the absence of a rostral plate posterior notch articulation for the mesethmoid, rostral plate reduced and thin, with an unplated area, medially between the dorsad and ventrad series of odontodes, vs. presence of a rostral plate with the posterior notch articulation with the mesethmoid, rostral plates thick. The new species is also distinguished from other species of Hisonotus, except H. laevior and H. taimensis, by its great number of median plate series 25-27 (Table 2), vs. low number of median plate series 20-25; by having four predorsal plates, vs. three predorsal plates, and by having 30-31 vertebrae, vs. 25-29. Hisonotus notopagos further differs from H. laevior and H. taimensis by having caudal peduncle slightly square in cross section, vs. round peduncle in cross section, and by having the area anterior to the nostrils covered by prenasal plates (Fig. 6c), vs. area anterior to nostrils naked (Fig. 6b).
Description. Morphometrics and meristics in Table 7. Adult size large for members of this genus (a female reaching 51.7 mm SL). Body elongate, without conspicuous keels. Caudal peduncle slightly square in cross section. Dorsal profile convex from snout to parieto-supraoccipital tip, slightly convex from that point to dorsal-fin origin; straight and posteroventrally sloped from dorsal-fin origin to caudal-fin origin. Greatest body depth at dorsal-fin origin. Least body depth at posterior end of caudal peduncle. Posterior profile of caudal-fin margin concave. Head and snout broad, snout rounded anteriorly in dorsal view, body progressively narrowing posterior to pelvicfin insertion. Snout region anterior to nares not depressed, interorbital region convex. Upper margin of orbit slightly elevated. Eye dorsolaterally positioned. Iris operculum present.
Pectoral fin I,6. Pectoral-fin posterior margin almost straight; when depressed tip extending beyond middle of pelvic fin (tip of pectoral-fin reaching just anterior third of pelvic fin in some specimens). Posterior margin of pectoral-fin spine serrate. In juveniles entire length of spine serrate, reduced to distal portion of spine in adults. Pectoral-fin axillary slit present, located below posterior bony margin of cleithral process. Pelvic fin i,5. Tip of depressed fin not reaching anal-fin origin in females, but extending beyond that point in males. Dorsal fin II,7. Dorsal-fin origin located slightly posterior of vertical through pelvic-fin origin. Dorsal-fin spinelet present, laterally extended. Anal fin i,5. First anal-fin pterygiophore exposed anterior to anal fin. Adipose fin absent. Caudal fin i,14,i.
Body almost entirely covered by plates except for region overlying opening of swim bladder capsule, area between pectoral girdle and lower lip, region around anus, and area around base of paired fins. Rostral plate without posterior notch articulation for mesethmoid, rostral plate reduced and thinned, rostral area between dorsad and ventrad series of odontodes unplated medially. Prenasal plates anterior to nares present, not reduced (Fig. 6c). Four rows of predorsal plates. Lateral line incomplete, with small gap without pores along middle length of body. Median plate series reaching the postrior end of caudal peduncle, not truncated (Fig. 4c). Median abdominal plates small, irregularly arranged, often leaving naked area between median and lateral abdominal plate series. Lateral abdominal plates larger, forming a regular series of about five to seven plate in each side (Fig. 8c ). Coracoid and cleithrum exposed and covered by odontodes, except for median region of cleithrum between arrector fossae opening.
Odontodes on posterior parieto-supraoccipital tip not enlarged in adults, slightly enlarged odontodes in juvenile specimens. Head, fin spines, and body plates covered with odontodes, larger on anterior surface of all fin spines, medially directed on pelvic fin. Odontodes on head and trunk of uniform size and distribution, except for enlarged odontodes on ventral margin of rostrum, ventrad series of odontodes sometimes absent in medial portion of rostral plate. Rostrum anterior margin with wide free-odontode band (Fig. 26 ). Compound pterotic with median-to-large size perforations from middle portion to ventral margin. Infraorbital canal entering infraorbital series via sphenotic. Lips roundish and papillose. Maxillary barbel present.
Premaxillary and dentary teeth slender proximally and flattened distally; bifid, major (medial) cusp large and rounded, minor (lateral) cusp pointed (Fig. 27c ). Accessory patch of teeth absent on dentary and premaxilla.
Compound ventral hypural plate (hypurals 1-2) and compound dorsal hypural plate (hypurals 3-5) completely fused to each other, but not completely fused in juveniles. Total vertebrae 31 (3 c&s).
Color in alcohol. Ground color of dorsal and lateral surfaces of body light to dark gray, brownish in some specimens. Dorsal and ventrolateral regions slightly lighter, lateral surface darker forming longitudinal band. Ventral surface of body less pigmented than lateral and dorsal portions, belly region with small scattered chromatophores. Dorsolateral surface of head and body with light longitudinal stripes. Stripes beginning on rostrum anterior to nares, passing above orbits and reaching the posterior end of parietosupraoccipital, bifurcating at this point and disappearing laterally bellow dorsal-fin base. Light longitudinal stripe on trunk located above lateral line. Tip of parieto-supraoccipital and corners of squared caudal peduncle lighter than surrounding areas. Paired, dorsal and anal fins mostly hyaline, except for chromatophores forming transverse dark bands (inconspicuous in pelvic fin); bands most conspicuous on unbranched rays. Caudal fin dark pigmented ventrally, unbranched rays with striped pattern. Upper branched rays of caudal fin almost hyaline except for transversal dark bands. Middle portion of caudal fin with hyaline transverse band formed by round light spots. Larger specimens with second hyaline band located posteriorly, on lower caudal lobe.
Sexual dimorphism. Urogenital papilla positioned just behind the anal opening in males. Adult males possess a fleshy flap along the dorsal margin of first thickened pelvic-fin ray, that is absent in females. Flap wide basally and progressively narrowing distally. Adult males with first branched ray of pelvic fin presenting a feeble fleshy flap in medial portion. In juvenile males, flaps are smaller or absent. Males have a longer pelvic-fin thickened unbranched ray that extends up to the anal-fin origin, never reaching that point in females.
Distribution and habitat.Hisonotus notopagos is known from the headwaters of rio Camaquã drainage in the laguna dos Patos system, southern Brazil (Fig. 22). This species inhabits slow to median flow, clear waters running over a sandy bottom, and is found in marginal or aquatic submersed vegetation (Fig. 10d). Hisonotus notopagos was collected together with H. armatus in some localities close to Lavras do Sul, and together with H. nigricauda and H. laevior close to Pinheiro Machado.
Geographic variation. Populations in the northern tributaries (Encruzilhada do Sul) and southern tributaries (Pinheiro Machado and Lavras do Sul) of rio Camaquã drainage differ to a degree in morphometrics and pigmentation. Specimens from the southern tributaries possess a longer pectoral-fin spine (19.2-25.7% SL, mean 23.5%, vs. 17.7-20.7% SL, mean 19.5% in the northern population). Also, the southwestern population is relatively darker and possesses a more conspicuous light transversal band on the middle portion of the caudal fin comparing to the population near Encruzilhada do Sul. In the absence of clear and discrete diagnostic features, we considered both populations as conspecific.
Etymology. The specific epithet, notopagos, from the Greek notos meaning South; and pagos meaning hills. In allusion to the hilly terrains on the southernmost portions of the Brazilian shield, from where this species is endemic.
Hisonotus carreiro, new species
Figs. 4d, 8d , 28 , and 29
Hisonotus
sp. 2. -Reis & Carvalho, 2007: 84 [listed].
Holotype. MCP 44515, 35.4 mm SL, female, Brazil, Rio Grande do Sul, Serafina Corrêa, rio Carreiro downstream Carreiro bathing spot, 28º42'10"S 51º50'57"W, 25 Oct 2006, T. P. Carvalho & V.A. Bertaco.
Paratypes. All from Brazil, Rio Grande do Sul, rio Carreiro drainage. ANSP 188702, 3, 27.7-28.3 mm SL; MCP 40495, 2 + 2 c&s, 27.5-31.2 mm SL; MZUSP 104944, 3, 27.8-28.6 mm SL; collected with the holotype. MCP 41548, 1, 32.0 mm SL, collected at type locality, 11 Jan 2006, J. P. Silva & T. P. Carvalho. MCP 40943, 3, 33.6-35.8 mm SL, Guabiju, arroio Guabiju on secondary road between Guabiju and Vila São Jorge, 28º30'49"S 51º41'22"W, 24 Oct 2006, T. P. Carvalho & V. A. Bertaco. MCN 16361, 3, 34.4-35.4 mm SL, Nova Araçá, arroio Guabiju on road between Guabiju and Vila São Jorge, 28º30'S 51º41'W, Out 2000, W. Koch. UFRGS 6961, 7, 31.7-37.8 mm SL, Serafina Corrêa, rio Carreiro, 28º44'S 51º50'W, Nov 2004, J. Anza.
Diagnosis.Hisonotus carreiro differs from its congeners, except H. francirochai, H. iota, H. leucophrys, and H. prata by the presence of a conspicuous tuft of enlarged odontodes on the tip of the parieto-supraoccipital, vs. odontodes on the tip of parieto-supraoccipital similar in size, not enlarged, or only slightly enlarged. It differs from H. francirochai, H. leucophrys, and H. iota by having the anterior portion of snout with a narrow odontode-free band (Fig. 29 ), vs. snout completely covered with odontodes, without an anterior band free of odontodes. Hisonotus carreiro can be distinguished from H. prata by having a longer dorsal-fin spine 24.329.0% SL, mean 26.6%, vs. 22.6-25.5% SL, mean 24.5% (Fig. 30); longer pectoral-fin spine 24.7-29.0% SL, mean 26.7 %, vs. 22.0-25.6% SL, mean 24.0% (Fig. 31); and by its general color pattern of the body, which is yellowish in life and pale yellow to light brown in alcohol preserved specimens, vs. a dark green general color pattern of the body in life and a dark gray to dark brown color in alcohol preserved specimens.
Description. Morphometrics and meristics in Table 8. Adult size small to medium for members of this genus (maximum size 37.8 mm SL). Body relatively stocky, not elongated, without conspicuous keels. Caudal peduncle round in cross section. Dorsal profile slightly concave from tip of snout to nares, convex from nares to tip of parieto-supraoccipital, almost straight and posterodorsally inclined from that point to dorsalfin origin. Dorsal-fin base straight and posteroventrally sloped, almost straight to slightly concave from posterior end of dorsalfin base to caudal-fin origin. Greatest body depth at dorsal-fin origin. Least body depth at middle of caudal peduncle. Posterior profile of caudal fin concave. Head and snout broad, snout rounded in dorsal view, body progressively narrowing posterior to pectoral-fin insertion. Snout region anterior of nares concave, interorbital region convex. Upper margin of orbit somewhat elevated. Eye dorsolaterally positioned. Iris operculum present.
