Abstract
A novel alkaline lipase-producing strain 1-7 identified as Acinetobacter calcoaceticus was isolated from soil samples collected from Bohai Bay, China, using an olive oil alkaline plate, which contained olive oil as the sole carbon source. The lipase from strain 1-7 showed the maximum activity at pH 9.0 under 40ºC. One interesting feature of this enzyme is that it exhibits lipase activity over a broad range of temperatures and good stability. It is also stable at a broad range of pHs from 4.0 to 10.0 for 24 h. Its catalytic activity was highly enhanced in the presence of Ca2+, Mg2+ and K+, but partially inhibited by Cu2+, Al3+, Fe3+ , Ba2+and Zn2+. The fact that it displays marked stability and activity in the presence of TritonX-100, Tween-20, Tween-80, SDS, Hydrogen peroxide, Sodium perborate, Sodium hypochlorite, Sodium citrate, Sodium taurocholate, Glycerine and NaCl suggests that this lipase is suitable as an additive in detergent formulations.
alkaline lipase; acinetobacter calcoaceticus; characterization; detergent
INDUSTRIAL MICROBIOLOGY
Screening and characterization of a novel alkaline lipase from Acinetobacter calcoaceticus 1-7 isolated from Bohai Bay in China for detergent formulation
Haikuan Wang; Shaojiong Zhong; Huijing Ma; Jie Zhang; Wei Qi* * Corresponding Author. Mailing address: Key Laboratory of Industrial Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China.; Tel.: 86-22-60601958; Fax: 86-22-60602298.; E-mail: haikuanwangcn@yahoo.com.cn
Key Laboratory of Industrial Microbiology, Ministry of Education, College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300457, China
ABSTRACT
A novel alkaline lipase-producing strain 1-7 identified as Acinetobacter calcoaceticus was isolated from soil samples collected from Bohai Bay, China, using an olive oil alkaline plate, which contained olive oil as the sole carbon source. The lipase from strain 1-7 showed the maximum activity at pH 9.0 under 40ºC. One interesting feature of this enzyme is that it exhibits lipase activity over a broad range of temperatures and good stability. It is also stable at a broad range of pHs from 4.0 to 10.0 for 24 h. Its catalytic activity was highly enhanced in the presence of Ca2+, Mg2+ and K+, but partially inhibited by Cu2+, Al3+, Fe3+ , Ba2+and Zn2+. The fact that it displays marked stability and activity in the presence of TritonX-100, Tween-20, Tween-80, SDS, Hydrogen peroxide, Sodium perborate, Sodium hypochlorite, Sodium citrate, Sodium taurocholate, Glycerine and NaCl suggests that this lipase is suitable as an additive in detergent formulations.
Key words: alkaline lipase; acinetobacter calcoaceticus; characterization; detergent.
INTRODUCTION
Lipases (triacylglycerol acylhydrolases; EC 3.1.1.3) are one of the most important classes of hydrolytic enzymes that catalyze both the hydrolysis and synthesis of esters (9, 10). The main reason for the steadily growing interest in lipases is because of their enantioselective, regioselective and chemoselective nature (28). Lipases occur widely in nature, but only microbial lipases are commercially significant, since they can be produced at low cost and exhibit improved stability (7).
The most commercially important field of application for hydrolytic lipases is their addition to detergents, which are used mainly in household and industrial laundry (8). Washing and degreasing by using lipases allows for smaller amounts of surfactants and operation at low temperatures (18). The lipase component causes an increase in detergency and prevents scaling, since enzymes can reduce the environmental load of detergent products (7). In 1994, Novo Nordisk introduced the first commercial lipase, Lipolase, which originated from the fungus T. lanuginosus and was expressed in A. oryzae. In 1995 two bacterial lipases were introducedLumafast from Pseudomonas mendocina and Lipomax from Pseudomonas alcaligenes, both produced by Genencor International, AU-KBC Research Center, Life Sciences, Anna University, Chennai, India (7). Lipases used as detergents also include those from Candida (17) and Chromobacterium (15). Laundering is generally carried out in alkaline media, lipases active under such conditions are preferred, and for example, the A. oryzae derived lipase (5, 21, 26). Alkaline Lipase produced by Acinetobacter radioresistens had an optimum pH of 10.0 and was stable over a pH range of 6.0-10.0; therefore have great potential for application in the detergent industry (3).
