Abstract
Enterotoxigenic Escherichia coli (ETEC) strains are leading causes of childhood diarrhea in developing countries. Adhesion is the first step in pathogenesis of ETEC infections and ETEC pili designated colonization factor antigens (CFAs) are believed to be important in the biofim formation, colonization and host cell adhesions. As a first step, we have determined the biofilm capability of ETEC expressing various types of pili (CFA/I, CfaE-R181A mutant/ CfaE tip mutant, CFA/II and CS2). Further, enzyme-linked immunosorbent assay (ELISA) assay were developed to compare the binding specificity of CFA/I, CFA/II (CS1 - CS3) and CS2 of ETEC, using extracted pili and piliated bacteria. CFA/II strain (E24377a) as well as extracted pili exhibited significantly higher binding both in biofilm and ELISA assays compared to non piliated wild type E24377a, CFA/I and CS2 strains. This indicates that co-expression of two or more CS2 in same strain is more efficient in increasing adherence. Significant decrease in binding specificity of DH5αF'lacIq/∆cotD (CS2) strain and MC4100/pEU2124 (CfaE-R181A) mutant strain indicated the important contribution of tip proteins in adherence assays. However, CS2 tip mutant strain (DH5αF'lacIq/pEU5881) showed that this specific residue may not be important as adhesions in these strains. In summary, our data suggest that pili, their minor subunits are important for biofilm formation and adherence mechanisms. Overall, the functional reactivity of strains co expressing various antigens, particularly minor subunit antigen observed in this study suggest that fewer antibodies may be required to elicit immunity to ETEC expressing a wider array of related pili.
ETEC; CFA/I; CFA/II; CS2; Biofilm formation; ELISA assays; asialo-GM1; R181-cotD; dsc19CotD(His)6
MEDICAL MICROBIOLOGY
Biofilm formation and binding specificities of CFA/I, CFA/II and CS2 adhesions of Enterotoxigenic Escherichia coli and CfaE-R181A mutant
Iram LiaqatI* * Corresponding Author. Mailing address: Department of Zoology, Government College University, Lahore, Pakistan.; E-mail: iramliaq@hotmail.com
IDepartment of Zoology, Government College University, Lahore, Pakistan
ABSTRACT
Enterotoxigenic Escherichia coli (ETEC) strains are leading causes of childhood diarrhea in developing countries. Adhesion is the first step in pathogenesis of ETEC infections and ETEC pili designated colonization factor antigens (CFAs) are believed to be important in the biofim formation, colonization and host cell adhesions. As a first step, we have determined the biofilm capability of ETEC expressing various types of pili (CFA/I, CfaE-R181A mutant/ CfaE tip mutant, CFA/II and CS2). Further, enzyme-linked immunosorbent assay (ELISA) assay were developed to compare the binding specificity of CFA/I, CFA/II (CS1 - CS3) and CS2 of ETEC, using extracted pili and piliated bacteria. CFA/II strain (E24377a) as well as extracted pili exhibited significantly higher binding both in biofilm and ELISA assays compared to non piliated wild type E24377a, CFA/I and CS2 strains. This indicates that co-expression of two or more CS2 in same strain is more efficient in increasing adherence. Significant decrease in binding specificity of DH5αF'lacIq/∆cotD (CS2) strain and MC4100/pEU2124 (CfaE-R181A) mutant strain indicated the important contribution of tip proteins in adherence assays. However, CS2 tip mutant strain (DH5αF'lacIq/pEU5881) showed that this specific residue may not be important as adhesions in these strains. In summary, our data suggest that pili, their minor subunits are important for biofilm formation and adherence mechanisms. Overall, the functional reactivity of strains co expressing various antigens, particularly minor subunit antigen observed in this study suggest that fewer antibodies may be required to elicit immunity to ETEC expressing a wider array of related pili.
Key words: ETEC; CFA/I; CFA/II; CS2; Biofilm formation; ELISA assays; asialo-GM1; R181-cotD; dsc19CotD(His)6
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) are leading causes of diarrhea in children living in developing countries and the most common cause of traveler's diarrhea (4). Infections with ETEC require proper adhesion of bacteria to the intestinal epithelium. This first step in the pathogenesis of ETEC diarrhea is mediated through specific surface structures called pili or fimbriae (11), which allow the bacteria to colonize the intestinal mucosa of small intestine. Hitherto, a large number of colonization factor antigens (CFAs) have been identified (27), which include CFA/I, CFA/II, CFA/III and CFA/IV, and a number of coli surface antigens (CS). CFA/I and CFA/III are rod like fimbrial antigens with diameters of 68 nm (12, 15). The CFA/II and CFA/IV are composed of antigenically distinct structures, of which CS1, CS2, and CS3 belong to CFA/II and CS4, CS5, and CS6 belong to CFA/IV (5, 15). Whereas CS1 and CS2 are morphologically similar to CFA/I, CS3 was shown to consist of fine fibrils with a diameter of 23 nm.