Pectoral fin I,6. Pectoral-fin posterior margin almost straight; when depressed tip extending beyond middle of pelvic fin. Posterior margin of pectoral-fin spine smooth in adults, juveniles with feeble serrae at distal portion. Pectoralfin axillary slit present, located below posterior bony margin of cleithral process. Pelvic fin i,5. Tip of depressed fin not reaching anal-fin origin in females, but extending beyond that point in males. Dorsal fin II,7. Dorsal-fin origin located just posterior of vertical through pelvic-fin origin. Dorsal-fin spinelet present, laterally extended, one c&s specimen lacking spinelet. Anal fin with i,5. First anal-fin pterygiophore exposed anterior to anal fin. Adipose fin absent. Caudal fin i,14,i.
Body almost entirely covered by plates except for region overlying opening of swim bladder capsule, area between pectoral girdle and lower lip, region around anus, and area around base of paired fins. Rostral plate with posterior notch articulation with mesethmoid. Rostral plate thickened, with narrow odontode-free band between dorsad and ventrad series of odontodes (Fig. 29 ), sometimes absent at medial portion of some specimens. Snout plates anterior to nares reduced, small unplated area between rostrum and nostril at lateral portion. Two or three rows of predorsal plates (rarely two). Lateral line incomplete, with small gap without pores along middle length of body. Median plate series truncated (Fig. 4d). Abdominal plates arranged in three rows anteriorly and irregularly arranged between pelvic-fin insertions. Lateral abdominal plates slightly larger and forming regular series. Median abdominal series usually formed by one plate row. Area between lateral and median abdominal plate series naked in some specimens (Fig. 8d ). Coracoid and cleithrum exposed and covered by odontodes, except for median region of cleithrum between arrector fossae openings and medial region of coracoids.
Odontodes on parieto-supraoccipital tip greatly enlarged, arranged in round patch. Odontodes approximately five times larger than those of surrounding areas. Head, fin-spines, and body plates covered with odontodes, larger on anterior surface of all fin spines, and on ventral and dorsal margin of rostrum medially. Odontodes medially directed in pelvic-fin thickened unbranched ray. Anteroventral margin of compound pterotic with median-to-large size perforations. Infraorbital series entering infraorbital canal via sphenotic. Lips roundish and papillose, posterior border of lower lip fimbriate. Maxillary barbel present.
Premaxillary and dentary teeth slender proximally and flattened distally; bifid, major (medial) cusp large and rounded, minor (lateral) minute pointed. Accessory patch of teeth absent on dentary and premaxilla.
Compound ventral hypural plate (hypurals 1-2) and compound dorsal hypural plate (hypurals 3-5) completely fused to each other, a median notch on the posterior margin of caudal-fin skeleton. Total vertebrae 28 (2 c&s).
Color in alcohol. Ground color of dorsal and lateral surfaces pale yellow to light brown. Dorsal and lateral body surfaces with scattered dark brown round spots. Dorsal and lateral portions of head darker than body. Ventral and ventrolateral surface of body yellowish except for scattered chromatophores, these grouped forming spots on ventral surface of caudal peduncle. Region anterior to nares lighter than surrounding areas, but not forming conspicuous longitudinal light stripes posterior to that point. Paired, anal, and dorsal fins mostly brown, sometimes with light transverse bands, forming striped pattern. Caudal fin almost completely brown, except for one pair of somewhat round hyaline areas in middle portion of upper and lower lobes. Unbranched rays of caudal fin with alternating transverse light bands and dark areas.
Sexual dimorphism. Urogenital papilla positioned just behind the anal opening in males. Adult males also possess a developed fleshy flap along the dorsal margin of first thickened pelvic-fin ray that is absent in females. Flap slightly wider basally and progressively narrowing distally. In males, first and second branched rays of pelvic fin with a fleshy flap in medial portion. In juvenile males, flaps are smaller or absent. Males have a longer pelvic-fin thickened unbranched ray that extends far beyond the anal-fin origin, with the pelvic-fin ray reaching just to the origin of anal fin in females.
Distribution and habitat.Hisonotus carreiro is endemic to rio Carreiro drainage, a tributary to the rio Taquari, which flows into the rio Jacuí basin in the laguna dos Patos system (Fig. 32). This species inhabits relative high altitudes, above 400 m. The habitat in rio Carreiro is a rapid flow watercourse, a wide river with about 0.5 m deep, with clear water and rocky bottom (Fig. 10a). The specimens were caught between aquatic vegetation of Echinodorus, which form green islands on the faults of the rocky substrate. The arroio Guabiju is a small stream about 3 m wide and up to 1 m deep, with clear water, running over a sandy and rocky bottom. There, the species is caught in marginal submersed vegetation. Hisonotus carreiro is sympatric with the undescribed Hypoptopomatinae Eurycheilichthys sp. 1 in arroio Guabiju. Its type locality is now flooded by the reservoir of the Caçador Hidroelectric Dam. The changes in the environment from a rapid running river to a lentic habitat is likely to promote the disappearance of this species in that region.
Etymology. The specific epithet, carreiro, refers to rio Carreiro, the river basin where this species is known. It is treated as a noun in apposition.
Hisonotus prata, new species
Figs. 4e, 8e , 27b , 33 , 34 , and 35
Hisonotus
sp. 1. -Reis & Carvalho, 2007: 84 [listed].
Holotype. MCP 44513, 31.8 mm SL, female, Brazil, Rio Grande do Sul, Nova Prata, rio da Prata on Passo do Despraiado, 28º38'01"S 51º36'51"W, 24 Oct 2006, T. P. Carvalho & V. A. Bertaco.
Paratypes. All from Brazil, Rio Grande do Sul, rio da Prata drainage, ANSP 188703, 3, 25.6-32.0 mm SL; MCP 40492, 18, 19.5-33.2 mm SL; MZUSP 104945, 3, 28.3 mm SL; collected with the holotype. MCP 22204, 9 + 3 c&s, 14.3-29.3 mm SL, collected at type locality, 20 Jan 2006, R. E. Reis, J. F. P. Silva & E. H. L. Pereira.
Diagnosis.Hisonotus prata differs from its congeners, except H. carreiro, H. francirochai, H. iota, and H. leucophris by the presence of a conspicuous tuft of enlarged odontodes on the tip of the parieto-supraoccipital (Fig. 34 ), vs. odontodes on the tip of parieto-supraoccipital similar in size, not enlarged or only slightly larger over the remaining odontodes on the parieto-supraoccipital. It differs from H. francirochai, H. iota, and H. leucophrys by having the anterior portion of snout with an odontode-free band (Fig. 35 ), vs. snout complete covered with odontodes, without an anterior band free of odontodes. Hisonotus prata can be distinguished from H. carreiro by having a shorter dorsal-fin spine 22.625.5% SL, mean 24.5%, vs. 24.3-29.0% SL, mean 26.6% (Fig. 30); shorter pectoral-fin spine 22.0-25.6% SL, mean 24.0 %, vs. 24.7-29.0% SL, mean 26.7%. (Fig. 31); and by its general color pattern of dorsal surface of body which is dark green in life specimens and dark gray to dark brown in alcohol preserved specimens, vs. a yellowish pattern in life, pale yellow to light brown coloration in alcohol preserved specimens.
Description. Morphometrics and meristics in Table 9. Adult size small for members of this genus (maximum size 33.2 mm in the SL). Body stocky, robust, without conspicuous keels. Caudal peduncle round in cross section. Dorsal profile convex from tip of snout to nares to parieto-supraoccipital, convex bump at parieto-supraoccipital tip, almost straight and posterodorsally inclined from that point to dorsal-fin origin. Dorsal-fin base straight and posteroventrally sloped, almost straight from posterior end of dorsal-fin base to caudal-fin origin. Ventral profile almost straight from snout tip to anal-fin origin, concave from that point to caudal-fin origin. Greatest body depth at dorsal-fin origin. Least body depth at middle of caudal peduncle. Posterior profile of caudal fin slightly concave. Head and snout broad, snout rounded in dorsal view, body progressively narrowing posterior to pelvic-fin insertion. Snout region anterior to nares concave, interorbital region convex. Upper margin of orbit not elevated. Eye dorsolaterally positioned. Iris operculum present.
Pectoral fin I,6. Pectoral-fin posterior margin slightly rounded; when depressed tip extending anterior to middle of pelvic fin. Posterior margin of pectoral-fin spine smooth in adults, juveniles with serrae along distal third of pectoral-fin spine. Pectoral-fin axillary slit present, located below posterior bony margin of cleithral process. Pelvic fin i,5. Tip of depressed fin just reaching anal-fin origin in females, but extending beyond that point in males. Dorsal II,7. Dorsal-fin origin located slightly posterior to vertical through pelvic-fin origin. Dorsal-fin spinelet present, laterally extended. Anal fin i,5. First anal-fin pterygiophore exposed anterior to anal fin. Adipose fin absent. Caudal fin i,14,i.
Body almost entirely covered by plates except for region overlying opening of swim bladder capsule, area between pectoral girdle and lower lip, region around anus, and area around base of paired fins. Rostral plate with posterior notch articulation with mesethmoid. Rostral plate thickened, with an odontode-free band between dorsad and ventrad series of odontodes (Fig. 35 ). Snout plates anterior to nares reduced, small unplated area between rostral plate and nostril. Two or three rows of predorsal plates (rarely two). Lateral line incomplete, with gap without pores along middle length of body, posterior portion of lateral line sometimes absent. Median plate series truncated, not reaching posterior end of caudal peduncle (Fig. 4e). Abdominal plates arranged in three rows anteriorly and irregularly arranged between pelvic-fin insertions. Lateral abdominal plates slightly larger and forming regular series. Median abdominal series usually formed by one plate row. Naked area between lateral and median abdominal plate series in most specimens (Fig. 8e ). Coracoid and cleithrum exposed and covered by odontodes, except for median region of cleithrum between arrector fossae openings and medial region of coracoids.
Odontodes on parieto-supraoccipital tip greatly enlarged, raised odontodes arranged in rounded patch, approximately five times larger than those of surrounding areas (Fig. 34 ). Head, fin-spines, and body plates covered with odontodes, larger on anterior surface of all fin spines, and on ventral and dorsal margin of rostrum. Odontodes medially directed on pelvic-fin thickened unbranched ray. Anteroventral margin of compound pterotic with median-to-large size perforations. Infraorbital canal entering infraorbital series via sphenotic. Lips roundish and papillose, posterior margin of lower lip fimbriate. Maxillary barbel present.