This study describes the production and characterization of a novel microbial lipase. We conducted an extensive screening of bacterial isolates collected from soils and isolated an alkaline lipase-producing strain 1-7, identified as Acinetobacter calcoaceticus. We provided experimental evidence that strain 1-7 produced a novel alkaline lipase capable of catalyzing the hydrolysis of esters at a broad range of temperatures and pHs in the presence of detergent ingredients.
MATERIALS AND METHODS
Enrichment of Lipase-producing Microorganisms
One gram of soil sample (collected from Bohai Bay, China) was added into a 250 mL Erlenmeyer flask containing 50 mL enriching medium with the composition (%) of yeast extract (Oxoid, England) 1, olive oil (Moreno, Spain) 2, NaCl 0.05, MgSO47H2O 0.02, K2HPO4 0.1, peptone (Oxoid, England) 2, initial pH 8.0. The mixture was incubated at 37ºC on a rotary shaker (HYGⅡ, Xinrui Co., China) at 180 rpm for 72 h.
Isolation, Screening and Identification of Lipase-producing Microorganisms
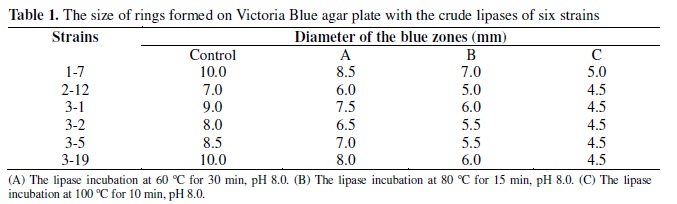
The grown microorganisms in the enrichment culture were isolated on the screening plates, which contain (%) K2HPO4 0.1, NaNO3 0.3, MgSO47H2O 0.05, FeSO47H2O 0.01, emulsion of olive oil (Moreno, Spain) (it contains 0.2% Victoria blue B (SCRC, China)) 2, agar (Solarbio, China) 2, initial pH 8.0. Plates were incubated at 37ºC for 24 h. First, the lipase activity was roughly estimated by Victoria Blue plate assay method (12). The growing colonies with blue zones were separately transferred to liquid medium, which contains (%) soluble starch (Shuangxuan Co., China) 1, bean flour (Shuangxuan Co., China) 2, corn flour (Shuangxuan Co., China) 1, (NH4)2SO4 0.5, K2HPO4 0.1, and emulsion of olive oil (Moreno, Spain) 2, initial pH 8.0. The mixture was incubated at 37ºC on a rotary shaker (HYGⅡ, Xinrui Co., China) at 180 rpm for 36 h. After that 5 mL aliquot was performed at 60ºC for 30 min, a second 5 mL aliquot was performed at 80ºC for 15 min and another 5 mL aliquot was performed at 100ºC for 10 min, which were respectively added to 4-mm-diameter holes of the plates with 20 μL. Plates were incubated at 37ºC for 24 h. Then, in order to select the best lipase producer for enzyme purification and characterization, strains with lipolytic activity on the plates were cultured in liquid medium, and lipase activity was determined with the spectrophotometric assay using p-NPP (Sigma, USA) as a substrate (26, 27, 28).
Identification of strain 1-7 was conducted using 16S ribosomal DNA (rDNA) analysis (4). The genomic DNA was extracted according to the method described by the instruction of the Genome DNA Extraction Kit (Biofuture, China). Two primers, F (5′- AGA GTT TGA TCC TGG CTC AG -3′) and R (5′- CTA CGG CTA CCT TGT TAC GA -3′), were used for PCR (24). PCR amplification was carried out as follow: 94ºC for 1 min; 94ºC for 45 s, 55ºC for 45 s, 72ºC for 90 s, 30 cycles; 72ºC for 10 min. PCR was analyzed by Agarose Gel Electrophoresis and DNA was screened by gel documentation system (GeneGenius, Syngene, USA). The sequence analysis was performed by Sunnybio Corporation (Shanghai, China). A homology search to reference strains registered in DDBJ/EMBL/ GenBank was performed using NCBI BLAST.
