Abstract
Tropical forests include remnants that should be characterized and monitored for long-term conservation. With this in mind, we performed a survey of the floristic composition and structure of the Mata Santa Elisa (Campinas, SP), and compared it to other fragments of semi-deciduous seasonal forest in the municipality. In Mata Santa Elisa, 506 living arboreal specimens were found, belonging to 100 species, 75 genera and 32 families. At the time of this work, the fragment was well-preserved and contained exclusive species and those of economic interest or potential, highlighting its importance in the region.
Keywords:
biodiversity; tropical forest; conservation
1. INTRODUCTION AND OBJECTIVES
Tropical forests have great biodiversity, containing between 70% and 90% of all plant species; they are among the most complex, fragile and threatened ecosystems in the world (Mittermeier et al., 2005Mittermeier RA, Gil PR, Hoffman M. Hotspots revisited: Earth’s biologically richest and most endangered terrestrial ecoregions. Washington, DC: Conservation International; 2005.). As such, the conservation of tropical forests is of particular interest.
Maintaining well-preserved large areas is essential to mitigating the loss of biodiversity. However, consideration should also be given to preserving small, fragmented areas that may contain relatively high levels of local biodiversity (Arroyo-Rodriguez et al., 2009Arroyo-Rodriguez V, Pineda E, Escobar F, Benitez-Malvido J. Value of small patches in the conservation of plant-species diversity in highly fragmented rainforest. Conservation Biology 2009; 23(3): 729-739. 10.1111/j.1523-1739.2008.01120.x
https://doi.org/10.1111/j.1523-1739.2008...
). These fragments are inserted in different matrices, and they are usually understudied, despite their biological importance due to a high concentration of the remaining information of the Atlantic Forest (Ribeiro et al., 2009Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM. The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation 2009; 142(6): 1141-1153. 10.1016/j.biocon.2009.02.021
https://doi.org/10.1016/j.biocon.2009.02...
).
Mata Santa Elisa (Campinas, SP) is an urban remnant of semi-deciduous seasonal forest, registered in 1991 and defined as a municipal environmental patrimony. This municipality has native vegetation cover consisting of fragments of forest, cerrado and small stretches of rock vegetation (Torres et al., 2014Torres RB, Adami SF, Coelho RM, editors. Atlas socioambiental da bacia do Ribeirão das Anhumas. Campinas: Pontes, 2014.), which has been reduced to 2.55% of its total area. In 1988 and 1994, fires were recorded in Mata Santa Elisa, and invasive exotic species such as Megathyrsus maximus (Jacq.) B. K. Simon & S. W. L. Jacobs and Urochloa decumbens (Stapf) R.D.Webster, respectively known as “capim-colonião” and “braquiária,” increased in the border and neighborhood of the fragment after this occurrence (personal observations). Studies of the flora and structure of 15 different areas in Campinas, with the same physiognomy as the Mata Santa Elisa, found 47 to 151 tree species (H’ diversity of 2.47 to 4.06). However, to date, studies of Mata Santa Elisa have not been conducted. Thus, the objective of this work was to characterize the arboreal component of Mata Santa Elisa and to verify its conservation status, given its importance in relation to the flora of the municipality.
2. MATERIALS AND METHODS
The Mata Santa Elisa (Figure 1), located in the Centro Experimental Central of the Instituto Agronômico de Campinas (IAC), has a total area of 14.81 ha (Souza et al., 2015Souza ACO, Torres RB, Bernacci LC, Jung-Mendaçolli SL. Espécies da flora nativa nas estações experimentais da Agência Paulista de Tecnologia dos Agronegócios, Instituto Agronômico de Campinas, estado de São Paulo, Brasil. Hoehnea 2015; 42(1): 59-92. 10.1590/2236-8906-29/2014
https://doi.org/10.1590/2236-8906-29/201...
). The municipality has two climates: subtropical hot with dry winter (Cwa) and subtropical hot without dry season (Cfa) (Rolim et al., 2007Rolim GS, Camargo MBP, Lima DG, Moraes JFL. Classificação climática de Köppen e de Thornthwaite e sua aplicabilidade na determinação de zonas agroclimáticas para o estado de São Paulo. Bragantia 2007; 66(4): 711-720. 10.1590/S0006-87052007000400022
https://doi.org/10.1590/S0006-8705200700...