Premaxillary and dentary teeth slender proximally and flattened distally; bifid, major (medial) cusp large and rounded, minor (lateral) minute pointed (Fig. 27b ). Accessory patch of teeth absent on dentary and premaxilla.
Compound ventral hypural plate (hypurals 1-2) and compound dorsal hypural plate (hypurals 3-5) completely fused to each other. Total vertebrae 28 (2 c&s).
Color in alcohol. Ground color of dorsal and lateral surfaces dark gray. Dorsal and lateral body surfaces with round dark gray spots. Dorsal and lateral portions of head darker than body, except for lighter cheek region. Ventral surface of body pale yellow, except for scattered chromatophores, these forming spots on ventral surface of caudal peduncle. Region anterior to nares lighter than surrounding areas, but not forming longitudinal light stripes beyond this point. Parietosupraoccipital tip lighter than remaining dorsal surface. Paired, anal, and dorsal fins mostly gray pigmented, with light transverse bands, forming striped pattern. Caudal fin almost completely, except for pair of round to somewhat triangular hyaline areas in middle portion of upper and lower lobes. Unbranched rays of caudal fin with striped pattern of transverse light bands. Ground color in life dark green.
Sexual dimorphism. Urogenital papilla, positioned just behind the anal opening in males. Adult males also possess a developed fleshy flap along the dorsal margin of first thickened pelvic-fin ray, that is absent in females. Flap slightly wider basally and progressively narrowing distally. In juvenile males, flap smaller or absent. Males have a longer thickened pelvic-fin unbranched ray that extends beyond anal-fin origin, with pelvic-fin ray reaching just to anal-fin origin in females.
Distribution and habitat.Hisonotus prata is endemic to the rio da Prata, tributary of rio das Antas, in the rio Taquari drainage, which is a tributary of the rio Jacuí basin in the laguna dos Patos system (Fig. 32). The rio da Prata, at the type locality, is a rapid flow watercourse, wide and shallow, with an average depth of 0.5 m, shallower in most of its extension, with clear water, rocky bottom and great amounts of submersed vegetation (Fig. 10b). The specimens were caught between aquatic vegetation, individuals being associated with plants of the genus Echinodorus, which form green islands in the faults of the rocky substrate. Hisonotus prata is collected in the vegetation with the undescribed Hypoptopomatinae Eurycheilichthys sp. 2.
Etymology. The specific epithet, prata, refers to rio da Prata, the river basin where this species is known. It is treated as a noun in apposition.
Hisonotus vireo, new species
Figs. 4f, 8f , 27a , 36 , 37 , and 38
Hisonotus sp. 4. -Reis & Carvalho, 2007: 84 [listed].
Hisonotus sp. 1. -Cramer et al., 2007: 54 [included in molecular phylogeny].
Holotype. MCP 44518, 38.2 mm SL, female, Brazil, Rio Grande do Sul, Caraá, rio dos Sinos, brigde 7 km north of Caraá, road to Fundo Quente, 29º47'S 50º19'W, 12 Jan 1995, L. R. Malabarba, P. A. Buckup, A. R. Cardoso & G. M. Guazelli.
Paratypes. All from Brazil, Rio Grande do Sul, rio Jacuí basin, rio dos Sinos drainage: MCP 17643, 6, 11.3-39.2 mm SL; MZUSP 104946, 4, 30.5-39.3 mm SL; collected with the holotype. ANSP 188704, 4, 31.0-34.3 mm SL; MCP 14619, 4 + 3 c&s, 28.3-42.0 mm SL; Caraá, rio do Sinos, about 5 km north of Caraá, 29º46'S 50º20'W, 17 Jan 1991, N. A. Menezes, R. E. Reis & E. H. L. Pereira. MCP 17620, 9, 30.0-40.3 mm SL, Caraá, rio dos Sinos, at praia João Fernandes, about 4 km of Caraá and 5 km of Vila Rodolfo Tetour, 29º45'53"S 50º25'41"W, 12 Jan 1995, L. R. Malabarba, P. A. Buckup, A. R. Cardoso & G. M. Guazelli.
Non-type material. All from Brazil, Rio Grande do Sul, laguna dos Patos system. Rio Jacuí drainage: MCP 25459 15, 16.3-38.7 mm SL and MCP 25719, 15 + 3 c&s, 24.1-39.1 mm SL, Ibarama, lageado do Gringo about 2 km from hidroeletrical power plant Dona Francisca, 29º26'49"S 53º15'36"W. MCP 26568, 6, 26.5-36.8 mm SL, Nova Palma, arroio Caemborá near Caemborá, 29º28'50"S 53º 17'50"W. Rio Taquari drainage: MCP 38766, 15 + 3 c&s, 16.9-38.2 mm SL, Lageado, arroio Saraquá, near Botanic Garden of Lageado, 29º27'42"S 52º00'14"W. UFRGS 8812, 14, 32.5-39.9 mm SL, Lageado, confluence of arroio Pinheirinho with rio Forqueta. 29º19'21"S 52º14'03"W. MCP 41550, 4, 14.6-20.0 mm SL, Cotiporã, rio Carreiro at bridge on road between Dois Lageados and Cotiporã, 29º59'43"S 51º45'16"W. Rio Caí drainage: MAPA 1750, 1, 40,6 mm SL, Nova Petrópolis, rio Cadeia, between Joaneta and Pinhal Alto. MAPA 1751, 2, 33.0-40.0 mm SL, Nova Petrópolis, affluent of rio Cadeia at Picada Café. MAPA 2625, 1, 40.4 mm SL, Nova Petrópolis, arroio Macaquinhos tributary of rio Cadeia. Rio dos Sinos drainage: MAPA 724, 1, 22.9 mm SL, Taquara, rio Rolante between Taquara and Rolante. MAPA 1868, 3, 32.9-41.2 mm SL, Santo Antônio da Patrulha, rio dos Sinos, bridge at Santo Antônio da Patrulha. MAPA 2422, 2, 17.3-23.4 mm SL, Taquara, rio da Ilha entre Padilha e rio da Ilha. MAPA 2627, 4, 29.7-41.2 mm SL, Santo Antônio da Patrulha, rio dos Sinos, bridge at Nossa Senhora de Monte Serrat. MCN 6455, 1, 38.7 mm SL, Três Coroas, arroio José Velho between Canastra Alta and Linha Café. MCN 6920, 1, 36.0 mm SL, Três Coroas, arroio Moreira at confluence of arroio Quilombo. MCN 6863, 1, 39,3 mm SL, Três Coroas, arroio Quilombo at Sander. Rio Gravataí drainage: MCP 23875, 3, 29.7-35.2 mm SL, arroio Itajacu, Glorinha, 29º47'34"S 50º42'07"W. Hisonotus cf. vireo: All from Brazil, Rio Grande do Sul, laguna dos Patos system. Rio Jacuí drainage: MCP 26855, 3, 36.0-41.7 mm SL, Júlio de Castilhos, arroio Tipiáia, about 13 km north of Júlio de Castilhos on road to Cruz Alta, 29º06'14"S 53º34'24"W. MCP 41074, 4, 39.0-44.2 mm SL, arroio Tipiáia (or arroio Passo dos Buracos), on road from Júlio de Castilhos to Cruz Alta, 29º06'48"S 53º39'01"W.
Diagnosis.Hisonotus vireo differs from its congeners, except from H. aky, H. brunneus, H. carreiro, H. charrua, H. heterogaster, H. laevior, H. megaloplax, H. montanus, H. nigricauda, H. notopagos, H. prata, H. ringueleti, and H. taimensis by having the anterior margin of the snout with an odontode-free band (Fig. 38 ); vs. anterior margin of the snout complete covered by odontodes. Differs from those above, except H. carreiro, H. prata, and H. ringueleti by the caudalfin pigmentation, when well defined, composed of a dark background with a pair of hyaline areas, in the middle portion of upper and lower lobes, vs. a dark background without hyaline areas or a transverse hyaline band at the middle of caudal-fin length. Hisonotus vireo differs from H. ringueleti by having the posterior margin of the pectoral-fin spine smooth in specimens between 25 and 35 mm SL, vs. posterior portion of spine serrate in specimens between 25 and 35 mm SL, and by having the abdomen completely covered by abdominal plates (Fig. 8f ), vs. abdomen having a large naked area medially on the anterior portion. Differs from H. carreiro and H. prata by the absence of a raised tuft of odontodes on the posterior parieto-supraoccipital tip on adults, juvenile specimens presenting slightly enlarged odontodes, vs. a conspicuous tuft of enlarged odontodes at posterior parietosupraoccipital tip, and median lateral plate series complete, not truncated (Fig. 4f), vs. median lateral plate series truncated (Fig. 4d-e).
Description. Morphometrics and meristics in Table 10. Adult size moderate for members of this genus (larger than 40.0 mm in SL). Body stocky, robust, without conspicuous keels. Caudal peduncle round in cross section. Dorsal profile convex from tip of snout to dorsal-fin origin, except for almost straight portion anterior to parieto-supraoccipital. Dorsal-fin base straight and posteroventrally sloped, straight to slightly concave from posterior end of dorsal-fin base to caudal-fin origin. Ventral profile almost straight from snout tip to analfin origin, concave from this point to caudal-fin origin. Greatest body depth at dorsal-fin origin. Least body depth at middle of caudal peduncle. Posterior profile of caudal-fin margin pronounced concave. Head and snout broad, snout rounded to slightly pointed in dorsal view, body progressively narrowing posterior of pelvic-fin insertion. Snout region anterior to nares slightly concave, interorbital region convex. Upper margin of orbit slightly elevate. Eye dorsolaterally positioned. Iris operculum present.
Pectoral fin I,6, posterior-fin margin almost straight, when depressed tip extending beyond middle of pelvic fin. Posterior margin of pectoral-fin spine smooth in adults, juveniles with serrae along distal half of pectoral-fin spine. Pectoral-fin axillary slit present, located below ventral margin of cleithral process. Pelvic fin i,5. Tip of depressed fin almost reaching anal-fin origin in females, but extending far beyond anal-fin origin in males. Dorsal II,7. Dorsal-fin origin located slightly posterior of vertical through pelvic-fin origin. Dorsal-fin spinelet somewhat rounded. Anal fin i,5. First anal-fin pterygiophore exposed anterior to anal fin. Adipose fin absent. Caudal fin i,14,i.