Lipase Production
Acinetobacter calcoaceticus 1-7 was grown in a liquid medium containing (%) soluble starch (Shuangxuan Co., China) 1, bean flour (Shuangxuan Co., China) 2, corn flour (Shuangxuan Co., China) 1, (NH4)2SO4 0.5, K2HPO4 0.1, and emulsion of olive oil (Moreno, Spain) 2, initial pH 9.0. Culture conditions were 37ºC and 180 rpm in a rotary shaker (HYGⅡ, Xinrui Co., China), in the 250 mL flask containing 30 mL of medium. An aliquot of 0.6 mL of a 10 h pre-culture in the culture (containing (%) peptone (Oxoid, England) 1, yeast extract (Oxoid, England) 0.5, NaCl 1, initial pH 9.0.) was used as inoculum.
Crude enzyme was obtained by centrifugation (CR21G, Hitachi, Japan) at 10,000 rpm at 4ºC for 10 min. The cell-free supernatant was considered as crude enzyme (20).
Lipase Activity
Lipase activity was determined by the spectrophotometric method with p-nitrophenyl palmitate (p-NPP) (Sigma, USA) as the substrate. Solution A contained p-NPP (30 mg) dissolved in propane-2-ol (10 mL), solution B contained Triton X-100 (Biofuture, China) (1 mL) dissolved in 90 mL buffer (0.1 mol/L Gly-NaOH, pH 9.0). The assay solution was prepared by adding solution A to solution B. The assay mixture contained 900 μL of the emulsion and 100 μL of the appropriately diluted lipase (boiled for 30 minutes as blanks) solution. The reaction was performed at 40ºC for 15 min and terminated at 4ºC for 10 min. The liberated p-nitrophenol was measured at 410 nm by spectrophotometer (TU-1810, Pgeneral Co., China). One unit (U/mL) of lipase was defined as the amount of lipase that releases 1 mmol p-nitrophenol per minute at 40ºC, pH 9.0 (26).
Properties of Lipase
The optimal temperature of the lipase was evaluated by using the lipase activity assay with p-NPP (Sigma, USA) at various temperatures from 20ºC to 50ºC under pH 8.0. The stability of the lipase was determined by measuring the residual activity after 60 h of pre-incubation in sodium phosphate buffer (pH 8.0) at various temperatures.
The optimal pH of the lipase was measured by incubating the lipase substrate at various pHs from 3.0 to 10.0. The following buffers (0.1 mol/L) were sodium acetate buffer (pH 3.0-5.0), sodium phosphate buffer (pH 6.0-8.0), and Gly-NaOH buffer (pH 9.0-10.0), and were adjusted to the optimal enzyme reaction temperature.
To determine the effect of metal ions on lipase activity, the enzyme solution was pre-incubated with metal ions (1 mmol/L) such as ZnCl2, CuCl2, MnCl2, AlCl3, KCl, FeCl2, FeCl3, BaCl2, MgCl2 and CaCl2 at 40ºC for 20 min and then the residual activity was determined.
In order to determine the potential application of lipase from Acinetobacter calcoaceticus 1-7 in detergent industry, its compatibility with various surfactants and oxidizing agents was investigated by respectively adding TritonX-100, Tween-20, Tween-80, SDS, Hydrogen peroxide, Sodium perborate, Sodium hypochlorite, Sodium citrate, Sodium taurocholate, Glycerine and NaCl into the activity assay mixture, then the lipase samples were incubated for 1 h at 40ºC and their activity was determined by the spectrophotometric assay at pH 9.0 and 40ºC. The residual lipase activity in each sample was determined.
RESULTS AND DISCUSSION
Screening and Identification of Lipase-producing Microorganisms
Fifty six strains with high lipase activity from 189 samples were isolated by enrichment cultures, among which 6 strains were shown to produce alkaline lipases. Strain 1-7 was selected for subsequent experiments due to its ability to produce a lipase with good stability at various temperatures (Table 1). The taxonomic identification of the strain 1-7 was conducted. The sequence of 16S rDNA of the strain 1-7 showed 99% homology to Acinetobacter calcoaceticus compared with the GenBank database.
Effects of Temperature on Lipase Activity
The lipase exhibited optimum lipolytic activity at 40ºC and has substantial activity from 20ºC to 50ºC with the relative activity of 87 % at 20ºC, 96 % at 30ºC, 100 % at 40ºC and 85 % at 50ºC. However, at low temperature (5ºC) and high temperature (70ºC), the lipase activity was greatly reduced (Figure 1). And as shown in Figure 2, stability test at various temperatures for 60 h at pH 8.0 showed that the enzyme was relatively stable, since it retained almost 90 % of its activity at 20ºC to 50ºC.