). The forest, as well as most of the municipality, is categorized under the Cwa climate, with an average annual rainfall of 1.381 mm (Ferreira et al., 2007Ferreira ICM, Coelho RM, Torres RB, Bernacci LC. Solos e vegetação nativa remanescente no município de Campinas, SP. Pesquisa Agropecuária Brasileira 2007; 2(9): 1319-1327. 10.1590/S0100-204X2007000900014
https://doi.org/10.1590/S0100-204X200700...
). The local relief is smooth wavy to wavy, with an altitude of about 670 m, and predominantly typical dystrophic Red Latosol (Ferreira et al., 2007Ferreira ICM, Coelho RM, Torres RB, Bernacci LC. Solos e vegetação nativa remanescente no município de Campinas, SP. Pesquisa Agropecuária Brasileira 2007; 2(9): 1319-1327. 10.1590/S0100-204X2007000900014
https://doi.org/10.1590/S0100-204X200700...
). Prior to this study, the last fire to occur in Mata Santa Elisa was in 1994.
We established fifty 10 × 10 m (0.5 ha; Figure 1) plots for a floristic and phytosociological survey. This survey was conducted between 2008 and 2009, approximately 15 years after the last recorded fire. All individuals, including standing dead trees, with breast height diameters (DBH) greater or equal to 4.8 cm were plated. Specimens were numbered, after having measured all the multiple stems that met the minimum diameter for inclusion.
Collection, processing of botanical material and incorporation into the IAC Herbarium collection followed the usual standards for this type of study. We made the identifications following the pertinent literature, comparisons with herbarium collections and expert consultations.
We checked species for valid names, spelling and authors in Flora do Brasil 2020, and organized them by family according to the Angiosperm Phylogeny Group IV classification system (2016Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 2016; 181: 1-20. 10.1111/boj.12385
https://doi.org/10.1111/boj.12385...
), with an indication of subfamilies in Fabaceae (LPWG, 2013LPWG - Legume Phylogeny Working Group. Legume phylogeny and classification in the 21st century: progress, prospects and lessons for other species-rich clades. Taxon 2013; 62: 217-248. 10.5167/uzh-78167
https://doi.org/10.5167/uzh-78167...
). We classified native species as pioneers or not pioneers, and exposed them to a dispersion syndrome (Barbosa et al., 2015Barbosa LM, Shirasuna RT, Lima FC, Ortiz PRT. Lista de espécies indicadas para restauração ecológica para diversas regiões do estado de São Paulo. In: Anais do VI Simpósio de Restauração Ecológica; 2015; São Paulo. São Paulo: Instituto de Botânica de São Paulo; 2015. p. 303-436.). When no classification was found for a particular species, we investigated the standard and used it at the generic level.
We analyzed the usual phytosociological parameters, Shannon’s diversity index (H’) and the Pielou equability index (J). We performed the calculations using FITOPAC 2.0 (Shepherd, 2008Shepherd GJ. Manual do FITOPAC 2.0. Campinas: Editora Unicamp; 2008.). And compared the results to 15 other forest fragments (Table 1) of semi-deciduous seasonal forest in the city of Campinas at 600-700 m altitude.
We verified the species names and revised their identifications when the material was available in the herbarium and a possible identification problem had been detected. Morphospecies were considered, since there was no possibility of dubiety with species in the other considered surveys (Coccoloba sp) (Cielo Filho & Martins, 2016Cielo Filho R, Martins FR. Elevation-based post-stratification in Atlantic forest sampling. Phytocoenologia 2016; 46(1): 21-31. 10.1127/phyto/2016/0053
https://doi.org/10.1127/phyto/2016/0053...
; Santos et al., 2007Santos K, Kinoshita L, Santos FAM. Tree species composition and similarity in semideciduous forest fragments of southeastern Brazil. Biological Conservation 2007; 135: 268-277. 10.1016/j.biocon.2006.10.027
https://doi.org/10.1016/j.biocon.2006.10...
). For species with undefined identifications (“cf.“ or “aff.“), we considered them to be the species mentioned, provided that it was not mentioned in the survey itself.