Body almost entirely covered by plates except for region overlying opening of swim bladder capsule, area between pectoral girdle and lower lip, region around anus, and areas around base of paired fins. Rostral plate with posterior notch articulation with mesethmoid. Rostral plate thickened, with odontode-free band between dorsad and ventrad series of odontodes (Fig. 38 ). Snout plates anterior to nares reduced, small unplated area at lateral portion between rostral plate and prenasal plates. Three rows of predorsal plates. Lateral line incomplete, with gap without pores along middle length of body. Median plate series complete, not truncated (Fig. 4f). Abdominal plates arranged in three rows anteriorly and irregularly arranged between pelvic-fin insertions. Lateral abdominal plates slightly larger and forming regular series. Median abdominal series usually formed by one row, pre-anal shield region formed by small to median size plates (Fig. 8f ). Coracoid and cleithrum exposed and covered by odontodes, except for median region of cleithrum between arrector fossae openings and medial region of coracoids.
Odontodes on parieto-supraoccipital tip slightly larger than those of surrounding areas, mostly in smaller specimens. Head, fin-spines, and body plates covered with odontodes, larger on anterior surface of all fin spines, and on ventral margin of rostrum, slightly enlarged on dorsal margin. Odontodes medially directed on pelvic-fin unbranched ray. Anterior margin of compound pterotic with median-to-large size perforations. Infraorbital canal entering infraorbital series via sphenotic. Lips roundish and papillose, posterior margin of lower lip fimbriate. Maxillary barbel present.
Premaxillary and dentary teeth slender proximally and flattened distally; bifid, major (medial) cusp rounded to spatulate, minor (lateral) minute pointed (Fig. 27a ). Accessory patch of teeth absent on dentary and premaxilla.
Compound ventral hypural plate (hypurals 1-2) and compound dorsal hypural plate (hypurals 3-5) completely fused to each other, with slight median notch on the posterior margin of caudal-fin skeleton. Total vertebrae 27-29 (3 c&s).
Color in alcohol. Ground color of dorsal and lateral surfaces of body light to dark brown. Dorsal and lateral surfaces of head darker than body, except for light area in ventrolateral region contrasting with dark blotches. Region anterior to nares lighter than surrounding areas, forming paired longitudinal light stripes from snout tip to posterior end of parieto-supraoccipital, bifurcating and inconspicuous posterior to that point. Midlateral surface of body darkish, forming darker brown wide longitudinal stripe above lateral line. Ventral surface of body pale yellow, sometimes with scattered round blotches, mostly on cheeks and belly. Unbranched rays of pectoral, pelvic, dorsal, and anal fins mostly brown pigmented, except for narrow lighter spots, forming striped pattern. Branched rays of these fins mostly hyaline except for darker transverse bands. Caudal fin mostly dark brown, except for pair of hyaline areas, somewhat triangular, on anterior portion of upper and lower lobes. Hyaline areas sometimes contacting each other at middle of caudal fin, forming hour-glass-like mark. Posterior portion of branched rays of caudal-fin hyaline. Unbranched rays of caudal fin with striped pattern of transverse light bands. In life, ground color of dorsum and flanks bright green (Fig. 37 ).
Sexual dimorphism. Urogenital papilla positioned just behind Unbranched rays of pectoral, pelvic, dorsal, and anal fins the anal opening in males. Adult males also possess a mostly brown pigmented, except for narrow lighter spots, developed fleshy flap along the dorsal margin of first forming striped pattern. Branched rays of these fins mostly thickened pelvic-fin ray, that is absent in females. Flap slightly wider basally and progressively narrowing distally. Presence of a fleshy flap on medial portion of the first and second branched rays of pelvic fin. In juvenile males, flaps are smaller or absent. Males have a longer pelvic fin that extends far beyond the anal-fin origin, with the pelvic fin never reaching the anal-fin origin in females.
Distribution and habitat.Hisonotus vireo is widely distributed in the rio Jacuí basin from the eastern rio Gravataí and rio dos Sinos drainages to the lower portions of the western rio Jacuí drainage (Fig. 32). In the rio Jacuí basin, the new species was not found in the southern tributaries or in the headwaters of rio Jacuí and rio Taquari drainages. This species inhabits medium to fast flow watercourses, with clear waters running over sandy or rocky bottoms, being collected in marginal or aquatic submersed vegetation. Hisonotus vireo is sympatric along almost its entire distribution with H. armatus.
Geographic variation. The specimens from arroio Tipiáia (MCP 26855 and MCP 41074) differ to a certain degree in pigmentation from other populations in the rio Jacuí basin. These specimens of arroio Tipiáia differ from other conspecifics by the darker pigmentation of caudal fin, which present reduced hyaline areas to complete dark brown in some specimens, contrasting with a pair of hyaline triangular areas on upper and lower lobes and no pigments at posterior portion of caudal fin rays. The condition observed in the caudal-fin pigmentation of specimens from arroio Tipiáia, resemble that of Hisonotus brunneus and H. heterogaster. However, when well defined, the hyaline areas in the specimens from arroio Tipiáia clearly are alike the triangular hyaline areas presented by other populations of H. vireo. A PCA was performed to compare morphometric data of that population to others of H. vireo, in rio Jacuí, rio Taquari, and rio dos Sinos (type locality). No discriminant differences were found between the populations of H. vireo, except when compared with the species H. brunneus (Fig. 39). For these reasons, we tentatively identified these specimens from arroio Tipiáia as belonging to the species H. vireo, and restricted the type series to specimens collected close to the type locality.
Etymology. The specific epithet, vireo, from the Latin meaning "greenish", in allusion to the greenish coloration of live specimens of this species.
Hisonotus brunneus, new species
Figs. 4g, 8g , 40 , and 41
Hisonotus
sp. 3. -Reis & Carvalho, 2007: 84 [listed].
Holotype. MCP 44516, 41.5 mm SL, female, Brazil, Rio Grande do Sul, Cruz Alta, rio Passo Novo, on road from Cruz Alta to Ibirubá, rio Jacuí drainage, 28º38'43"S 53º33'35"W, 2 Apr 1999, R. E. Reis, E. H. L. Pereira & V. A. Bertaco.
Paratypes. All from Brazil, Rio Grande do Sul, rio Jacuí drainage: MCP 22701, 27 + 3 c&s, 26.3-41.1 mm SL, collected with the holotype. ANSP 188705, 4, 34.6-39.0 mm SL; MCP 41072, 13, 29.0-41.0 mm SL; MZUSP 104947, 4, 37.2-40.9 mm SL; collected at the type locality, 13 Dec 2006, T. P. Carvalho & A. R. Cardoso.
Diagnosis.Hisonotus brunneus differs from its congeners, except from H. aky, H. brunneus, H. carreiro, H. charrua, H. heterogaster, H. laevior, H. megaloplax, H. montanus, H. nigricauda, H. notopagos, H. prata, H. ringueleti, and H. taimensis by having the anterior portion of snout with an odontode-free band (Fig. 41 ), vs. snout completely covered with odontodes. It differs from those above, except from H. laevior, H. taimensis, H. megaloplax, and H. heterogaster by a darkly pigmented caudal fin, without a hyaline area in the middle portion, vs. caudal fin with hyaline areas in the middle portion. Hisonotus brunneus differs from H. heterogaster, H. laevior, H. megaloplax, and H. taimensis by having the abdominal median plate series large, one or two series of plates regularly arranged between the lateral abdominal series (Fig. 8g ), vs. abdominal median plate series with several small plates irregularly arranged between the lateral abdominal plates in H. laevior and H. taimensis, or abdominal medium series absent, midline portion of the belly naked in H. heterogaster (Fig. 8h ) or covered by the enlarged lateral series in H. megaloplax, respectively. Also, it differs from H. heterogaster, H. laevior, H. megaloplax, and H. taimensis by having median lateral plate series incomplete, truncated (Fig. 4g), vs. median lateral plate series complete, not truncated.
Description. Morphometrics and meristics in Table 11. Adult size small to median for members of this genus (usually less than 40.0 mm SL). Body relatively stocky, robust, without conspicuous keels. Caudal peduncle round in cross section. Dorsal profile convex from tip of snout to parietosupraoccipital tip, slightly convex from that point to dorsalfin origin. Dorsal-fin base straight and posteroventrally sloped, straight from posterior end of dorsal-fin base to caudal-fin origin. Ventral profile almost straight from snout tip to anal-fin origin, concave from this point to caudal-fin origin. Greatest body depth at dorsal-fin origin. Least body depth at posterior end of caudal peduncle. Posterior profile of caudal fin concave to slightly forked. Head and snout broad, snout rounded to slightly square in dorsal view, body progressively narrowing posterior of pelvic-fin insertion. Snout region anterior of nares concave, interorbital region convex. Upper margin of orbit slightly elevated. Eye dorsolaterally positioned. Iris operculum present.
Pectoral fin I,6. Pectoral-fin posterior margin slightly rounded to straight, when depressed tip extending just anterior to middle of pelvic fin. Posterior margin of pectoral-fin spine smooth in adults, juveniles with feeble serrae along distal third of pectoralfin spine. Pectoral-fin axillary slit present, located below posterior bony margin of cleithral process. Pelvic fin i,5. Tip of depressed fin just reaching anal-fin origin in females, but extending far beyond that point in males. Dorsal-fin II, 7. Dorsalfin origin located slightly posterior to vertical through pelvicfin origin. Dorsal-fin spinelet slight-laterally extended. Anal i,5. First anal-fin pterygiophore exposed anterior to anal fin. Adipose fin absent. Caudal fin i,14,i.
Body almost entirely covered by plates except for region overlying opening of swim bladder capsule, area between pectoral girdle and lower lip, region around anus, and area around base of paired fins. Rostral plate with posterior notch articulation of mesethmoid. Rostral plate thickened, with odontode-free area between dorsad and ventrad series of odontodes (Fig. 41 ). Snout plates anterior to nares reduced, small unplated area at lateral portion between rostral plate and nostril. Three rows of predorsal plates. Lateral line incomplete, with gap without pores along middle length of body. Median plate series truncated, not reaching posterior end of caudal peduncle (Fig. 4g). Abdominal plates arranged in three rows anteriorly and irregularly arranged between pelvic-fin insertions. Lateral abdominal plates slightly larger and forming regular series. Median abdominal series formed by one or two plate series, pre-anal shield region formed by median to large plates (Fig. 8g ). Coracoid and cleithrum exposed and covered by odontodes, except for median region of cleithrum between arrector fossae openings and medial region of coracoids.
Odontodes on parieto-supraoccipital tip slightly larger than those of surrounding areas, most in smaller specimens. Head, fin-spines, and body plates covered with odontodes, larger on anterior surface of all fin spines, and on ventral margin of rostrum slightly enlarged in dorsal margin. Odontodes medially directed on pelvic-fin unbranched ray. Anterior margin of compound pterotic with median-to-large size perforations. Infraorbital canal entering infraorbital series via compound pterotic. Lips roundish and papillose, posterior margin of lower lip fimbriate. Maxillary barbel present.