Effects of pH on Lipase Activity
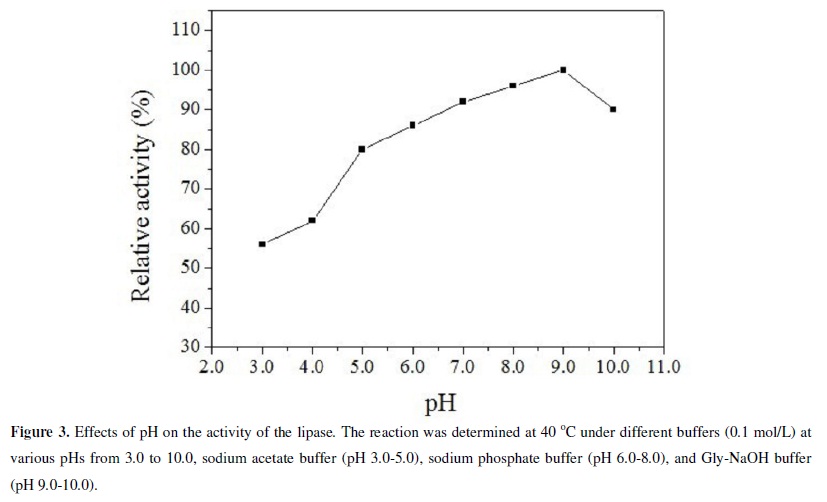
The optimum activity of the lipase was observed at pH 9.0, consistent with the results based on the plate method. The lipase was also highly active over a wide pH range (4.0-10.0) (Figure 3). It retained 80% of its activity at pH 5.0, 86 % at pH 6.0, 92 % at pH 7.0, 96 % at pH 8.0, 100 % at pH 9.0 and 90 % at pH 10.0 after 24 h at 40ºC. It is noteworthy that the lipase retained 56 % activity even at pH 3.0. And as shown in Figure 4, stability test at various pHs for 24 h at 40ºC shown that the lipase was relatively active over a wide pH range. The remarkable wide pH range of the Acinetobacter calcoaceticus lipase justifies its further investigation for commercial applications.
As has been reported for other lipases, the enzyme from Bacillus sp. RSJ-1 showed good stability as it retained > 90 % activity at 60ºC for 1 h under alkaline conditions and also exhibited a half life of > 150 min at 60ºC and 45 min at 70ºC, respectively. The enzyme exhibited the good stability in an alkaline pH range, as it retained >95 % activity at pH 9.0, which reduced to 78 % at pH 10.0 after 1 h of incubation (23). The results showed the lipase from Pseudomonas aeruginosa SRT 9 that exhibited fairly stable activity ranging from 55 to 65ºC, and the enzyme was remarkably stable in the pH rang 6.0 to 7.5 retaining 68 % of the residual activity at pH 8.0 (2). The lipase from Geobacillus thermoleovorans was stable at temperature range 40ºC to 60ºC with no loss of activity, whereas increasing incubation temperature up to 100ºC led to 70 % loss of activity after 60 min of incubation. However, its activity of lipase was only observed at pH 7.0-8.0 (1). Actually, preferred lipases for detergent components are those which show sufficient lipase activity in washing solutions and washing conditions. Generally the pH of washing solutions is in the alkaline region. in some countries such as Japan, the temperature of washing water is 40ºC (25). Therefore, the stability of Acinetobacter calcoaceticus lipase in alkaline pH and at various temperatures suggests its potential utility in industrial applications.
Effects of Metal ions on Lipase Activity
Effects of different metal ions on the activity of the lipase were shown in Table 2. K+, Ca2+, and Mg2+ were found to enhance the lipase activity. While Al3+, Fe3+, Ba2+and Zn2+ partially inhibited the enzymatic activity, Cu2+ completely inhibited the lipase activity. Lipase activity was not affected by the presence of Fe2+ and Mn2+.
As has been reported for other lipases, Ca2+ salts increased activity immediately (59 %) and after 1 h of incubation at 30ºC (35 %) (6, 13, 14).The calcium-induced increase on lipase activity could be attributed to the complex action of calcium ions on the released fatty acids, and on enzyme structure stabilization due to the binding of calcium ions to the lipase, bridging the active region to a second subdomain of the protein and hence stabilizing enzyme tertiary structure (11). Another reported that Ca2+ showed stimulatory effect whereas Mg2+, Mn2+, Ba2+ had negligible effect on the enzyme activity. However, Fe2+, Cu2+ and Zn2+ reduced enzyme activity to less than 37 % of its relative activity (2).