CS1 pili are composed almost entirely of the major pilin called CooA. A minor pilin, CooD, is found only at the pilus tip contributing only one subunit per pilus. CooD is essential for the transport of CooA across the outer membrane, and the level of CooD expression determines the number of assembled pili on cell surface, hence indicating its significance for CS1 pilus assembly initiation (24). Modeling of CFA/I has placed the CfaB subunits in a recurring interaction pattern with the tip protein at the end acting as an adhesion, resulting in a helical structure (19). While conflicting data exists supporting a role for CfaB and/or CfaE in mediating specific target cell binding, convincing results from recent studies indicate CfaE as critical binding subunit. In vitro studies of CFA/I fimbriae expressed in DH5α suggested CfaE mediated haemagglutination. When CFA/I was expressed in DH5α with a single-amino-acid mutation R181A in CfaE resulted in haemagglutination without affecting pilus assembly (24).
Hemagglutination has also been proven useful in the determination of the colonization factor (CF) carbohydrate receptor specificity. The CF and the receptor molecules act as logical targets for inhibiting the interaction between pathogen and host cell. Thus, the use of CF, CF analogs, receptors, or receptor analogs could prevent adherence of pathogens (26). Alternatively, antibody to CF could prevent the initial attachment to the host cell. The glycosphingolipid receptor candidates for P. aeruginosa adhesions include gangliotetraosyl ceramide (asialo-GM), gangliotriaosyl ceramide (asialo-GM2), lactosylceramide and sialic acid-containing glycosphingolipids (2). However, as for the majority of the receptors for adhesive factors of the enterovirulent bacteria, the intestinal receptors for the adhesive factors of ETEC remain unknown. Little is known about receptors specific for CS1 and CFA/I strains. Some studies have shown that the latter bind to sialic acid-containing glyconjugates (29). In erythrocytes, CFA/I (26), can recognize sialic acid, sialic acid-containing glycopeptides (8), the GM2-like glycoconjugate or the asialo-GM1 (2, 3). An analogy between the carbohydrate specificities of the CFA/I and CFA/II adhesins was observed, since hemagglutination inhibition is successfully obtained using same preparations of complex carbohydrates (20). The ETEC adhesins utilizing these glycosphingolipids as receptors have not been unambiguously identified. It has been reported previously that this binding activity in CFA/I is exclusively mediated by the minor pilin subunit (24). Colonization factors also enable ETEC to adhere and colonize resulting in biofilm formation (22) Biofilm-formation in ETEC strains is responsible for many serious infections in patients with indwelling bladder catheters and bowel diseases (17). Also intracellular biofilm-like aggregates formed inside bladder cells by these strains, make them hard to reach by both host defence mechanisms and antibiotics (1).
Hence, in this background, very first objective of our study was to examine the biofilm-forming capacity of ETEC strains expressing various types of pili (CS2, CFA/I, CFA/II (CS1-CS3) on abiotic surfaces. Notably, we have probed the ability of MC4100/pJGX15W (CFA/I) to compete with MC4100/pEU2124 (CfaE-R181A) mutant piliated strains during biofilm formation.
Also we showed that ETEC pili can utilize asialo-GM1, as a receptor. Hence, we tested the binding of piliated recombinant bacteria with asialo-GM1. Additionally we observed that interactions between CFA/I pili and the asialo-GM, receptor occur at the tip of the pili. The rationale for this approach is the known ability of CFA/I and CFA/II expressing recombinant ETEC bacteria and tip mutants to attach to both human and rabbit intestinal mucosae (29). Whilst the previous studies suggested involvement of R181A in the minor, tip-associated pilin, critical for hemagglutination of bovine erythrocytes and reported it a conserved feature in CS1 and CFA/I strains (23, 24), we found that site directed mutagenesis at R181 point of DH5αF'lacIq/pEU588 (called as DH5αF'lacIq/pEU5881 in this study) minor pilin subunit has resulted in no effect on bovine haemagglutination. Further, to investigate whether minor pilin subunit of CS2 pili may contribute amino acid residues to a combined pilin receptor-binding site, we generated a self complimented CotD using the same strategy described for CfaE and CfaB from the CFA/I system (21). Our aim was to compare its binding specificity to bovine erythrocytes compared to purified CS2 pili. However, work is in progress in our lab to express it in stable, naturally-folded form and compare its binding specificity with that of purified CS2 pili.
MATERIALS AND METHODS
Bacterial strains and growth conditions
ETEC K12 strains MC4100, MC4100/pJGX15W (cfaABCE), MC4100/pEU2124 (cfaABCE1) (24); DH5αF'lacIq/pEU588 and DH5αF'lacIq/pBAD33-cotD were grown in LuriaBertani (LB) medium without or with appropriate antibiotics at concentrations as follows. Antibiotics added to liquid or solid media were ampicillin (Ap) 100 μg ml−1, chloramphenicol (Cm) 50 μg ml−1, or tetracycline (Tc) 20 μg ml−1. CF expression of ETEC E24377a (CS1+CS3+LT+ST+) was obtained by growing in CFA medium (Becton Dickinson, Franklin Lakes, NJ). For solid media, 2% agar was added. Plasmids used in this study are listed in Table1.
Antisera
Rabbit anti CFA/I pili antibody was custom made by immunizing a rabbit (Millipore Corporation, Millipore)with crude CFA/I pili extracted from MC4100/pJGX15W (cfaABCE). Nonspecific antibodies were removed by adsorption with sonicated MC4100/pJGX15W. Antisera against CS2 pili was available from previous studies in the lab.