Premaxillary and dentary teeth slender proximally and flattened distally; bifid, major (medial) cusp large and rounded, minor (lateral) minute pointed. Accessory patch of teeth absent on dentary and premaxilla.
Compound ventral hypural plate (hypurals 1-2) and compound dorsal hypural plate (hypurals 3-5) completely fused to each other, with small median notch on the posterior margin of caudal-fin skeleton. Total vertebrae 27-28 (2 c&s).
Color in alcohol. Ground color of dorsal and lateral surfaces pale to dark brown. Dorsal and lateral body surfaces with rounded gray spots. Dorsal and lateral portions of head darker than body, except for lighter area in ventrolateral region contrasting with dark blotches. Ventral surface of body pale yellow, except for scattered chromatophores. Region anterior to nares lighter than surrounding areas, forming longitudinal light stripe from snout tip to posterior end of parietosupraoccipital. Unbranched rays of fins mostly brown pigmented, sometimes presenting hyaline transverse bands, forming striped pattern. Caudal fin almost completely brown, except for hyaline area on posterior end of upper caudal fin rays. Unbranched rays of caudal fin with alternating transverse light bands and dark areas. General color pattern in life, dark green.
Sexual dimorphism. Urogenital papilla, positioned just behind the anal opening in males. Adult males also possess a developed fleshy flap along the dorsal margin of first thickened pelvic-fin ray, that is absent in females. Flap slightly wider basally and progressively narrowing distally. Presence of a fleshy flap in the medial portion of the first and second branched rays of pelvic fin. In juvenile males, flaps smaller or absent. Males have longer pelvic-fin unbranched ray that extends far beyond anal-fin origin, with pelvic-fin reaching just to anal-fin origin in females.
Distribution and habitat.Hisonotus brunneus is endemic to the upper rio Jacuí basin, in the laguna dos Patos system. The new species is only known from the rio Passo Novo, an affluent of the rio Ingaí, tributary to the upper portion of rio Jacuí basin (Fig. 32). This species inhabits a median to fast flowing watercourse, with clear to brown waters running over sand or stones. Being found in marginal submersed grasses, H. brunneus is collected in the vegetation together with Eurycheilichthys limulus Reis & Schaefer. The rio Passo Novo at the type locality is a small creek, about 3 m wide and shallow with a maximum depth of 0.5 m, at 400 m above sea level. The stream is somewhat polluted, crossing the urban area of Cruz Alta, and has deforested riparian vegetation and amounts of rubbish along the margins.
Etymology. The specific epithet brunneus, from the Latin, means tawny and refers to overall brownish pigmentation of the species. It is treated as an adjective.
Hisonotus heterogaster, new species
Figs. 4h, 8h , 42 , and 43
Holotype. MCP 44514, 43.0 mm SL, female, Brazil, Rio Grande do Sul, Júlio de Castilhos, arroio Felício on road from Nova Palma to Júlio de Castilhos, 29º19'04"S 53º37'54"W, 12 Dec 2006, T. P. Carvalho & A. R. Cardoso.
Paratypes. All from Brazil, Rio Grande do Sul, rio Jacuí drainage: ANSP 188706, 3, 37.1-40.7 mm SL; MCP 41073, 5 + 2 c&s, 37.4-44.3 mm SL; MZUSP 104948, 3, 40.8-41.3 mm SL; collected with the holotype. MCP 26802, 1, 43.0 mm SL, collected at type locality, 28 Nov 2000, L. R. Malabarba, V. A. Bertaco, M. A. Azevedo, J. R. H. Bastos & C. Ricken.
Diagnosis.Hisonotus heterogaster differs from its congeners, except from H. aky, H. brunneus, H. carreiro, H. charrua, H. heterogaster, H. laevior, H. megaloplax, H. montanus, H. nigricauda, H. notopagos, H. prata, H. ringueleti, and H. taimensis by having the anterior margin of the snout with an odontode-free band (Fig. 43 ), vs. anterior margin of the snout completely covered by odontodes. It differ from those above by its lack of the median abdominal plate series, leaving a large naked abdominal area, plates at ventral midline restricted to small platelets at pre-anal shied region (Fig. 8h ); vs. presence of the median abdominal plate series, pre-anal shield region plated.
Description. Morphometrics and meristics in Table 12. Adult size moderate to large for members of this genus (larger than 40.0 mm in the SL). Body robust, without conspicuous keels. Caudal peduncle round in cross section, slightly flattened dorsally. Dorsal profile convex from tip of snout to dorsal-fin origin. Dorsal-fin base straight and posteroventrally sloped, straight from posterior end of dorsal-fin base to caudal-fin origin. Ventral profile somewhat concave from snout tip to posterior portion of head, almost straight from that point to anal-fin origin. Concave at anal fin base and straight from that point to caudal-fin origin. Greatest body depth at dorsal-fin origin. Least body depth at middle of caudal peduncle. Posterior profile of caudal-fin margin slightly concave. Head and snout broad, snout rounded to slightly pointed in dorsal view, body progressively narrowing posterior to pectoral-fin insertion. Snout region anterior to nares straight, not depressed; interorbital region straight to slightly convex. Upper margin of orbit not elevated. Eye dorsolaterally positioned. Iris operculum present.
Pectoral fin I,6. Pectoral-fin posterior margin almost straight, when depressed tip extending beyond middle of pelvic fin. Posterior margin of pectoral-fin spine smooth in adults, smaller specimens with feeble serrae along posterior third of pectoral-fin spine. Pectoral-fin axillary slit present, located below ventral margin of cleithral process. Pelvic fin i,5. Tip of depressed fin just reaching anal-fin origin in females, but extending far beyond that point in males. Dorsal II,7. Dorsal-fin origin located slightly posterior to vertical through pelvic-fin origin. Dorsal-fin spinelet laterally extended. Anal fin i,5. First anal-fin pterygiophore exposed anterior to anal fin. Adipose fin absent. Caudal fin i,14,i.
Body almost entirely covered by plates except for region overlying opening of swim bladder capsule, area between pectoral girdle and lower lip, region around anus, and base of paired fins, and belly region between lateral abdominal plate series. Rostral plate with posterior notch articulation with mesethmoid. Rostral plate thickened, with odontode-free area between dorsad and ventrad series of odontodes (Fig. 43 ).
Snout plates anterior to nares reduced, small unplated area at lateral portion between rostral plate and prenasal plates. Three rows of predorsal plates. Lateral line incomplete, with gap without pores along middle length of body. Median plate series not truncated, reaching posterior end of caudal peduncle (Fig. 4h). Median abdominal plate series absent. Irregularly arranged platelets in preanal shield region, absent in some specimens. Lateral abdominal plates relative small and forming regular series of three to six plates in each side (Fig. 8g ). Coracoid and cleithrum exposed and covered by odontodes, except for median region of cleithrum between arrector fossae openings and medial region of coracoids.
Odontodes on parieto-supraoccipital tip slightly larger than those of surrounding areas. Head, fin-spines, and body plates covered with odontodes, larger on anterior surface of all fin spines, and on ventral margin of rostrum, slightly enlarged on dorsal margin of rostrum. Anteroventral margin of compound pterotic with median-to-large size perforations. Infraorbital canal entering infraorbital series via sphenotic. Lips roundish and papillose, posterior margin of lower lip fimbriate. Maxillary barbel present.
Premaxillary and dentary teeth slender proximally and flattened distally; bifid, major (medial) cusp round; minor (lateral) minute pointed. Accessory patch of teeth absent on dentary and premaxilla.
Compound ventral hypural plate (hypurals 1-2) and compound dorsal hypural plate (hypurals 3-5) completely fused to each other, with median notch on posterior margin of caudal-fin skeleton. Total vertebrae 29 (2 c&s).
Color in alcohol. Ground color of dorsal and lateral surfaces light brown to gray. Dorsal and lateral portions of head darker than body, except for yellowish area, contrasting with dark blotches, in ventrolateral region of head. Region anterior to nares lighter than surrounding areas, forming paired longitudinal light stripe from snout tip to posterior end of parieto-supraoccipital, bifurcating and inconspicuous from that point. Ventral surface of body pale yellow, with scattered chromatophores, mostly grouped on cheeks and base of pectoral fins forming dark blotches. Unbranched rays of pectoral, pelvic, dorsal, and anal fins mostly brown, except for narrow light bands, forming banded pattern. Branched rays of these fins mostly hyaline except for darker transverse bands. Caudal mostly dark brown, except for hyaline area on posterior portion of upper lobe. Unbranched rays of caudal fin with banded pattern of transverse light bands. In life, ground color of dorsum and flanks dark green.
Sexual dimorphism. Urogenital papilla, positioned just behind the anal opening in males. Adult males also possess a developed fleshy flap along the dorsal margin of first thickened pelvic-fin ray, that is absent in females. Flap slightly wider basally and progressively narrowing distally. Presence of a fleshy flap in the medial portion of first and second branched rays of pelvic fin. In juvenile males, flaps smaller or absent. Males have longer pelvic fin that extends far beyond anal-fin origin, with the pelvic fin just reaching anal-fin origin in females.
Distribution and habitat.Hisonotus heterogaster is known from the arroio Felício, a stream affluent to the rio Soturno that is a western tributary of the rio Jacuí basin (Fig. 32). This species inhabits a median to fast flowing watercourse, with clear water running over sand or rocks, being collected mostly in the marginal submersed vegetation composed of bamboos.
Etymology. The specific epithet heterogaster, from the Greek heteros meaning distinct, deviating, abnormal, and gaster meaning belly, in allusion to the specific shape of plate covering of the abdominal plates. It is treated as a noun in apposition.
Key to the species of Hisonotus from the laguna dos Patos system
Discussion
Hisonotus was resurrected by Schaefer (1998) and diagnosed by having reduced or absent snout plates anterior to nares, yielding a large paired unplated region in either side of midline from rostrum to nostril, and by having the margins of rostral plates thickened with enlarged odontodes. However, the monophyly of the genus is questioned by several authors (Britski & Garavello, 2003, 2007; Gauger & Buckup, 2005; Azpelicueta et al., 2007; Cramer et al., 2007; Chiachio et al., 2008). Britski & Garavello (2007) stated that some species of the genus do not present the diagnostic characters proposed by Schaefer (1998), including Hisonotus notatus its type species. Moreover, these authors argue that the condition of the thickened rostral plates is shared with other Hypoptopomatinae, as for example Parotocinclus.