Effects of Surfactants, Oxidizing Agents and Detergent Ingredients on Lipase Activity
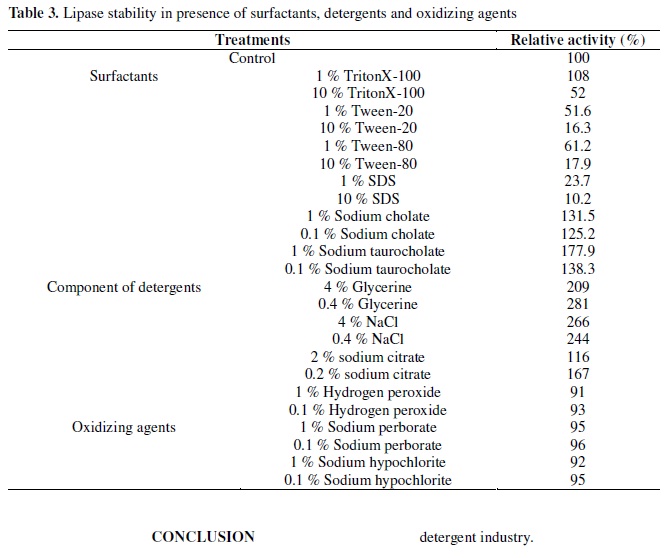
Besides pH and temperature stability, a good detergent lipase should also be stable in the presence of various surfactants. The effects of various surfactants, oxidizing agents and detergent ingredients on lipase activity are depicted in Table 3. Addition of 1 % Triton X-100 to the lipase mixture increased the enzyme activity, but 10 % Triton X-100 inhibited its activity, while it was completely inhibited in the presence of 1 % and 10 % SDS, Tween-80 and Tween-20, the effects was apparent under increased concentrations. However, addition of 0.1 % and 1 % Sodium cholate and Sodium taurocholate were found to enhance the enzyme activity apparently. Glycerine, NaCl and Sodium citrate were also found to highly enhance the enzyme activity. All of the oxidizing agents shown in Table 3 had little effect on the lipase activity.
As has been reported for other lipases, Schmidt-Dannert et al. (22) reported a total loss of lipolytic activity in the presence of Tween-20 and Tween-80, but no effect was observed when was incubated with Triton X-100. Prazeres et al. (19) observed Tween-40, Tween-80 and surfactin inhibited up to 30 % lipase activity, but Triton X-100 and Triton X-114 showed an activating effect. Nawani et al. (16) found a total loss of activity in the presence of SDS. In contrast, activity was enhanced in the presence of TritonX-100, Tween-20 and Tween-80. Hydrogen peroxide, Sodium perborate and Sodium hypochlorite had little effect on the enzyme activity. Sodium cholate, Sodium taurocholate, Glycerine and NaCl were found to enhance the enzyme activity. Thus, the lipase from Acinetobacter calcoaceticus 1-7 suggested a good potential application in the detergent industry.
CONCLUSION
In aqueous solution, the lipase of Acinetobacter calcoaceticus 1-7 shows some interesting properties such as stability at a broad range of pHs (4.0-10.0) with the optimum activity at pH 9.0. It also showed good stability ranging from 20ºC to 50ºC with the maximum activity at 40ºC. Our experimental evidence clearly indicated that the lipase produced by the strain 1-7 is a moderate thermophilic alkaline lipase. Its activity was highly enhanced at the presence of Ca2+, Mg2+ and K+. In contrast, it was almost completely inhibited by Cu2+, while Al3+, Fe3+, Ba2+and Zn2+ partially inhibited the enzymatic activity. Moreover, it was resistant to various surfactants, oxidizing agents and enzyme inhibitors, such as Tween-20, Tween-80, Triton X-100, and SDS, while Sodium cholate, Sodium taurocholate, Glycerine, NaCl, Sodium citrate enhance its enzymatic activity. The present studies showed that this novel lipase has the potential for applications in the detergent industry.
ACKNOWLEDGEMENTS
This work was supported by Tianjin Natural Science Foundation (No. 09JCZDJC17800 and No. 07JCYBJC07900).
REFERENCES
1. Abdel-Fattah, Y.R.; Gaballa, A.A. (2008). Identification and over-expression of a thermostable lipase from Geobacillus thermoleovorans Toshki in Escherichia coli. Microbiol. Res. 163 (1), 13-20.