Biofilm assay in microtitre plates
Biofilm formation of ETEC strains was monitored following Liaqat et al. (16). Briefly, Cells were grown for 18 h in respective media and two hundred fifty micro-litres were transferred to 25 ml fresh medium, transferred to 96-well flat-bottom microplates (Beckton Dickinson, USA), and incubated at 37ºC for 62, 117 and 170 h. Adhered cells were then stained with 0.1 % crystal violet for 30 min. Crystal violet was then solubilized by the addition of 33% glacial acetic acid and the OD563 was measured. Each strain was assayed in eight wells on each plate and all experiments were repeated three times.
Hemagglutination and piliation assays
For slide hemagglutination, 18 h old cultures were resuspended in PBS (0.24% Tris-HCl, 0.88% NaCl) pH 7.4, to give an OD600 of 10. In glass slides, 20 μl of bacterial suspension was mixed with 20 μl of TBS containing 0.1 M D-mannose and 20 μl of washed bovine erythrocyte suspension. For detection of pili by slide agglutination, 25 μl of bacterial suspension in TBS (OD600 of 10) was incubated for 1 min. at room temperature with 25 μl of anti-CS2 or anti CFA/I serum in glass slides (24). The degree of hemagglutination was observed visually based on the clump size and time of agglutination.
Extraction of pili, SDS-PAGE gel electrophoresis and Immunoblotting
CS2 pili from 18 h old LB cultures of DH5αF'lacIq/pEU588, CFA/I from MC4100/pJGX15W (cfaABCE), MC4100/pEU2124 (cfaABCE1) and CFA/II from E24377A were extracted using the method as described previously ( Sakellaris et al.24). Crude pili preparations from the extraction steps and normalized whole-cell lysates or heat extract were separated by SDS-PAGE gels. Samples of 10 μl were loaded on a 15% SDS polyacrylamide gel and electrophoresed at 160 V. Immunoblotting was carried out using polyclonal anti CS2 or anti CFA/I antibody at a dilution of 1:1,000 and goat anti-rabbit immunoglobulin G whole molecule (1:1,000) as secondary antibody. Blots were developed with BCIP/NBT (Roche) substrate according to manufacturer instructions.
ELISA assay for whole cell and pili binding
Polyvinyl chloride plates (Falcon; Becton Dickinson) were coated with washed erythrocytes (OD600=1) and incubated for 16 h at 4ºC. For asialo-GM1 (GgO4Cer) binding, solvent was suspended in methanol (5 μgml-1), added to wells of microtiter plates and allowed to evaporate for 18 h at room temperature. Wells were blocked with gelatin blocking buffer (Sigma, USA) for 1 h at RT and were washed five times with TBS. Blocking was followed by washing and 100 μl of two fold serially diluted bacterial suspensions (OD600=30 in the first well) or crude pili solution (50 μg of pili in the first well) was added. After wells were washed as previously described, goat pAb to E. coli in gelatin blocking buffer (1:5,000) (or for wells with fimbriae, anti-CS2/CFA/I [2nd bleed/Ist bleed respectively; 1:1,000) was added for 1 h at RT. Following another washing step, alkaline phosphatase-conjugated rabbit anti-goat immunoglobulin G whole molecule (Sigma) or goat anti-rabbit immunoglobulin G whole molecule (Sigma) in gelatin blocking buffer (1:1,000) was added for 1 h at 37ºC. Wells without erythrocytes, or without asialo-GM1 were used as controls. The bound enzyme was detected by the addition of alkaline phosphatase substrate (PNPP, Sigma 71768) diluted in diethanolamine buffer per well for 5 to 30 min at 37ºC. The control wells were treated in the same manner except that blank control wells had no bacteria or pili. Absorbance was measured at OD405 nm with a microplate reader (POLAR star Omega). Each experiment was repeated twice with six replicates per plate.
Site directed mutagenesis of CotD
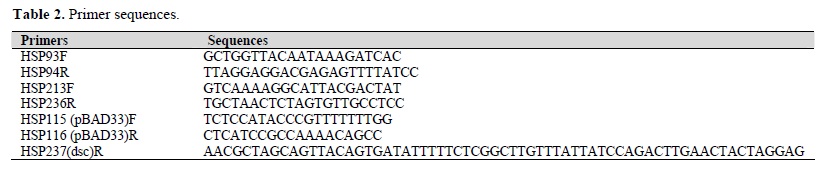
Site-directed mutagenesis of changing Arg-181 of CotD to Ala was performed with the template plasmid pEU588, and mutagenic primers (HSP213F, 236R). Plasmid pEU588 was digested with DpnI and purified using PCR purification kit (Roche Diagnostic, Germany), self ligated and transformed to DH5αF'lacIq. The presence of R181A mutation was confirmed by nucleotide sequencing of the entire CotD gene and of the region surrounding the directed mutation for R181 allele. Primers used are listed in Table 2.