We constructed a presence/absence matrix for species demonstrating at least three occurrences. We also built a quantitative data matrix (absolute density) for the 45 species with the highest Importance Value Index (IVI), or with the highest coverage value index (CVI) in the absence of frequency. We used Principal Coordinates Analysis as the ordering method, simple Euclidean distances for qualitative data and Bray Curtis coefficients for quantitative data (Felfili et al., 2011Felfili JM, Eisenlohr PV, Melo MMRF, Andrade LA, Meira Neto JAA, editors. Fitossociologia no Brasil: métodos e estudo de casos. Viçosa: Editora UFV; 2011.). We used the Mantel test, within the PC-ORD program, to verify the correlation between floristic composition and the geographic distance between the fragments (McCune & Mefford, 1999McCune B, Mefford MJ. PC-ORD: multivariate analysis of ecological data. Versão 5. [software]. Gleneden Beach, OR, USA; 1999.).
3. RESULTS
In the Mata Santa Elisa, we found 556 arboreal specimens (1.112 trees.ha-1), including 506 living individuals (1.012 trees.ha-1) belonging to 100 species, 75 genera and 32 families (Table 2). The most species-rich families were Fabaceae (23 species), Meliaceae (eight species), Rutaceae and Lauraceae (six species) and Euphorbiaceae, Malvaceae and Myrtaceae (represented by five species each). Five of these families were also the most abundant in the area: Euphorbiaceae (258 individuals.ha-1, 25.49% of live trees), Fabaceae (256, 25.30%), Malvaceae (122, 12.05%), Meliaceae (60, 5.93%) and Myrtaceae (42, 4.15%).
Eleven families (Siparunaceae - magnoliids, Rosaceae, Chrysobalanaceae, Combretaceae, Sapotaceae, Boraginaceae, Bignoniaceae, Verbenaceae and Cardiopteridaceae - eudicots) and the eudicotiledon species Nyctaginaceae and Rubiaceae (two species each) were represented by only one individual (2 individuals.ha-1). Standing dead trees were represented by 50 individuals (100 trees.ha-1, 8.99%). Euphorbiaceae (51 individuals species.ha-1), Malvaceae (24), Urticaceae (17), Anacardiaceae (13), Bignoniaceae (12) and Fabaceae (11) had the highest mean number of individuals per species per hectare.
The most abundant species were “caixeta” (Croton piptocalyx, 116 individuals.ha-1, 11.46%), followed by “capixingui” (Croton floribundus, 88, 8.70%), “mutamba” (Guazuma ulmifolia, 86, 8.50%), “pau-jacaré” (Piptadenia gonoacantha, 54, 5.34%) and “araribá” (Centrolobium tomentosum, 48, 4.74%). The Shannon’s diversity index was 3.82 and the Pielou equability index was 0.83 (considering only living individuals). In Mata Santa Elisa, 66 species were classified as non-pioneers, 30 were pioneers and three were exotic, two of which were invasive (I3N Brasil, 2016I3N Brasil. Base de dados nacional de espécies exóticas invasoras I3N Brasil. 2016 June 10 [cited 2018 Apr. 20]. Available from: Available from: https://bit.ly/2jQYAMa
https://bit.ly/2jQYAMa...
). Although the number of non-pioneer species was higher, their abundance (412 individuals.ha-1, 40.71%) was lower than that of the pioneer species (542, 53.56%).
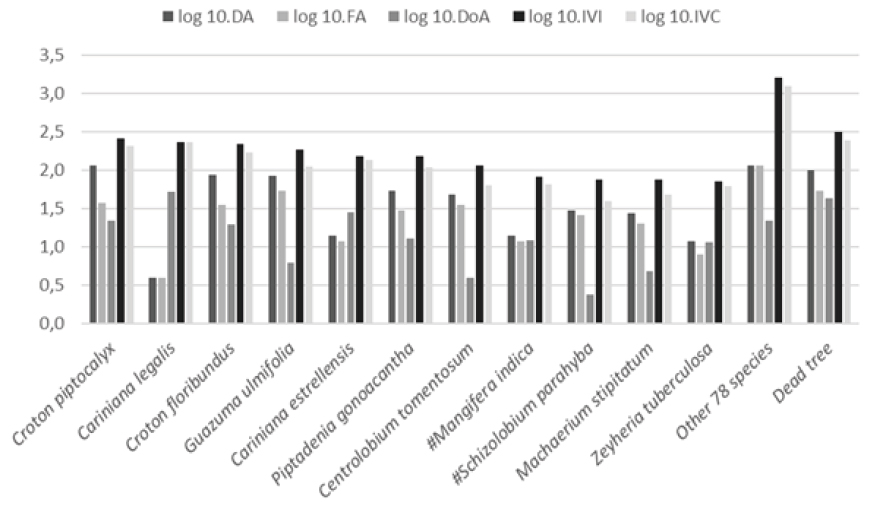
With respect to the 10 species with the highest IVI (Figure 2), seven are among the most abundant species, with 10 or more individuals sampled (20 or more individuals.ha-1). Croton piptocalyx, C. floribundus and Piptadenia gonoacantha had high IVIs due to their density and dominance (70-80% total), whereas density and frequency were the largest contributions (70-80% total) from Guazuma ulmifolia, Centrolobium tomentosum, Schizolobium parahyba (“guapuruvu”) and Machaerium stipitatum (“pau-de-malho”). Cariniana legalis (“jequitibá-rosa”; 96%) and C. estrellensis (“jequitibá-branco”; 80%) obtained their IVIs almost exclusively due to their dominance, which was also very strong (63%) for Mangifera indica (“mangueira”).