2. Borkar, P.S.; Bodade, R.G.; Rao,S.R.; Khobragade, C.N. (2009). Purification and characterization of extracellular lipase from a new strain - Pseudomonas aeruginosa SRT 9. Braz. J. Microbiol. 40 (2), 358-366.
3. Chen, S.J.; Cheng, C.Y.; Chen, T.L. (1998). Production of an alkaline lipase by Acinetobacter radioresistens. J. Ferment. Bioeng. 86 (3), 308-312.
4. Eltaweel, M.A.; Rahman, R.A.; Salleh, A.B.; Basri, M. (2005) An organic solvent-stable lipase from Bacillus sp. strain 42. Ann. Microbiol. 55 (3), 187-192. Gerhartz, W. (1990). Enzymes in industry: Production and Application. VCH Press, Weinheim.
5. Handelsman, T.; Shoham, Y. (1994). Production and characterization of an extracellular thermostable lipase from a thermophilic Bacillus sp. J. Gen. Appl. Microbiol. 40 (5), 435-443.
6. Hasan, F.; Shah, A.A.; Hameed, A. (2006). Industrial applications of microbial lipases. Enz. Microb Technol. 39 (2), 235-251.
7. Ito, S.; Kobayashi, T.; Ara, K.; Ozaki, K.; Kawai, S.; Hatada, Y. (1998). Alkaline detergent enzymes from alkaliphiles: enzymatic properties, genetics, and structures. Extremophiles. 2 (3), 185-190.
8. Jaeger, K.E.; Dijkstra, B.W.; Reetz, M.T. (1999). BACTERIAL BIOCATALYSTS: Molecular Biology, Three-Dimensional Structures, and Biotechnological Applications of Lipases. Annu. Rev. Microbiology. 53 (1), 315-351.
9. Jaeger, K.E.; Reetz, M.T. (1998). Microbial lipases form versatile tools for biotechnology. Trends. Biotechnol. 16 (9), 396-403.
10. Kim, M.H.; Kim, H.K.; Lee, J.K.; Park, S.Y.; Oh, T.K. (2000). Thermostable lipase of Bacillus stearothermophilus: high level production, purification, and calcium-dependent thermostability. Biosci. Biotechnol. Biochem. 64 (2), 280-286.
11. Kouker, G.; Jaeger, K.E. (1987). Specific and sensitive plate assay for bacterial lipases. Appl. Environ. Microb. 53 (1), 211-213.
12. Lee, D.W.; Kim, H.W.; Lee, K.W.; Kim, B.C.; Choe, E.A.; Lee, H.S.; Kim, D.S.; Pyun, Y.R. (2001). Purification and characterization of two distinct thermostable lipases from the gram-positive thermophilic bacterium Bacillus thermoleovorans ID-1. Enz. Microb. Technol. 29 (6-7), 363-371.
13. Lee, D.W.; Koh, Y.S.; Kim, K.; Kim, B.; Choi, H.; Kim, D.; Suhartono, M.T.; Pyun, Y. (1999). Isolation and characterization of a thermophilic lipase from Bacillus thermoleovorans ID-1. FEMS. Microbiol. Lett. 179 (2), 393-400.
14. Minoguchi, M.; Mukaiyama, T. November 1989. Immobilization of lipase on polyacrylamide and its use in detergents. J.P. Pat. 1, 285, 188.
15. Nawani, N.; Dosanjh, N.S.; Kaur, J. (1998). A novel thermostable lipase from a thermophilic Bacillus sp.: characterization and esterification studies. Biotechnol. Lett. 20 (10), 997-1000.
16. Nishioka, M.; Takama, M. April 1990. Lipase manufacture with Candida for use in detergents. J.P. Pat 292, 281.
17. Novak, J.; Kralova, B.; Demnerova, K.; Prochazka, K.; Vodrazka, Z.; Tolman, J.; Rysova, D.; Smidrkal, J.; Lopata, V. February 1990. Enzyme agent based on lipases and oxidoreductases for washing, degreasing and water reconditioning. E.U. Pat. 355, 228.
19 Prazeres, J.N.; Cruz, J.A.B.; Pastore, G.M. (2006). Characterization of alkaline lipase from Fusarium oxysporum and the effect of different surfactants and detergents on the enzyme activity. Braz. J. Microbiol.37 (4), 505-509.