Hisonotus notatus cited in the material examined by Schaefer (1998: 399, CAS 56717 Brazil: rio Ribeira do Iguape) is likely a misidentification since that species does not occurs in rio Ribeira de Iguape, and the only species of the genus occurring in that basin is Hisonotus leucofrenatus (Oyakawa et al., 2006). In fact, our examination indicates that H. notatus as well as other species of the genus do not present an unplated area anterior to nares. However, the reduction in different degrees of the prenasal plates anterior to nostril occurs in several species of Hisonotus (Fig. 6). This character-state condition should be tested to find out whether it constitutes a synapomorphy for Hisonotus with posterior reversions or rather a derived feature for some species within the genus. Another character used by Schaefer (1998) to diagnose the genus refers to the interruption of the median lateral plate series on the posterior end of caudal peduncle (Schaefer, 1998, ch. 33). According to Schaefer's topology (1998) the interruption involving the median series evolved independently in Hisonotus and Microlepidogaster. This character was discussed by Britski & Garavello (2003, 2007), and reported as being highly variable. These authors also noted that although some individuals of H. notatus and H. nigricauda have a complete median plate series, a posteriorly interrupted median plate series is variable present in other specimens, even bilaterally. Accordingly, they conclude that this character is inappropriate to define the genus. We agree with those authors and confirm the presence of the complete median series posteriorly in H. notatus and other Hisonotus (e. g., H. armatus and H. leucofrenatus, see also Fig. 4). However, from 13 cleared and stained specimens examined of H. nigricauda, only three (two specimens in just one side) present a complete median series, while in most specimens the last one or two plates in dorsal and ventral series contact each other at lateral midline. Among the species of Hisonotus having the truncation of median series, we found almost no variation within species. For these reason, that characteristic could be useful to diagnose Hisonotus species and, if not derived for the whole genus as stated by Schaefer (1998), is likely an apomorphic feature shared by some species.
In the context of a non-diagnosable Hisonotus, Britski & Garavello described three species: H. insperatus (2003), and H. chromodontus plus H. luteofrenatus (2007), which do not present some diagnostic features of the genus. According to Schaefer (1998: 387), his "New Taxon 3" can be defined by its pointed and elongated rostrum; thickened paired rostral plate; dorsal and ventral margins of trunk plates without odontode ridges; and pectoral arrector fossae open. This features fit with those presented by H. luteofrenatus and it is likely that this species represent Schaefer's "New Taxon 3". Moreover, most of the material examined by Schaefer (1998) come from the rio Xingu basin, neighbor to the rio Tapajós basin, in which H. luteofrenatus is also present. In Schaefer's analysis (1998), the "New Taxon 3" is basal within Otothyrini and not closely related to Hisonotus. In the same manner, Gauger & Buckup (2005) included both H. luteofrenatus (as "New Taxon 3") and H. chromodontus (as "New Taxon 6") in a phylogenetic analysis of Hypoptopomatinae. These species of Hisonotus do not grouped with H. notatus in both hypotheses proposed by Gauger & Buckup (2005). These examples illustrate well the non-monophyly of Hisonotus, and future phylogenetic analysis are likely to make extensive nomenclatural changes in this group.
Some character states shared by species of Hisonotus could be tentatively polarized and recognized as synapomorphies for some species groups. Three species of Hisonotus in the laguna dos Patos system (H. laevior, H. notopagos, and H. taimensis) have an increased number of vertebrae, predorsal plates, and lateral plates, contrasting with other species of Hisonotus. These species have 30-32 vertebrae, which seems to be a derived feature contrasting with the low number of other species of Hypoptopomatinae (29 or less vertebrae; Schaefer, 1997). Moreover, this putative clade presents 3-4 (modally 4) predorsal plates, and 25-29 lateral plates, versus 2-3 (modally 3) predorsal plates, and 2025 lateral plates in other species of Hisonotus, which may be related to their high number of vertebrae.
Other derived feature presented by species of Hisonotus, which was described by Aquino et al. (2001) is the odontodefree area between the dorsad and ventrad series of odontodes. Most hypoptopomatines having thickened rostral plates with no odontode-free band, being its anterior portion of rostrum completely covered by odontodes (e. g., Epactionotus bilineatus, Hisonotus notatus, and Parotocinclus maculicauda). Contrastingly, numerous species of Hisonotus presents a band devoid of odontodes on the anterior portion of the snout. The width of the odontode-free band is somewhat variable between species of Hisonotus, however, its presence seems to be homologous.
Some species of Hisonotus present a peculiar green coloration in life. That color pattern and the capacity to change color was described and discussed by Azpelicueta et al. (2004) for Hisonotus aky. The greenish coloration was previously observed among hypoptopomatines by Retzer et al. (1999), who shown that Acestridium dichromum is able to change color (greenish to brownish) for camouflage.
Field examination of this feature revealed that the green color pattern is more widespread among species of Hisonotus and not exclusive to H. aky. In the other hand, some species of Hisonotus never presented this green pattern, even when collected syntopically with species presenting the green coloration (e. g., H. armatus collected with the greenish H. vireo). This feature is shared by several species of Hisonotus inhabiting the rio Uruguay basin (Casciotta et al., 2006; Carvalho & Reis, 2009) and the laguna dos Patos system, and its homology should be tested in a phylogenetic framework.
Geographic distribution. Twelve species of Hisonotus are herein reported for the laguna dos Patos system. This diversity greatly surpasses that presented by other hydrographic basins of comparable size. As commented previously by Carvalho et al. (2008), Hisonotus is not the only high diverse genus of loricariids in that system, which indicates a species rich region. From the species of Hisonotus included in this review, only three are not endemic to the laguna dos Patos system (H. charrua, H. leucofrenatus, and H. nigricauda).
Hisonotus nigricauda has a wide distribution compared to other congeners. The species is present in the marshlands of the rio Vacacaí drainage and rio Ibicuí headwaters. The watershed of these drainages is located in a flat region separated from each other by no more than a few hundred meters. During periods of floodings, both drainages maybe connected permitting fish, which lives in these marsh habitats, to disperse from one to another. Recently, several fishes pertaining to rio Uruguay basin (e. g., Acestrorhynchus pantaneiro; Saccol-Pereira et al., 2006) were caught in the rio Jacuí drainage. The probable cause of these recent introductions is the connection of these two drainages by irrigation channels used in the rice culture. Since Hisonotus nigricauda has a wide distribution in the system, and syntypes collection predates the extensive rice culture in Rio Grande do Sul, the human-influenced introduction hypothesis is rejected. Therefore its dispersal is likely to be part of a natural event.
Hisonotus charrua is known in the laguna dos Patos system in two localities in the headwaters of rio Jaguarão and rio São Gonçalo drainages. The distribution of H. charrua is puzzling, being widely distributed in the rio Uruguay basin contrasting with a restricted distribution in the laguna dos Patos system. The headwaters of the rio Piratiní and Jaguarão are close together to the headwaters of the rio Negro, a tributary of the rio Uruguay, so that a stream capture event between those drainages may be invoked as the responsible for the interchange of H. charrua from rio Uruguay basin to laguna dos Patos system. This suggests that dispersal through stream capture events, without subsequent dispersion may be frequent. Additional samples from the headwater fish fauna of those drainages are necessary for a better understanding of the complex faunal pattern of these regions.
Several species of Hisonotus presented in this review are endemic to the headwaters of the rio Jacuí basin. Two of them (H. carreiro and H. prata) are collected in fast flowing watercourses of high altitudes, which are uncommon habitats to Hisonotus. Both can be caught syntopically with species of Eurycheilichthys. That genus is endemic to southern Brazil and restricted to the high altitudes of the Serra Geral formation in Rio Grande do Sul and Santa Catarina States (Reis & Schaefer, 1998), presenting a great species diversity in the rio Taquari drainage (Liedke, 2006; Reis & Carvalho, 2007). The headwaters of the Taquari basin present a high declivity where most species are isolated from each other by waterfall barriers. For both Eurycheilichthys and Hisonotus the mountain relief on which the Taquari drainage flows is responsible for dividing species distribution. This allopatric distribution is observed in Hisonotus carreiro, whose distribution in the rio Carreiro drainage is limited to the upper reaches upstream the waterfall Salto do Carreiro. The distribution of some species of Hisonotus is coincident with that presented by some Eurycheilichthys. Hisonotus brunneus can be collected with E. limulus in the upper rio Jacuí drainage, as well as H. carreiro and H. prata are sympatric with two different undescribed species of Eurycheilichthys. The events, which promoted the distribution and alopatric diversification of both groups, are likely to be correlated.
Contrasting with the species of Hisonotus present in the headwaters of the rio Jacuí basin, a putative clade of the genus formed by H. laevior, H. notopagos, and H. taimensis is distributed in relatively lower portions of the laguna dos Patos system. Along with the fact that these species are not alopatric, the lower portions of the laguna dos Patos system do not present clear geographical barriers. Hisonotus laevior and H. taimensis are morphologically very similar to each other and can be differed only by morphometric features of a relatively more elongated and lower body of the latter species. A similar body form is observed between Rineloricaria cadeae and R. longicauda, also occurring in the laguna dos Patos system, that are distinguished only by morphometric traits also related to a lower body shape of the later (cf. Rodriguez & Reis, 2008). Geographic distributions of H. taimensis and R. longicauda species are mostly overlapping, with species inhabiting the coastal plains of southern Brazil. That similarity in body shape seems to play a significant role in the adaptation for lowland habits for both H. taimensis and R. longicauda. In the same manner, Heptapterus sympterigium, which is distributed throughout the coastal lowlands of eastern Rio Grande do Sul State (Buckup, 1988), present some morphological specializations compared to its relative, Hepapterus mustelinus, which inhabits the upstream portions of the laguna dos Patos system. The eastern coastal plain of laguna dos Patos system is a relatively recent terrain (Schwarbold & Schäfer, 1984), therefore the speciation events of species inhabiting this portion should postdate the pleistocene formation of the coastal plain. Malabarba & Isaia (1992) recorded several species shared between the lower portions of rio Tramandai system and laguna dos Patos system, and discussed the relationships between those areas. According to them the dispersion between the laguna dos Patos system and the rio Tramandaí system was the main source of the fish fauna of coastal plain. Another Hisonotus present in this region is H. leucofrenatus. Widely distributed in the lowlands of coastal drainages in São Paulo, Paraná, Santa Catarina and Rio Grande do Sul States, its presence in the laguna dos Patos system is likely to be due to dispersion through the coastal plain of southern Brazil. According to Schwarbold & Schäfer (1984), the eastern coastal plain in southern Brazil was formed during the pleistocene glaciations. In that period, the laguna Mirim and laguna dos Patos were formed as freshwater habitats. The presence of H. leucofrenatus in the laguna dos Patos system is relatively recent, since it area of distribution in the eastern laguna dos Patos coastal plain was submerged by seawater during the Sangamon interglacial period (80.000 years). As demonstrated by Hisonotus, the fish fauna of the laguna dos Patos system seems to have a hybrid nature, sharing elements from rio Uruguay basin, as well as from coastal Atlantic drainages of southeastern Brazil.