Construction and expression of donor strand compliment CotD
Complementary primers (top strand) AACGCTAGCAGTTACAGTGATATTTTTCTCGGCTTGTTTATTATCCAGACTTGAACTACTAGGAG and bottom strand GATCCAACTATCGATCTGATGCAACATCATCATCATCATCATTAATGCGGGAGACTTTATCTGC which contain the coding sequence for a hairpin linker (DNKQ) followed by the first 19 residues of mature CotA, were used to amplify DH5αF'lacIq/pBAD33-cotD. The amplicon was digested with DpnI, ligated and transformed to DH5αF'lacIq following methods as described previously (24). Sequencing of entire DH5αF'lacIq/dsc19CfaE(His)6 was done to confirm the correct construction.
The strains expressing His6-tagged CotD was grown in LB medium with Cm50 at 37ºC to late logarithmic phase (OD600=0.8), followed by induction for 3 h by addition of 0.1-0.5% L-arabinose. Heat extracts were prepared by boiling at 65ºC for 20 minutes. After transfer to nitrocellulose, Immuno blot analysis was performed by chemiluminescence using monoclonal antipolyhistdine, antibody produced in mouse (1:2,000 dilution) and HRP labeled anti-mouse IgG as secondary antibody (1:5,000).
RESULTS
Biofilm formation studies
The biofilm-forming capacity of ETEC strains was assessed in microtitre plates by quantitative crystal violet staining. A range of ETEC strains including E24377a (CFA/II), DH5aF'lacIq/pEU588 (CS2), DH5aF'lacIq/pEU588∆CotD, MC4100/pJGX15W (CFA/I) and MC4100/pEU2124 (CfaE-R181A) were included for comparison. It transpired that among tested E. coli isolates, E24377a produced maximum biofilm after 60 hours, followed by DH5aF'lacIq/pEU588 and MC4100/pJGX15W. Significant decrease (P<0.05) in biofilm formation of MC4100/pJGX15W was observed compared to E24377a and DH5aF'lacIq/pEU588 after 117 hours (Figure 1a). The decreasing rate of biofilm formation observed in DH5aF'lacIq/pEU588 and MC4100/pJGX15W might correspond to presence of single type of fimbriae hence lowering their ability to adhere and form biofilm.
Having analysed the biofilm-forming capacity of a range of different piliated ETEC isolates, we proceeded with investigating whether after 60 hours recombinant piliated CFA/II, CS2 and CFA/I strains could outperform their respective non piliated CFA/II (E24377a grown in LB), CotD mutant (DH5aF'lacIq/pBAD33∆cotD) or CfaE-R181 mutant (MC4100/pEU2124) strains respectively. It was observed that piliated E24337a produced highly significant (P< 0.001) biofilm compared to non piliated E24337a strain. Also significant decrease in biofilm formation was observed in DH5aF'lacIq/pBAD33∆cotD and MC4100/pEU2124 respectively compared to recombinant wild type strains (Figure 1b).
SDS-PAGE and Immunoblotting of CS2, CFA/I and CFA/II pili
About 6.8, 3.1, 1.7 and 7.5 mgml-1 of crude pili from MC4100/pEU2124 (cfaABCE1); MC4100/pJGX15W (cfaABCE); DH5αF'lacIq/pEU588 and E24377a were obtained from 1 L of culture fluid. SDS-PAGE of the extracted ETEC pili showed band that migrated at a position corresponding to a molecular mass of 17 kDa (CFA/II and CS2) and 16kDA (CFA/I). Pili concentration was measured using Imaje J. Immunoblotting of the crude CFA/I recombinant and tip mutant pili fractions employing anti CFA/I serum revealed reacting protein bands at 16 kDa. Interestingly, CFA/II crude pili were found to be cross reacted with anti-CS2 serum (Data not shown).
Haemagglutination
CS2 pili expression on DH5αF'lacIq/pEU588 ETEC mediate specific adherence to bovine erythrocytes in a mannose-resistant manner. However, DH5αF'lacIq/pEU5881 clones bearing CotD-R181A mutants exhibited either strong or no haemagglutination activity, indicating that R 181A is not critical for formation of a receptor binding epitope on CotD of CS2 strains.
Expression of DH5αF'lacIq/dsc19CotD(His)6
Following Poole et al. (21), we constructed a plasmid that expresses a CotD variant containing a C-terminal extension consisting of a hairpin tetrapeptide linker followed by the 19 residue donor strand from the N terminus of mature CotA, and a terminal hexahistidine affinity tag. Restriction digestion and sequencing confirmed the correct sequence and validated accuracy of dsc19CotD(His)6. Immunoblotting of DH5αF'lacIq/dsc19CotD(His) showed an obvious band upon induction with 0.4% L-arabinose (Data not shown).
Mannose-resistant haemagglutination assay (MRHA) of DH5αF'lacIq/dsc19CotD(His) showed strong agglutination of bovine erythrocytes as well as anti CFA/I serum without induction on CFA media however on LB media agglutination was observed when induced with 0.4% L-arabinose.
Adherence of CS2, CFA/I and CFA/II pili and piliated bacteria with bovine erythrocytes and asialo-GM1
The ability of purified pili and piliated bacteria to bound with bovine erythrocytes and asialo-GM1 was studied in microtiter plate assay. Both pili and bacteria bound to erythrocytes and asialo-GM1 in a dose-dependent manner (Figure 2a, b). We found that wild-type E24377a bound abundantly to bovine erythrocytes and asialo-GM1. In contrast, non significant difference in binding capacity of MC4100/pJGX15W and DH5αF'lacIq/pEU588 was observed at OD600=5. However, highly significant decrease in binding capacity of DH5αF'lacIq/pEU588 was observed at OD600=2.5.