Mata Santa Elisa species and standing dead trees in IVI order. Log: log base 10; DA: absolute density; FA: absolute frequency, 10: 10 times; DoA: absolute dominance; IVI: importance value index; IVC: coverage value index; #: exotic species.
In addition, “jequitibás” had the highest dominance averages per individual (1.30 and 0.20 m2.ha-1, respectively), which represented at least twice that of other species (< 0.01 m2.ha-1 to Peltophorum dubium or 0.10 m2.ha-1 to Zeyhera tuberculosa). Among these species (Figure 2), four were classified as pioneers (C. piptocalyx, C. floribundus, G. ulmifolia and P. gonoacantha), four as non-pioneers (C. legalis, C. estrellensis, M. stipitatum and C. tomentosum) and the other two as exotic (S. parahyba and M. indica). When considering CVI, we observed changes in the positions, in relation to IVI, of C. legalis (96% of CVI due to dominance, moving from second to first), C. estrellensis (90%, from fifth to fourth), M. indica (79%, from eighth to seventh) and Zeyhera tuberculosa (81%, from eleventh to ninth).
Trees (Figure 3) were predominately 5 to 10 m in height (about 62.5%), with some emergent individuals (> 20 m) exceeding the regular forest canopy for more light (e.g. Zeyheria tuberculosa, Cariniana legalis). The arithmetic mean was 12.6 cm. The estimated basal area was 27.26 m2.ha-1.
The matrix based on absolute density data consisted of 199 species (https://figshare.com/s/b5b304a83795bd3afb04). The ordering analysis resulted in a 42% explanation of the first two axes (Figure 4) and 11% of the third axis (figure not shown). Through this analysis, it was possible to observe separation between the studied remnants. The Mata Santa Elisa showed greater similarity with the Bosque dos Alemães, remaining to the left side of the graph. Still on the left side, in the upper part, there were three fragments of environmental protection area of Campinas (Pequenos 1 and 3 and Ribeirão Cachoeira, Sample 2), besides Fazenda São Vicente, Bosque dos Jequitibás and Reserva Santa Genebra. The other eight fragments of the Campinas APA remained condensed in a single cluster.
Analysis of principal coordinates using the Bray Curtis coefficient of the 45 species with the highest IVI of the selected fragments in Campinas, SP. Fragment forest codes are the same as in Table 1.
Trichilia clausseni (−0.8) and Esenbeckia leiocarpa Engl. (0.8) were highly correlated with the first axis. Myrcia splendens (−0.8), Copaifera langsdorffii Desf., Ocotea corymbosa (Meisn.) Mez, Piptadenia gonoacantha and Leucochloron incuriale (Vell.) Barneby & J.W.Grimes (−0.7) were correlated with the second axis. Qualitative analyses showed less explanatory power (28% in the two main axes, considering the Euclidean distances), indicating that the floristic composition of the fragments is the same, except in the structure of the tree community.
Although the Mantel test indicated a positive association between geographic distance and density matrices (r = 0.2046; Z observed = 0.1738E+00; t = 1.6986), the p value was high (p = 0.09), indicating that the association was not significant. Thus, we must assume that the floristic similarity between the areas is not determined by the proximity between them.
4. DISCUSSION
Mata Santa Elisa was subject to disturbance factors, including fires, which could have decharacterized its vegetation. Fabaceae, which was the richest and had the second highest IVI, is cited as one of the most important in terms of number of species in other states in the southeast (Bernacci et al., 2006Bernacci LC, Franco GADC, Catharino ELM, Durigan G, Metzger JP. O efeito da fragmentação florestal na composição e riqueza de árvores na região da Reserva Morro Grande (Planalto de Ibiúna, SP). Revista do Instituto Florestal 2006; 18(1): 121-166.; Medeiros et al., 2016Medeiros AS, Pereira MG, Braz DM. Estrutura e conservação de um trecho de floresta estacional em Piraí, RJ. Floresta e Ambiente 2016; 23(3): 330-339. 10.1590/2179-8087.106214
https://doi.org/10.1590/2179-8087.106214...