20. Sangeetha, R.; Geetha, A.; Arulpandi, I. (2010). Concomitant production of protease and lipase by Bacillus Licheniformis VSG 1: Production, purification and characterization. Braz. J. Microbiol. 41 (1), 179-185.
21. Satsuki, T.; Watanabe, T. (1990). Application of lipase to laundry detergents. Bio. Ind. 7, 501-507.
22. Schmidt-Dannert, C.; Sztajer, H.; Stocklein, W.; Menge, U.; Schmid, R.D. (1994). Screening, purification and properties of a thermophilic lipase from Bacillus thermocatenulatus. Biochim. Biophys. Acta. 1214 (1), 43-53.
23. Sharma, R.; Soni, S.K.; Vohra, R.M.; Gupta, L.K.; Gupta, J.K. (2002). Purification and characterization of a thermostable alkaline lipase from a new thermophilic Bacillus sp. RSJ-1. Process. Biochem. 37 (10), 1075-1084.
24. Sun, T.S.; Zhao, S.P.; Wang, H.K.; Cai, C.K.; Chen, Y.F.; Zhang, H.P. (2009) ACE-inhibitory activity and gamma-aminobutyric acid content of fermented skim milk by Lactobacillus helveticus isolated from Xinjiang koumiss in China. Eur. Food Res. Technol. 228 (4), 607-612.
25. Suzuki, M. October 2001. Alkaline lipase and detergent composition active at low temperature. U.S. Pat. 6, 306, 813.
26. Umehara, K.; Masago, Y.; Mukaiyama, T.; Okumura, O. (1990). Behavior of alkaline lipase on detergency. Yukagaku. 39, 322-326.
27. Vorderwülbecke, T.; Kieslich, K.; Erdmann, H. (1992). Comparison of lipases by different assays. Enz. Microb. Technol. 14 (8), 631-639.
28. Winkler, U.K.; Stuckmann, M. (1979). Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 138 (3), 663-670.
Submitted: June 25, 2010; Returned to authors for corrections: December 15, 2011; Approved: January 16, 2012.
- 1. Abdel-Fattah, Y.R.; Gaballa, A.A. (2008). Identification and over-expression of a thermostable lipase from Geobacillus thermoleovorans Toshki in Escherichia coli Microbiol. Res 163 (1), 13-20.
- 2. Borkar, P.S.; Bodade, R.G.; Rao,S.R.; Khobragade, C.N. (2009). Purification and characterization of extracellular lipase from a new strain - Pseudomonas aeruginosa SRT 9. Braz. J. Microbiol 40 (2), 358-366.
- 3. Chen, S.J.; Cheng, C.Y.; Chen, T.L. (1998). Production of an alkaline lipase by Acinetobacter radioresistens J. Ferment. Bioeng 86 (3), 308-312.
- 4. Eltaweel, M.A.; Rahman, R.A.; Salleh, A.B.; Basri, M. (2005) An organic solvent-stable lipase from Bacillus sp. strain 42. Ann. Microbiol 55 (3), 187-192.
- Gerhartz, W. (1990). Enzymes in industry: Production and Application. VCH Press, Weinheim.
- 5. Handelsman, T.; Shoham, Y. (1994). Production and characterization of an extracellular thermostable lipase from a thermophilic Bacillus sp. J. Gen. Appl. Microbiol 40 (5), 435-443.
- 6. Hasan, F.; Shah, A.A.; Hameed, A. (2006). Industrial applications of microbial lipases. Enz. Microb Technol. 39 (2), 235-251.
- 7. Ito, S.; Kobayashi, T.; Ara, K.; Ozaki, K.; Kawai, S.; Hatada, Y. (1998). Alkaline detergent enzymes from alkaliphiles: enzymatic properties, genetics, and structures. Extremophiles 2 (3), 185-190.
- 8. Jaeger, K.E.; Dijkstra, B.W.; Reetz, M.T. (1999). BACTERIAL BIOCATALYSTS: Molecular Biology, Three-Dimensional Structures, and Biotechnological Applications of Lipases. Annu. Rev. Microbiology 53 (1), 315-351.
- 9. Jaeger, K.E.; Reetz, M.T. (1998). Microbial lipases form versatile tools for biotechnology. Trends. Biotechnol 16 (9), 396-403.
- 10. Kim, M.H.; Kim, H.K.; Lee, J.K.; Park, S.Y.; Oh, T.K. (2000). Thermostable lipase of Bacillus stearothermophilus: high level production, purification, and calcium-dependent thermostability. Biosci. Biotechnol. Biochem 64 (2), 280-286.