Binding assay of purified fimbriae and fimbriated bacteria with asialo-GM1 indicated binding in a concentration dependent manner with maximum binding observed at 30 μgml-1 in E24377a. DH5αF'lacIq/pEU588 and crude pili of this strain exhibited the lowest binding capacity (Figure 3a, b). Together, this data strongly suggest that the carbohydrate residues of glycolipids may be the binding site for piliated bacteria.
Adherence of CFA/I and CfaE-R181A mutant piliated bacteria and pili with bovine erythrocytes and asialo-GM1
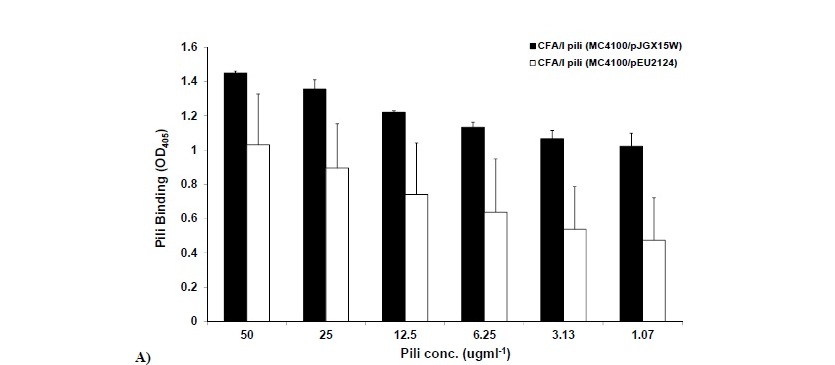
The adherence assays were performed employing MC4100/pJGX15W (CFA/I) and Mc4100/pEU2124 (CfaER181A) strains and pili as mentioned above. According to our preliminary data, there was significant decrease (P<0.001) in binding capacity of MC4100/pEU2124 to bovine erythrocytes compared to MC4100/pJGX15W. However, binding to asialo-GM1 was significantly low only at maximum OD600=20, afterwards non significant decrease was observed (Figure 4a, b).
The adherence assays using crude pili of MC4100/pJGX15W and MC4100/pEU2124 showed the same trend as observed for piliated and CfaE R181A mutant piliated bacteria. Significant (P< 0.05) and highly significant (P<0.001) decrease in binding of MC4100/pEU2124 pili to bovine erythrocytes and asailoGM1 was observed respectively (Figure 5a, b).
DISCUSSION
This study was undertaken to obtain insights into adherence mechanisms of ETEC strains. In ETEC, cell surface structures such as fimbriae/pili have been shown to be necessary for initial colonization on biotic and abiotic surfaces resulting in well-established biofilms (6). In our study, all tested strains produced maximum biofilm after 60 hours. This may be due to greater production of glycocalyx in these strains in biofilm mode (14). Also significantly higher biofilm production by E24377a compared to MC4100/pJGX15W and DH5aF'lacIq/pEU588 may be related to presence of two antigenic types (i.e., CS1 and CS3), hence contributing towards greater adherence and or/glycocalyx production. Also significantly high biofilm produced by piliated and recombinant piliated strains may be due to the fact that piliated E. coli organisms are more hydrophobic compared to their non-piliated counterparts (14). A comparison between MC4100/pJGX15W (CFA/I) and (CfaE-R181 mutant) showed significantly decreased biofilm formation in MC4100/pEU2124. Likewise decreased biofilm formation observed in DH5aF'lacIq/pEU588∆cotD compared DH5aF'lacIq/pEU588 can be justified with the fact that biofilm formation may be a minor pili/tip-associated event as noticed for PAK pili of P. aeruginosa (28).
It has previously been reported that R181A mutation in CFA/I pili manifest MRHA inhibition of bovine erythrocytes so we tested that whether CS2 bearing the same mutation affected the agglutination of bovine erythrocytes in MRHA assay. Using strain DH5αF'lacIq/pEU588, we found that R181 residue is not essential for the agglutination of bovine erythrocytes since tested clones having R181A mutation showed either very strong or no agglutination at all. This indicates R181 which specifies binding to both bovine and human erythrocytes in CFA/I and CS1 pili (23, 24), is not a conserved feature in CS2 pili and minor pilins of CS2 does not inhibit pilus-mediated hemagglutination.