; Pennington et al., 2009Pennington RT, Lavin M, Oliveira-Filho A. Woody plant diversity, evolution, and ecology in the tropics: perspectives from seasonally dry tropical forests. Annual Review of Ecology, Evolution and Systematics 2009; 40(4): 37-57. 10.1146/annurev.ecolsys.110308.120327
https://doi.org/10.1146/annurev.ecolsys....
). The other families that have been highlighted by their number of species (Meliaceae, Rutaceae, Lauraceae, Euphorbiaceae, Myrtaceae and Malvaceae) or their IVIs (Euphorbiaceae, Lecythidaceae, Malvaceae and Meliaceae) are frequently observed in inventories of semi-deciduous seasonal forest (Oliveira-Filho & Fontes, 2000Oliveira-Filho A, Fontes MAL. Patterns of floristic differentiation among Atlantic Forests in Southeastern Brazil and the influence of climate. Biotropica 2000; 32: 793-810. 10.1111/j.1744-7429.2000.tb00619.x
https://doi.org/10.1111/j.1744-7429.2000...
).
Croton piptocalyx, the most abundant species with a high IVI, as well as Piptadenia gonocantha, Guazuma ulmifolia and C. floribundus, are pioneers; they are generally heliophytes (Tabarelli, 1997Tabarelli M, Mantovani W. Ocupação de clareiras naturais na Serra da Cantareira - SP. Naturalia 1997; 22: 89-102.) and function to recover disturbed environments, as is the case for this studied fragment (Rodrigues, 1995Rodrigues RR. A sucessão florestal. In: Morellato PC, Leitão Filho HF, editors. Ecologia e preservação de uma floresta tropical urbana: Reserva de Santa Genebra. Campinas: Editora Unicamp; 1995. p. 30-35.). Centrolobium tomentosum and Machaerium stipitatum, also abundant and with high IVI values, are both early secondary species, related to the intermediate stage of succession (Gandolfi et al., 1995Gandolfi S, Leitão Filho HF, Bezerra CLE. Levantamento florístico e caráter sucessional das espécies arbustivo arbóreas de uma floresta mesófila semidecídua no município de Guarulhos, SP. Revista Brasileira de Biologia 1995; 55(4): 753-767.). The two “jequitibás” species (Cariniana legalis and C. estrellensis) are characteristic of mature forest areas (Gandolfi et al., 1995Gandolfi S, Leitão Filho HF, Bezerra CLE. Levantamento florístico e caráter sucessional das espécies arbustivo arbóreas de uma floresta mesófila semidecídua no município de Guarulhos, SP. Revista Brasileira de Biologia 1995; 55(4): 753-767.). In this successional mosaic, the high density of pioneer species indicates young forest stretches (Gandolfi et al., 1995Gandolfi S, Leitão Filho HF, Bezerra CLE. Levantamento florístico e caráter sucessional das espécies arbustivo arbóreas de uma floresta mesófila semidecídua no município de Guarulhos, SP. Revista Brasileira de Biologia 1995; 55(4): 753-767.). On the other hand, the survival and longevity of large trees, such as “jequitibás,” allows for the occurrence of reproductive events and increases the possibility of maintaining these populations in the area.
Still, it should be noted that two exotic invasive species are among the species with the highest IVIs in the Mata Santa Elisa, and the occurrence of both species represents anthropic interference in the forest. The occurrence of Mangifera indica may be due to humans discarding their seeds, since all the sampled individuals were close to local trails. Schizolobium parahyba was planted in the area by IAC researchers between 2006 and 2008. Although they have not been studied since, Schizolobium parahyba have developed in the forest (Dr. Wilson Barbosa, CEC Director, Fazenda Santa Elisa, 2007-2012 - personal information).