- 11. Kouker, G.; Jaeger, K.E. (1987). Specific and sensitive plate assay for bacterial lipases. Appl. Environ. Microb 53 (1), 211-213.
- 12. Lee, D.W.; Kim, H.W.; Lee, K.W.; Kim, B.C.; Choe, E.A.; Lee, H.S.; Kim, D.S.; Pyun, Y.R. (2001). Purification and characterization of two distinct thermostable lipases from the gram-positive thermophilic bacterium Bacillus thermoleovorans ID-1. Enz. Microb. Technol 29 (6-7), 363-371.
- 13. Lee, D.W.; Koh, Y.S.; Kim, K.; Kim, B.; Choi, H.; Kim, D.; Suhartono, M.T.; Pyun, Y. (1999). Isolation and characterization of a thermophilic lipase from Bacillus thermoleovorans ID-1. FEMS. Microbiol. Lett 179 (2), 393-400.
- 14. Minoguchi, M.; Mukaiyama, T. November 1989. Immobilization of lipase on polyacrylamide and its use in detergents. J.P. Pat. 1, 285, 188.
- 15. Nawani, N.; Dosanjh, N.S.; Kaur, J. (1998). A novel thermostable lipase from a thermophilic Bacillus sp.: characterization and esterification studies. Biotechnol. Lett 20 (10), 997-1000.
- 16. Nishioka, M.; Takama, M. April 1990. Lipase manufacture with Candida for use in detergents. J.P. Pat 292, 281.
- 17. Novak, J.; Kralova, B.; Demnerova, K.; Prochazka, K.; Vodrazka, Z.; Tolman, J.; Rysova, D.; Smidrkal, J.; Lopata, V. February 1990. Enzyme agent based on lipases and oxidoreductases for washing, degreasing and water reconditioning. E.U. Pat. 355, 228.
- 19 Prazeres, J.N.; Cruz, J.A.B.; Pastore, G.M. (2006). Characterization of alkaline lipase from Fusarium oxysporum and the effect of different surfactants and detergents on the enzyme activity. Braz. J. Microbiol37 (4), 505-509.
- 20. Sangeetha, R.; Geetha, A.; Arulpandi, I. (2010). Concomitant production of protease and lipase by Bacillus Licheniformis VSG 1: Production, purification and characterization. Braz. J. Microbiol 41 (1), 179-185.
- 21. Satsuki, T.; Watanabe, T. (1990). Application of lipase to laundry detergents. Bio. Ind 7, 501-507.
- 22. Schmidt-Dannert, C.; Sztajer, H.; Stocklein, W.; Menge, U.; Schmid, R.D. (1994). Screening, purification and properties of a thermophilic lipase from Bacillus thermocatenulatus Biochim. Biophys. Acta 1214 (1), 43-53.
- 23. Sharma, R.; Soni, S.K.; Vohra, R.M.; Gupta, L.K.; Gupta, J.K. (2002). Purification and characterization of a thermostable alkaline lipase from a new thermophilic Bacillus sp. RSJ-1. Process. Biochem 37 (10), 1075-1084.
- 24. Sun, T.S.; Zhao, S.P.; Wang, H.K.; Cai, C.K.; Chen, Y.F.; Zhang, H.P. (2009) ACE-inhibitory activity and gamma-aminobutyric acid content of fermented skim milk by Lactobacillus helveticus isolated from Xinjiang koumiss in China. Eur. Food Res. Technol 228 (4), 607-612.
- 25. Suzuki, M. October 2001. Alkaline lipase and detergent composition active at low temperature. U.S. Pat. 6, 306, 813.
- 26. Umehara, K.; Masago, Y.; Mukaiyama, T.; Okumura, O. (1990). Behavior of alkaline lipase on detergency. Yukagaku 39, 322-326.
- 27. Vorderwülbecke, T.; Kieslich, K.; Erdmann, H. (1992). Comparison of lipases by different assays. Enz. Microb. Technol 14 (8), 631-639.
- 28. Winkler, U.K.; Stuckmann, M. (1979). Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens J. Bacteriol 138 (3), 663-670.
Publication Dates
-
Publication in this collection
02 May 2012 -
Date of issue
Mar 2012
History
-
Received
25 June 2010 -
Accepted
16 Jan 2012 -
Reviewed
15 Dec 2011