Adapting a strategy used by Poole et al. (21), we produced dsc19CotD, a stabilized variant of the CS2 pilin minor subunit, extending the C terminus with the N-terminal β-strand from the CotA major subunit. This modification facilitated folding of CotD into an erythrocyte-binding-ready conformation in the absence of its chaperone, CotB. In the Chaperon-usher (CU) pathway the chaperone is necessary for transit of proper subunit and catalysing incorporation into a filament (25), except where subverted by providing the in cis missing donor β-strand (10). Immunoblotting showed expression at 0.4% L-arabinose induction. MRHA assay exhibited rapid agglutination on CFA medium without induction. Demonstration that donor strand complementation is common to the CU and AC pathways reopens the evolutionary question about whether these assembly processes have arisen along convergent lineages or by divergent routes in which the ancestral relationship is so remote that primary sequence similarity has been lost. Purification and expression in a stable, naturally-folded will further determine relative binding of purified CotD to bovine and porcine blood mirrors that of whole CS2 pili. It will suggest us whether that CotA plays no role in determining erythrocyte binding specificity. The ongoing work in our lab is continued to prove the validity of above hypothesis.
The present study suggests that biofilm production and adherence mechanisms of ETEC isolates are associated with colonization factors and multiple CF genes located on the plasmid (CFA/I, CFA/II), as well as on its chromosome (CS2). The fact that biofilm formation and adherence was observed in mutant/tip mutant indicates that other adhesins may act simultaneously or at distinct steps of the adherence process (18). Also this study provides corroborative evidence that in contrast to CS1 and CFA/I, R181A mutation in CotD of CS2 is not necessary for receptor binding moiety.
In conclusion, it is clear that pili are important structures in adhesion by ETEC strains. Several questions about the assembly of these unusual covalently linked structures remain to be addressed. Efforts have been made in this study to study pili role in vitro using recombinant pilin subunits and strains. In vitro adhesion studies using bovine erythrocytes and asailoGM1 have clarified the role of the pili in these processes and strengthen the rationale for using pilus proteins as vaccine components. An additional advantage suggested by the functional reactivity of minor subunit antigen is that fewer antibodies may be required to elicit immunity to ETEC expressing a wider array of related pili. Finally, genome sequencing and comparison will lead to a better understanding of the evolution of the pilus-encoding pathogenicity islands and how they have spread through Gram-negative pathogens considering E. coli as model organism.
ACKNOWLEDGEMENTS
This work was supported by Endeavour Award (Australian scholarships), 2010.
REFERENCES
1. Anderson, G.G.; Palermo, J.J.; Schilling, J.D. et al. (2003). Intracellular bacterial biofilm-like pods in urinary tract infections. Science., 301:105107.
2. Baker, N.; Hansson, G.C.; Leffler, H. et. Al. (1990). Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect. Immun., 58:2361-2366.
3. Buhler, T.; Hoschutzky, H.; Jann, K. (1991). Analysis of colonization factor antigen I, an adhesin of enterotoxigenic Escherichia coli O78:H11 fimbrial morphology and location of the receptor-binding site. Infect. Immun., 59:38763882.
4. Clavijoa, A.P.; Baia, J.; Gómez-Duarte, O.G. (2010). The Longus type IV pilus of enterotoxigenic Escherichia coli (ETEC) mediates bacterial self-aggregation and protection from antimicrobial agents. Microbial. Pathogenesis., 48:230-238.
5. de Lorimier, A.J.; Byrd, W.; Hall, E.R. et al. (2003). Murine antibody response to intranasally administered enterotoxigenic Escherichia coli colonization factor CS6. Vaccine., 21:25482555.
6. Domka, J.; Lee, J.; Bansal, T. et al. (2007). Temporal gene-expression in Escherichia coli K-12 biofilms. Environ. Microbiol., 9:332-346.
7. Evans, D.G.; Evans, D.J.; Clegg, S. et al. (1979). Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect. Immun., 25:738748.
8. Gonzales, E.A.; Blanco, J. (1985). Comparative study of inhibition of mannose-resistant hemagglutination caused by CFA/I, CFA/II, K88, and K99-positive Escherichia coli strains. FEMS. Microbiol. Lett., 29:115121.
9. Jones, C.H.; Jacob-Dubuisson, F.; Dodson, K. et al. (1992). Adhesin presentation in bacteria require molecular chaperones and ushers. Infect. Immun., 60:4445-4451.
10. Jedrzejczak, R.; Dauter, Z.;, Dauter, M. et al. (2006). Structure of DraD invasin from uropathogenic Escherichia coli: a dimer with swapped beta-tails. Acta. Crystallogr. D. Biol. Crystallogr., 62:157164.
11. Jonson, A.B.; Normark, S.; Rhen, M. (2005). Fimbriae, pili, flagella and bacterial virulence. Contrib. Microbiol., 12:6789.
12. Knutton, S.; Lloyd, D.R.; Candy, D.C.;et al. (1985). Adhesion of enterotoxigenic Escherichia coli to human small intestinal enterocytes. Infect. Immun., 48:824831.
13. Lee, K.K.; Sheth, H.B.; Wong, W.Y. et al. (1994). The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol., 11:705-713.
14. Leung, J.W.; Liu, Y.L.; Desta, T. et al. (1998). Is there a synergistic effect between mixed bacterial infection in biofilm formation on biliary stents? Gastrointest. Endosc., 48:250-257.
15. Levine, M.M.; Ristaino, P.; Marley, G. et al. (1984). Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect. Immun., 44:409420.
16. Liaqat, I.; Bachmann, R.T.; Sabri, A.N. et al. (2010). Isolate-specific effects of patulin, penicillic Acid and EDTA on biofilm formation and growth of dental unit water line biofilm isolates. Curr. Microbiol., 61:148-156.