The species that correlated with the PCO axes were abundant in all or most of the similar fragments from the Mata Santa Elisa (Bosque dos Alemães, Bosque dos Jequitibás, Reserva Santa Genebra etc.), but were not abundant in the other fragments of the environmental protection area of Campinas in general. However, some of these species were not sampled in Mata Santa Elisa, namely: Trichilia clausseni, Esenbeckia leiocarpa, Copaifera langsdorfii, Ocotea corymbosa, Leucochloron incuriale, and Syagrus romanzoffiana. All are zoocorous species except for Leucochloron incuriale, which can be reintroduced or their populations increased in the area.
Schizolobium parahyba was abundant only in the Mata Santa Elisa, which is another indication that it is not a native species. This species has been at risk and caused conflict with neighboring municipalities due to the fall of branches or plants (personal observation), whereas surveys (Abreu et al., 2014Abreu RCR, Santos FFM, Durigan G. Changes in plant community of Seasonally Semideciduous Forest after invasion by Schizolobium parahyba at southeastern Brazil. Acta Oecologica 2014; 54: 57-64. 10.1016/j.actao.2013.03.013
https://doi.org/10.1016/j.actao.2013.03....
) show that the species is invasive in areas of semi-deciduous seasonal forest, displacing successional forest species and modifying the composition and structure of the vegetation; this justifies the suppression of “guapuruvu” individuals, as well as “mangueira” and other exotic species, in Mata Santa Elisa.
Among the compared fragments, Annona mucosa, Celtis pubescens, Terminalia triflora, Croton rottlerifolius, Albizia polycephala, Inga striata, Tachigali denudata, Piper claussenianum and Matayba juglandifolia were sampled exclusively in the Mata Santa Elisa. In addition, the forest also contained noble wood species (e.g. Cedrela fissilis, Hymenaea courbaril) with economic, medicinal and aromatic potentials, among other properties (Perigo et al., 2016Perigo CV, Torres RB, Bernacci LC, Guimarães EF, Haber LL, Facanali R et al. The chemical composition and antibacterial activity of eleven Piper species from distinct rainforest areas in Southeastern Brazil. Industrial Crops and Products 2016; 94: 528-539. 10.1016/j.insoeop.2016.09.028
https://doi.org/10.1016/j.insoeop.2016.0...
; Souza et al., 2015Souza ACO, Torres RB, Bernacci LC, Jung-Mendaçolli SL. Espécies da flora nativa nas estações experimentais da Agência Paulista de Tecnologia dos Agronegócios, Instituto Agronômico de Campinas, estado de São Paulo, Brasil. Hoehnea 2015; 42(1): 59-92. 10.1590/2236-8906-29/2014
https://doi.org/10.1590/2236-8906-29/201...
). Thus, this highlights the importance of Mata Santa Elisa in terms of species of interest and general biodiversity. Even small fragments of forest can be good representatives of local biodiversity and, in addition, preserve a good percentage of regional biodiversity (Arroyo-Rodriguez et al., 2009Arroyo-Rodriguez V, Pineda E, Escobar F, Benitez-Malvido J. Value of small patches in the conservation of plant-species diversity in highly fragmented rainforest. Conservation Biology 2009; 23(3): 729-739. 10.1111/j.1523-1739.2008.01120.x
https://doi.org/10.1111/j.1523-1739.2008...
).
In Mata Santa Elisa, we observed patterns of abundant concentration of a few species and the occurrence of a large number of species represented by few individuals. These patterns are observed in inventories of semi-deciduous seasonal forests (Oliveira-Filho & Fontes, 2000Oliveira-Filho A, Fontes MAL. Patterns of floristic differentiation among Atlantic Forests in Southeastern Brazil and the influence of climate. Biotropica 2000; 32: 793-810. 10.1111/j.1744-7429.2000.tb00619.x
https://doi.org/10.1111/j.1744-7429.2000...
) and in tropical forests; species have preferential environments and their abundance increases in the places where most of their biotic and abiotic requirements are met (Kreft & Jetz, 2007Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. PNAS 2007; 104(14): 5925-5930. 10.1073/pnas.0608361104
https://doi.org/10.1073/pnas.0608361104...
).
Through the H’ and J indices, we confirmed that the tree community has high diversity and low dominance. Index values were close to those of well-preserved areas of the region, such as Ribeirão Cachoeira Forest and Fazenda São Vicente. In the histogram distribution of diameters, the lowest class contained the highest frequency of individuals, and in general, the number of individuals decreased as the diameter classes increased, following a typical distribution for tropical forests (Felfili et al., 2011Felfili JM, Eisenlohr PV, Melo MMRF, Andrade LA, Meira Neto JAA, editors. Fitossociologia no Brasil: métodos e estudo de casos. Viçosa: Editora UFV; 2011.).