Comparative material.Epactionotus bilineatus: MCP 29293, 29 + 3 c&s, Brazil, Rio Grande do Sul, arroio das Bananeiras. Eurycheilichthys pantherinus: MCP 35042, 17 + 3 c&s, Brazil, Rio Grande do Sul, rio dos Touros on road from Rondinha to Silveira. Lampiella gibosa: MCP 31588, 1 + 1 c&s, Brazil, São Paulo, rio Bonito tributary to rio Pardo. Eurycheilichthys sp. 1: MCP 40973, 1, Brazil, Rio Grande do Sul, arroio Guabiju. Eurycheilichthys sp. 2: MCP 35062, 25, Brazil, Rio Grande do Sul, rio da Prata at Passo do Respraiado. Hisonotus aky: AI 124, holotype of Epactionotus aky, Argentina, Missiones, arroyo Garibaldi. AI 125, 7 + 1 c&s, paratypes of Epactionotus aky, collected with the holotype. UFRGS 10802, 3, 34.7-41.1 mm SL, Brazil, Rio Grande do Sul, Entre Ijuis, rio Ijuizinho. Hisonotus chromodontus: INPA 29097, 17, Brazil, Mato Grosso, Juruena, rio Arinos. MCP 35873, 194 + 5 c&s, Brazil, Mato Grosso, rio Sauê-Uina on highway BR-364. Hisonotus depressicauda: MCP 20100, 2, Brazil, São Paulo, rio Taiaçupeba near eletrical station of Tijuco Preto, tributary of rio Tiête drainage. Hisonotus depressinotus: MZUSP 86167, 6, Brazil, Paraná, creek tributary to rio Tibagi. Hisonotus francirochai: MZUSP 3258, lectotype of Otocinclus francirochai, Brazil, São Paulo, creeks by Pirangy, headwaters of rio Turvo. MCP 41341, 4, Brazil, São Paulo, stream tributary of rio Mogi-Guaçu. Hisonotus insperatus: MZUSP 78957, holotype, Brazil, São Paulo, rio Capivara. MZUSP 78958, 5, collected with the holotype. MZUSP 78966, 7, paratypes, Brazil, São Paulo, rio Capivara. Hisonotus luteofrenatus: MCP 32670, 9 + 1 c&s, Brazil, Mato Grosso, igarapé Ribeirão Preto on highway MT-338. MCP 32666, 2, Brazil, Mato Grosso, rio Azul on highway MT-140. INPA 7307, 5, Brazil, Pará, Pimental, rio Tapajós. Hisonotus cf. maculipinnis: BMNH 1934.8.20.321-5, San Lorenzo, Paraguay. BMNH 1934.8.20:220240, +500, Paraguay, near Asuncion. Hisonotus maculipinnis: BMNH 1909.4.2.19-22, syntypes of Otocinclus maculipinnis, La Plata. ANSP 187011, 507, Argentina, Corrientes, side channels and backwaters of río Paraná and lower río Guayquiraro, about 25 km south from Esquina. ILPLA 235, 6 + 1, Argentina, Corrientes, arroyo Batel. MACN 3240, +50, Argentina, Buenos Aires. MACN 7594, +50, Argentina Santa Fé, arroyo Ciquenã. Hisonotus notatus: BMNH 1904.1.28.13-16; MNRJ 28882, 3; NWM 45380-4, NMW 45380-7, NMW 45381-6, NMW 45381-4; ANSP 166924, 6; all syntypes, Brazil, Rio de Janeiro, rio Grande (arroio Fundo) on fazenda Santa Cruz. MCP 18098, 204 + 4 c&s, Brazil, Espírito Santo, rio São José das Torres on road BR-101. Hisonotus paulinus: BMNH 1907.7.6.9, holotype of Otocinclus paulinus, Brazil, São Paulo, rio Piracicaba. Hisonotus ringueleti: ILPLA 886, holotype, Uruguay, Rivera, creek at km 18 of route joining Santana do Livramento to Rivera. ILPLA 883, 95, and MLP 9536, 4, paratypes, collected with the holotype. ZVC-P 5595, holotype of Hisonotus candombe, Uruguay, Salto, arroyo Palomas. MCP 11215, 128 + 4 c&s, Brazil, Quaraí, arroio Quaraí Mirim on road between Quaraí and Alegrete. Hisonotus yasi: AI 159, 4, paratypes of Epactionotus yasi, Argentina, Missiones, arroyo Lobo. NUP 790, 15, Brazil, Paraná, Caxias reservoir. UFRGS 4187, 2, Brazil, Paraná, Ampére, small creek tributary to rio dos Macacos. Microlepidogaster perforatus: MCP 17717, 4 + 1 c&s, Brazil, Minas Gerais, rio Carandaí. Otothyropsis marapoama: MCP 38303, paratypes, 9 + 1 c&s, Brazil, São Paulo, córrego Cubatão. Otocinclus flexilis: MCP 17414, 11 + 2 c&s, Brazil, Rio Grande do Sul, Capão do Leão, arroio Itaetá at Passo das Pedras. Parotocinclus maculicauda: MCP 31591, 50 + 4 c&s, Brazil, São Paulo, rio Bonito tributary to rio Pardo. MCP 20087, 17, Brazil, Paraná, Cerro Azul, arroio Ribeirão Bonito.
Acknowledgements
We would like thank the following people for their help and support while visiting their institutions and/or for the loan of specimens: M. Sabaj-Pérez and J. Lundberg (ANSP), J. Maclaine (BMNH), M. Cheffe, A. Miquelarena (ILPLA), G. Chiaramonte and F. Firpo (MACN), F. Meyer (MAPA), M. Azevedo (MCN), M. Azpelicueta (MLP), P. Buckup and M. Britto (MNRJ), O. Oyakawa (MZUSP), H. Wellendorf (NMW), C. Pavanelli (NUP), J. Ferrer, G. Frainer and L. Malabarba (UFRGS), L. Py-Daniel and M. Rocha (INPA). We are grateful to F. Mayer, J. Verba, J. Wingert, C. Lucena and M. Lucena for support at the MCP collection. Thanks also to V. Bertaco, J. Wingert, C. Cramer, and especially to A. Cardoso for help in collecting Hisonotus in field trips. Thanks to L. Lanés and P. Lehmann for providing Hisonotus material from southeastern Rio Grande do Sul State. Thanks to L. Malabarba and J. Pezzi for providing photos of live specimens of H. vireo and H. armatus, respectively. Thanks to R. M. F. Alberto for the identification of the isopod in Fig. 37 . We are grateful to the Centro de Microscopia e Microanálises - CEMM, PUCRS for the SEM preparations. M. Azpelicueta, M. Britto, and J. Armbruster were members of the master's thesis committee of TPC and contributed with their corrections and suggestions for this work. This paper was financially supported by the "All Catfishes Species Inventory" Project (NSF DEB 0315963) that provided funding to visit museum collections. Thanks are also due to the Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq, for a studentship to TPC (process #132879/2006-9). RER is partially financed by CNPq (process #303362/2007-3). This work was part of first author's Master in Sciences dissertation at the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), titled taxonomic review of the species of Hisonotus (Siluriformes: Loricariidae) from the rio Uruguay basin and the laguna dos Patos system.
Literature Cited
Accepted November 26, 2010
Published March 31, 2011
- Abell, R., M. L. Thieme, C. Revenga, M. Bryer, M. Kottelat, N. Bogutskaya, B. Coad, N. Mandrak, S. Contreras Balderas, W. Bussing, M. L. J. Stiassny, P. Skelton, G. R. Allen, P. Unmack, A. Naseka, R. Ng, N. Sindorf, J. Robertson, E. Armijo, J. V. Higgins, T. J. Heibel, E. Wikramanayake, D. Olson, H. L. Lopez, R. E. Reis, J. G. Lundberg, M. H. Sabaj Pérez & P. Petry. 2008. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. Bioscience, 58: 403-414.
- Almirón, A. E., M. M. Azpelicueta, J. R. Casciotta & T. Litz. 2006. A new species of Hisonotus (Siluriformes, Loricariidae, Otothyrini) from the Republica Oriental de Uruguay. Revue suisse de Zoologie, 113: 87-94.
- Andreata, A. A., C. Oliveira & F. Foresti. 2006. Karyological characterization of four Neotropical fish species of the genus Hisonotus (Teleostei, Loricariidae, Hypoptopomatinae) from distinct Brazilian river basins. Genetics and Molecular Biology, 29: 62-66.
- Aquino, A. E., S. A. Schaefer & A. M. Miquelarena. 2001. A new species of Hisonotus (Siluriformes, Loricariidae) of the Upper Río Uruguay Basin. American Museum Novitates, 3333: 1-12.
- Azpelicueta, M. M., A. E. Almirón, J. R. Casciotta & S. Koerber. 2007. Hisonotus hungy sp. n. (Siluriformes, Loricariidae) a new species from arroyo Tirica, Misiones, Argentina. Revue suisse de Zoologie, 114: 591-598.
- Azpelicuta, M. M., J. R. Casciotta, A. E. Almirón & S. Koerber. 2004. A new species of Otothyrini (Siluriformes: Loricariidae: Hypoptopomatinae) from the Río Uruguay basin, Argentina. Verhandlungen der Gesellschaft für Ichthyologie, 2004: 81-90.
- Bemvenuti, M. A. & A. Moresco. 2005. Peixes: áreas de banhados e lagoas costeiras do Extremo Sul do Brasil. Editora ABRH, Porto Alegre, Brazil.
- Boulenger, G. A. 1891. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proceedings of the Zoological Society of London, 1891: 231-235.
- Brannan, T. B., L. J. Althof, J. Jacobs, J. Norby & S. Rubenstein. 2000. SigmaPlot. Exact Graphics For Exact Science. Version 6.1 for Windows. SPSS.INC.
- Britski, H. A. & J. C. Garavello. 2003. Hisonotus insperatus: New species from the upper Rio Paraná Basin (Pisces: Ostariophysi: Loricariidae). Copeia, 2003: 588-593.
- Bristski, H. A. & J. C. Garavello. 2007. Description of two new sympatric species of the genus Hisonotus Eigenmann & Eigenmann, 1889 from the upper rio tapajós, Mato Grosso state, Brazil (Pisces: Ostariophysi: Loricariidae). Brazilian Journal of Biology, 67: 631-637.
- Buckup, P. A. 1981. Microlepidogaster taimensis sp. n., novo Hypoptopomatinae da Estação Ecológica do Taim, Rio Grande do Sul, Brasil (Ostariophysi, Loricariidae). Iheringia, 60: 19-31.