17. Macfarlane, S.; Dillon, J.F. (2007). Microbial biofilms in the human gastrointestinal tract. J. Appl. Microbiol., 102:11871196.
18. Mazariego-Espinosa, K.; Cruz, A.; Ledesma, M.A. et al. (2010). Longus, a type IV pilus of enterotoxigenic Escherichia coli, is involved in adherence to intestinal epithelial cells. J. Bacteriol., 192:2791-800.
19. Mu, X.Q.; Savarino, S.J.; Bullitt, E. (2008). The three-dimensional structure of CFA/I adhesion pili: traveler's diarrhea bacteria hang on by a spring. J. Mol. Biol., 376:614620.
20. Orø, H.S.; Kolstø, A.B.; Wennerås, C. et al. (1990). Identification of asialo-GM1 as a binding structure for Escherichia coli colonization factors antigens. FEMS. Microbiol. Lett., 72:289292.
21. Poole, ST, McVeigh AL, Anantha RP et al (2007). Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol Microbiol 63:1372-1384.
22. Proft, T.; Baker, E.N. (2009). Pili in Gram-negative and Gram-positive bacteria - structure, assembly and their role in disease. Cell. Mol. Life. Sci., 66:613-635.
23. Sakellaris, H.; Balding, D.P.; Scott, J.R. (1996). Assembly proteins of CS1 pili of enterotoxigenic Escherichia coli. Mol. Microbiol., 21:529-541.
24. Sakellaris, H.; Penumalli, V.R.; Scott, J.R. (1999). The level of expression of the minor pilin subunit, CooD, determines the number of CS1 pili assembled on the cell surface of Escherichia coli. J. Bacteriol., 181:1694-1697.
25. Sauer, F.G.; Pinkner, J.S.; Waksman, G. et al. (2002). Chaperone priming of pilus subunits facilitates a topological transition that drives fiber formation. Cell., 111:543551.
26. Sharon, N.; Lis, H. (1993). Carbohydrates in cell recognition. Sci. Amer., 268:82-89.
27. Thomas, L.V.; McConnell, M.M.; Rowe, B. et al. (1985). The possession of three novel coli surface antigens by enterotoxigenic Escherichia coli strains positive for the putative colonization factor PCF8775. J. Gen. Microbiol., 131:23192326.
28. van Schaik, E.J.; Giltner, C.L.; Audette, G.F. et al. (2005). DNA binding: a novel function of Pseudomonas aeruginosa type IV pili. J. Bacteriol., 187:1455-1464.
29. Wennerås, C.; Holmgren, J.; Svennerholm, A.M.; (1990). The binding of colonization factor antigens of enterotoxigenic Escherichia coli to intestinal cell membrane proteins. FEMS. Microbiol. Lett., 54:107-112.
Submitted: August 12, 2011; Returned to authors for corrections: December 01, 2011; Approved: June 07, 2012.
ERRATA
Referente ao artigo "Biofilm formation and binding specificities of CFA/I, CFA/II and CS2 adhesions of Enterotoxigenic Escherichia coli and CfaE-R181A mutant" publicado no Brazilian Journal of Microbiology volume 43(3)12 - edição de Julho-Setembro.
Página 969
Linha 7: retirar o nome do autor Harry Sakellaris2
Onde se lê: Iram Liaqat1,2*, Harry Sakellaris2
Leia se: Iram Liaqat1,2*
Linha 9 e 10: retirar a instituição 2The University of Western Australia, School of Biomedical, Biomolecular & Chemical Sciences Microbiology M502, 35 Stirling Hwy Crawley WA 6009.
Onde se lê:1Department of Zoology, Government College University, Lahore, Pakistan; 2The University of Western Australia, School of Biomedical, Biomolecular & Chemical Sciences Microbiology M502, 35 Stirling Hwy Crawley WA 6009.
Leia se:1Department of Zoology, Government College University, Lahore, Pakistan.
- 1. Anderson, G.G.; Palermo, J.J.; Schilling, J.D. et al. (2003). Intracellular bacterial biofilm-like pods in urinary tract infections. Science, 301:105107.
- 2. Baker, N.; Hansson, G.C.; Leffler, H. et. Al. (1990). Glycosphingolipid receptors for Pseudomonas aeruginosa Infect. Immun, 58:2361-2366.
- 3. Buhler, T.; Hoschutzky, H.; Jann, K. (1991). Analysis of colonization factor antigen I, an adhesin of enterotoxigenic Escherichia coli O78:H11 fimbrial morphology and location of the receptor-binding site. Infect. Immun, 59:38763882.
- 4. Clavijoa, A.P.; Baia, J.; Gómez-Duarte, O.G. (2010). The Longus type IV pilus of enterotoxigenic Escherichia coli (ETEC) mediates bacterial self-aggregation and protection from antimicrobial agents. Microbial. Pathogenesis, 48:230-238.
- 5. de Lorimier, A.J.; Byrd, W.; Hall, E.R. et al. (2003). Murine antibody response to intranasally administered enterotoxigenic Escherichia coli colonization factor CS6. Vaccine, 21:25482555.