Based on the floristic and vegetation parameters at the time of this study, we would categorize Mata Santa Elisa as being at an advanced stage of regeneration, according to the CONAMA No. 1 (1994Conselho Nacional do Meio Ambiente - CONAMA. Resolução Conama nº 1, de 31 de janeiro de 1994. Diário Oficial da União [Internet], Brasília, DF (1994 Jan. 31) [cited 2019 Sept. 2]; Sec. 1: 1684-1685. Available from: Available from: https://bit.ly/2lzy3mZ
https://bit.ly/2lzy3mZ...
) legislation. Main diameter was the only evaluated parameter that was lower than that established for advanced stage regeneration. Analysis of satellite images indicated the advanced regeneration of Mata Santa Elisa from 1991 to 2011 (Andrade & Sanches, 2011Andrade DD, Sanches ID. Vulnerabilidade do patrimônio ambiental tombado em Campinas-SP e sua relação com índice de vegetação. In: Simpósio Brasileiro de Sensoriamento Remoto; 2011; Curitiba. São José dos Campos: INPE; 2011. p. 1666-1673.); these images showed that forest cover had increased in comparison to 1991 (the tipping point), even with the fire in 1994.
Mata Santa Elisa is located in the State of São Paulo, administered by the Secretariat of Agriculture, which includes sustainability in its mission (Veiga et al., 2006Veiga RFA, Vieira MJFR, Costa AA, Barbosa W, Tombolato AFC, Torres RB et al. A educação ambiental no IAC. O Agronômico 2006; 58(1-2): 5-10.). In addition, the use of forest fences is a key factor in the prevention of forest fires. After this study, a new fire hit Mata Santa Elisa, possibly caused by the fall of lantern balloons, and affected about half of its area (Campinas, 2014Campinas. Defesa Civil e outros órgãos públicos controlam fogo na Mata Santa Elisa [Internet]. 2014 [cited 2017 July 5]. Available from: Available from: https://bit.ly/2lssR4A
https://bit.ly/2lssR4A...
). In 2015-2016, financial difficulties hampered the maintenance of firebreaks, which compromised fire protection and relief to the forest. Fortunately, since 2017, the change of the Municipal Secretariat for Public Works, in the vicinity of the forest, has made this maintenance possible (personal observation).
The remaining fragments of semi-deciduous seasonal forest account for only 7.1% of the original cover in the state (Ribeiro et al., 2009Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM. The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation 2009; 142(6): 1141-1153. 10.1016/j.biocon.2009.02.021
https://doi.org/10.1016/j.biocon.2009.02...
), further highlighting the importance of Mata Santa Elisa. These forest fragments are fundamental in the qualitative recovery of the landscape, with an aim towards sustainability and quality of life for the population. Data presented here can act as an important reference for new studies of the composition and structure of Mata Santa Elisa, which are expected to occur momentarily.
5. CONCLUSIONS
In 2007, the Mata Santa Elisa fragment had high diversity and low dominance, as well as species of economic interest or potential. The conservation of the remnant represents a refuge for species that found adequate conditions to establish themselves and develop stable populations, contributing to the preservation of the flora of the region. The relative protection of the area due to its tipping and its location on state property did not prove to be sufficiently effective, as it did not prevent the occurrence of fires in the area. As such, there is a need for other fire prevention measures, such as environmental education for the local community, trained personnel, and equipment for fire control to guarantee its long-term sustainability. In this study, we observed the urgency of monitoring forest dynamics and the suppression of invasive alien species to increase the preservation of the native vegetation of this valuable forest fragment.
ACKNOWLEDGEMENTS
To dr. Márcia Ortiz Mayo Marques for financial support in the initial phases of the project. To support assistant José de Freitas Benedito, for collaboration in field activities. To research assistant Carla F. Nardin for assistance with multivariate analysis.
REFERENCES
- Abreu RCR, Santos FFM, Durigan G. Changes in plant community of Seasonally Semideciduous Forest after invasion by Schizolobium parahyba at southeastern Brazil. Acta Oecologica 2014; 54: 57-64. 10.1016/j.actao.2013.03.013
» https://doi.org/10.1016/j.actao.2013.03.013 - Andrade DD, Sanches ID. Vulnerabilidade do patrimônio ambiental tombado em Campinas-SP e sua relação com índice de vegetação. In: Simpósio Brasileiro de Sensoriamento Remoto; 2011; Curitiba. São José dos Campos: INPE; 2011. p. 1666-1673.