- Buckup, P. A. 1988. The genus Heptapterus (Teleostei, Pimelodidae) in southern Brazil and Uruguay with the description of a new species. Copeia, 1988: 641-653.
- Buckup, P. A. & L. R. Malabarba. 1983. A list of the fishes of the Taim Ecological Station, Rio Grande do Sul, Brazil. Iheringia, 63: 103-113.
- Carvalho, T. P., P. A. Lehmann, E. H. L. Pereira & R. E. Reis. 2008a. A new species of Hisonotus (Siluriformes: Loricariidae: Hypoptopomatinae) from the Laguna dos Patos Basin, Southern Brazil. Copeia, 2008: 510-516.
- Carvalho, T. P., P. A. Lehmann, E. H. L. Pereira & R. E. Reis. 2008b. Gymnotocinclus anosteos, a new uniquely-plated genus and species of loricariid catfish (Teleostei: Siluriformes) from the upper rio Tocantins basin, central Brazil. Neotropical Ichthyology, 6: 329-338.
- Carvalho, T. P. & R. E. Reis. 2009. Four new species of Hisonotus (Siluriformes: Loricariidae) from the upper rio Uruguay, southeastern South America, with a review of the genus in the rio Uruguay basin. Zootaxa, 2112: 1-40.
- Casciotta J. R., M. M. Azpelicueta, A. E. Almirón & T. Litz. 2006. Hisonotus candombe, a new species from the rio Uruguay basin in the Republica Oriental del Uruguay. Spixiana, 29: 147-152.
- Chiachio, M. C., C. Oliveira & J. I. Montoya-Burgos. Molecular systematic and historical biogeography of the armored Neotropical catfishes Hypoptopomatinae and Neoplecostominae (Siluriformes: Loricariidae). Molecular Phylogenetics and Evolution, 49: 606-617.
- Cramer, C. A., A. M. R. Liedke, S. L. Bonatto & R. E. Reis. 2007. Phylogenetic relationships of the Hypoptopomatinae and Neoplecostominae (Siluriformes: Loricariidae) as inferred from mitochondrial cytochrome c oxidase I sequences. Bulletin of Fish Biology, 9: 51-59.
- Cope, E. D. 1894. On the Fishes obtained by the Naturalist Expedition in Rio Grande do Sul. Proceedings of American Philosophical Society of Philadelphia, 33: 84-108.
- Eigenmann, C. H. 1910. Catalogue of the fresh-water fishes of tropical and south temperate America. Pp. 375-511. In: Reports of the Princeton University expeditions to Patagonia 1896-1899, Zoology, vol. 3. Princeton University, 632p.
- Eigenmann, C. H. & R. S. Eigenmann. 1889. Preliminary notes on South American Nematognathi. 2. Proceedings of the California Academy of Sciences, 1: 119-172.
- Fowler, H. W. 1915. Notes on nematognathous fishes. Proceedings of the Academy of Natural Sciences of Phildelphia, 67: 203-243.
- Fowler, H. W. 1940. Zoological resultsof the second Bolivian expedition for the Academy of natural Sciences of Philadelphia 1936-1937. Proceedings of the Academy of Natural Sciences of Phildelphia, 92: 43-103.
- Fowler, H. W. 1954. Os peixes de água doce do Brasil (4Ş entrega). Arquivos de Zoologia do Estado de São Paulo, 9: 1-400.
- Ferraris, C. J. 2007. Checklist of Catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa, 1418: 1-628.
- Gauger, M. F. W. & P. A. Buckup. 2005. Two new species of Hypoptopomatinae from rio Paraíba do Sul basin, with comments on the monophyly of Parotocinclus and Otothyrini (Siluriformes: Loricariidae). Neotropical Ichthyology, 3: 509-518.
- Gomes, A. L. 1947. A small collection of fishes from Rio Grande do Sul Brazil. Miscellaneous Publications of the University of Michigan Museum of Zoology, 67: 1-39.
- Gosline, W. A. 1945. Catálogo dos nematognatos de água-doce da América do Sul e Central. Boletim do Museu Nacional do Rio de Janeiro, 33: 1-138.
- Grosser, K. M., W. R. Koch & S. Drügg-Hahn. 1994. Ocorrência e distribuição de peixes na estação ecológica do Taim, Rio Grande do Sul, Brasil (Pisces, Teleostomi). Iheringia, 77: 89-98.
- Isbrücker, I. J. H. 1980. Classification and catalogue of the mailed Loricariidae (Pisces, Siluriformes, Loricariidae). Verslagen em Techniche Gegevens, 22: 1-170.
- Liedke A. M. R. 2006. Filogenia e Filogeografia gênero Eurycheilichthys (Siluriformes: Loricariidae. Unpusblished Master Thesis. Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil.
- Lucena, C. A. S., A. S. Jardim & E. S. Vidal. 1994. Ocorrência, distribuição e abundância da fauna de peixes da praia de Itapuã, Viamão, Rio Grande do Sul, Brasil. Comunicações do Museu de Ciências e Tecnologia da PUCRS, 7: 3-27.
- Malabarba, L. R. 1989. Histórico sistemático e lista comentada das espécies de peixes de água doce do sistema da laguna dos Patos, Rio Grande do Sul, Brasil. Comunicações do Museu de Ciências da PUCRS, 2: 107-179.
- Malabarba, L. R. & E. A. Isaia. 1992. The fresh water fish fauna of the rio Tramandaí drainage, Rio Grande do Sul, Brazil, with a discussion of its historical origin. Comunicações do Museu de Ciências da PUCRS, 5: 197-223.
- Menezes, N. A., S. H. Weitzmann, O. T. Oyakawa, F. C. T. Lima, R. M. C. Castro & M. J. Weitzmann. 2007. Peixes de água doce da Mata Atlântica. Museu de Zoologia/USP, São Paulo, Brazil. 407p.
- Miranda-Ribeiro, A. 1908. Peixes da Ribeira. Resultados de excursão do Sr. Ricardo Krone, membro correspondente do Museu Nacional do Rio de Janeiro. Kosmos, 5: 1-5.
- Miranda-Ribeiro, A. 1911. Fauna brasiliense. Peixes. Tomo IV (A) [Eleutherobranchios Aspirophoros]. Arquivos do Museu Nacional Rio de Janeiro, 16: 1-504.
- Oyakawa, O. T., A. Akama, K. C. Mautari & J. C. Nolasco. 2006. Peixes de riachos da Mata Atlântica nas Unidades de Conservação do Vale do Rio Ribeira de Iguape no Estado de São Paulo. Editora Neotrópica, São Paulo, Brazil, 201p.
- Papavero, N. 1973. Essays on the history of Neotropical Dipterology, with special reference to collectors (1750-1905), V. II. Museu de Zoologia Universidade de São Paulo, São Paulo, Brazil.
- Regan, C. T. 1904. A monograph of the fishes of the family Loricariidae. Transactions of the Zoological Society of London, 17: 191-350.
- Reis, R. E. & T. P. Carvalho. 2007. Hypoptopomatinae. Pp. 83-84. In: Buckup, P. A., N. A. Menezes & M. S. Ghazzi (Eds.). Catálogo das espécies de peixes de água doce do Brasil. Museu Nacional (Série Livros), Rio de Janeiro, 195p.
- Reis, R. E. & S. A. Schaefer. 1992. Eurycheilichthys pantherinus (Siluriformes: Loricariidae), a new genus and species of Hypoptopomatinae from Southern Brazil. Copeia, 1992: 215-223.
- Reis, R. E. & S. A. Schaefer. 1998. New cascudinhos from southern Brazil: systematics, endemism, and relationships (Siluriformes, Loricariidae, Hypopotopomatinae). American Museum Novitates, 3254: 1-25.
- Retzer, M. E., L. G. Nico & F. R. Provenzano. 1999. Two new species of Acestridium (Siluriformes: Loricaridae) from southern Venezuela, with observations on camouflage and color change. Ichthyological Exploration of Freshwaters, 10: 313-326.
- Ribeiro, M. F., A. Köhler, A. Dupont & E. C. G. Azevedo. 2007. Os peixes do rio Pardinho. Santa Cruz do Sul, EdUnisc.
- Rodriguez, M. S. & R. E. Reis. 2008. Taxonomic review of Rineloricaria (Loricariidae: Loricariinae) from the Laguna dos Patos drainage, Southern Brazil, with the descriptions of two new species and the proposition of two species groups. Copeia, 2008: 333-349.
- Ryan, P. D., D. A. T. Harper & J. S. Whalley. 1995. PALSTAT, Statistics for paleontologists. Chapman & Hall, London, England.
- Saccol-Pereira, A., P. C. C. Milani & C. B. Fialho. 2006. First record of Acestrorhynchus pantaneiro Menezes 1992 (Characiformes, Acestrorhynchidae) in the system of the laguna dos Patos, Rio Grande do Sul, Brazil. Biota Neotropica, 6: 1-4.
- Schaefer, S. A. 1991. Phylogenetic analysis of the loricariids subfamily Hypoptopomatinae (Pisces: Siluroidei: Loricariidae), with comments on generic diagnoses and geographic distribution. Zoological Journal of the Linnean Society, 102: 1-41
- Schaefer, S. A. 1997. The Neotropical cascudinhos: Systematics and biogeography of the Otocinclus catfishes (Siluriformes: Loricariidae). Proceedings of the Academy of Natural Sciences of Philadelphia, 148: 1-120.
- Schaefer, S. A. 1998. Conflict and resolution: Impact of new taxa on phylogenetic studies of the neotropical cascudinhos (Siluriformes: Loricariidae). Pp. 375-400. In: Malabarba, L. R., R. E. Reis, R. P. Vari, C. A. S. Lucena & Z. M. S. Lucena (Eds.). Phylogeny and Classification of Neotropical Fishes. Porto Alegre, Edipucrs, 603p.
- Schaefer, S. A. 2003. Loricariidae - Hypoptopomatinae (Armored catfishes). Pp. 321-329. In: Reis, R. E., S. O. Kullander & C. J. Ferraris, Jr. (Eds.). Checklist of Freshwater Fishes of the South and Central America. Porto Alegre, Edipucrs, 729p.
- Schwarzbold, A. & A. Schäfer, 1984. Gênese e Morfologia das Lagoas Costeiras do Rio Grande do Sul - Brasil. Amazoniana, 9: 87-104.
- Strauss, R. E. 1985. Evolutionary allometry and variation in body form in the South American catfish genus Corydoras (Callichthyidae). Systematic Zoology, 34: 381-396.
- Taylor, W. R. & G. C. van Dyke. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium, 9: 107-119.
Publication Dates
-
Publication in this collection
24 May 2011 -
Date of issue
Mar 2011
History
-
Received
26 Nov 2010 -
Accepted
31 Mar 2011