- 6. Domka, J.; Lee, J.; Bansal, T. et al. (2007). Temporal gene-expression in Escherichia coli K-12 biofilms. Environ. Microbiol, 9:332-346.
- 7. Evans, D.G.; Evans, D.J.; Clegg, S. et al. (1979). Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli Infect. Immun., 25:738748.
- 8. Gonzales, E.A.; Blanco, J. (1985). Comparative study of inhibition of mannose-resistant hemagglutination caused by CFA/I, CFA/II, K88, and K99-positive Escherichia coli strains. FEMS. Microbiol. Lett, 29:115121.
- 9. Jones, C.H.; Jacob-Dubuisson, F.; Dodson, K. et al. (1992). Adhesin presentation in bacteria require molecular chaperones and ushers. Infect. Immun, 60:4445-4451.
- 10. Jedrzejczak, R.; Dauter, Z.;, Dauter, M. et al. (2006). Structure of DraD invasin from uropathogenic Escherichia coli: a dimer with swapped beta-tails. Acta. Crystallogr. D. Biol. Crystallogr, 62:157164.
- 11. Jonson, A.B.; Normark, S.; Rhen, M. (2005). Fimbriae, pili, flagella and bacterial virulence. Contrib. Microbiol, 12:6789.
- 12. Knutton, S.; Lloyd, D.R.; Candy, D.C.;et al. (1985). Adhesion of enterotoxigenic Escherichia coli to human small intestinal enterocytes. Infect. Immun, 48:824831.
- 13. Lee, K.K.; Sheth, H.B.; Wong, W.Y. et al. (1994). The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol, 11:705-713.
- 14. Leung, J.W.; Liu, Y.L.; Desta, T. et al. (1998). Is there a synergistic effect between mixed bacterial infection in biofilm formation on biliary stents? Gastrointest. Endosc, 48:250-257.
- 15. Levine, M.M.; Ristaino, P.; Marley, G. et al. (1984). Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect. Immun, 44:409420.
- 16. Liaqat, I.; Bachmann, R.T.; Sabri, A.N. et al. (2010). Isolate-specific effects of patulin, penicillic Acid and EDTA on biofilm formation and growth of dental unit water line biofilm isolates. Curr. Microbiol, 61:148-156.
- 17. Macfarlane, S.; Dillon, J.F. (2007). Microbial biofilms in the human gastrointestinal tract. J. Appl. Microbiol, 102:11871196.
- 18. Mazariego-Espinosa, K.; Cruz, A.; Ledesma, M.A. et al. (2010). Longus, a type IV pilus of enterotoxigenic Escherichia coli, is involved in adherence to intestinal epithelial cells. J. Bacteriol, 192:2791-800.
- 19. Mu, X.Q.; Savarino, S.J.; Bullitt, E. (2008). The three-dimensional structure of CFA/I adhesion pili: traveler's diarrhea bacteria hang on by a spring. J. Mol. Biol, 376:614620.
- 20. Orø, H.S.; Kolstø, A.B.; Wennerås, C. et al. (1990). Identification of asialo-GM1 as a binding structure for Escherichia coli colonization factors antigens. FEMS. Microbiol. Lett, 72:289292.
- 21. Poole, ST, McVeigh AL, Anantha RP et al (2007). Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol Microbiol 63:1372-1384.
- 22. Proft, T.; Baker, E.N. (2009). Pili in Gram-negative and Gram-positive bacteria - structure, assembly and their role in disease. Cell. Mol. Life. Sci., 66:613-635.
- 23. Sakellaris, H.; Balding, D.P.; Scott, J.R. (1996). Assembly proteins of CS1 pili of enterotoxigenic Escherichia coli Mol. Microbiol, 21:529-541.
- 24. Sakellaris, H.; Penumalli, V.R.; Scott, J.R. (1999). The level of expression of the minor pilin subunit, CooD, determines the number of CS1 pili assembled on the cell surface of Escherichia coli J. Bacteriol, 181:1694-1697.
- 25. Sauer, F.G.; Pinkner, J.S.; Waksman, G. et al. (2002). Chaperone priming of pilus subunits facilitates a topological transition that drives fiber formation. Cell, 111:543551.
- 26. Sharon, N.; Lis, H. (1993). Carbohydrates in cell recognition. Sci. Amer, 268:82-89.
- 27. Thomas, L.V.; McConnell, M.M.; Rowe, B. et al. (1985). The possession of three novel coli surface antigens by enterotoxigenic Escherichia coli strains positive for the putative colonization factor PCF8775. J. Gen. Microbiol, 131:23192326.
- 28. van Schaik, E.J.; Giltner, C.L.; Audette, G.F. et al. (2005). DNA binding: a novel function of Pseudomonas aeruginosa type IV pili. J. Bacteriol, 187:1455-1464.
- 29. Wennerås, C.; Holmgren, J.; Svennerholm, A.M.; (1990). The binding of colonization factor antigens of enterotoxigenic Escherichia coli to intestinal cell membrane proteins. FEMS. Microbiol. Lett, 54:107-112.
Publication Dates
-
Publication in this collection
10 Jan 2013 -
Date of issue
Sept 2012
History
-
Received
12 Aug 2011 -
Accepted
07 June 2012 -
Reviewed
01 Dec 2011