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 2016; 181: 1-20. 10.1111/boj.12385
» https://doi.org/10.1111/boj.12385 - Arroyo-Rodriguez V, Pineda E, Escobar F, Benitez-Malvido J. Value of small patches in the conservation of plant-species diversity in highly fragmented rainforest. Conservation Biology 2009; 23(3): 729-739. 10.1111/j.1523-1739.2008.01120.x
» https://doi.org/10.1111/j.1523-1739.2008.01120.x - Barbosa LM, Shirasuna RT, Lima FC, Ortiz PRT. Lista de espécies indicadas para restauração ecológica para diversas regiões do estado de São Paulo. In: Anais do VI Simpósio de Restauração Ecológica; 2015; São Paulo. São Paulo: Instituto de Botânica de São Paulo; 2015. p. 303-436.
- Bernacci LC, Franco GADC, Catharino ELM, Durigan G, Metzger JP. O efeito da fragmentação florestal na composição e riqueza de árvores na região da Reserva Morro Grande (Planalto de Ibiúna, SP). Revista do Instituto Florestal 2006; 18(1): 121-166.
- Bernacci LC, Leitão Filho HF. Flora fanerogâmica da floresta da Fazenda São Vicente, Campinas, SP. Revista Brasileira de Botânica 1996; 19(2): 149-164.
- Campinas. Defesa Civil e outros órgãos públicos controlam fogo na Mata Santa Elisa [Internet]. 2014 [cited 2017 July 5]. Available from: Available from: https://bit.ly/2lssR4A
» https://bit.ly/2lssR4A - Cielo Filho R, Martins FR. Elevation-based post-stratification in Atlantic forest sampling. Phytocoenologia 2016; 46(1): 21-31. 10.1127/phyto/2016/0053
» https://doi.org/10.1127/phyto/2016/0053 - Cielo Filho R, Santin DA. Estudo florístico e fitossociológico de um fragmento florestal urbano: Bosque dos Alemães, Campinas, SP. Revista Brasileira de Botânica 2002; 25(3): 291-301. 10.1590/S0100-84042002000300005
» https://doi.org/10.1590/S0100-84042002000300005 - Conselho Nacional do Meio Ambiente - CONAMA. Resolução Conama nº 1, de 31 de janeiro de 1994. Diário Oficial da União [Internet], Brasília, DF (1994 Jan. 31) [cited 2019 Sept. 2]; Sec. 1: 1684-1685. Available from: Available from: https://bit.ly/2lzy3mZ
» https://bit.ly/2lzy3mZ - Farah FT, Rodrigues RR, Santos FAM, Tamashiro JY, Shepherd GJ, Siqueira T et al. Forest destructuring as revealed by the temporal dynamics of fundamental species: case study of Santa Genebra Forest in Brazil. Ecological Indicators 2014; 37: 40-44. 10.1016/j.ecolind.2013.09.011
» https://doi.org/10.1016/j.ecolind.2013.09.011 - Felfili JM, Eisenlohr PV, Melo MMRF, Andrade LA, Meira Neto JAA, editors. Fitossociologia no Brasil: métodos e estudo de casos. Viçosa: Editora UFV; 2011.
- Ferreira ICM, Coelho RM, Torres RB, Bernacci LC. Solos e vegetação nativa remanescente no município de Campinas, SP. Pesquisa Agropecuária Brasileira 2007; 2(9): 1319-1327. 10.1590/S0100-204X2007000900014
» https://doi.org/10.1590/S0100-204X2007000900014 - Gandolfi S, Leitão Filho HF, Bezerra CLE. Levantamento florístico e caráter sucessional das espécies arbustivo arbóreas de uma floresta mesófila semidecídua no município de Guarulhos, SP. Revista Brasileira de Biologia 1995; 55(4): 753-767.
- I3N Brasil. Base de dados nacional de espécies exóticas invasoras I3N Brasil. 2016 June 10 [cited 2018 Apr. 20]. Available from: Available from: https://bit.ly/2jQYAMa
» https://bit.ly/2jQYAMa - Jardim Botânico do Rio de Janeiro. Flora do Brasil 2020 [Internet]. 2012 Aug. 25 [cited 2017 Dec. 13]. Available from: Available from: https://bit.ly/2OSYpuK
» https://bit.ly/2OSYpuK - Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. PNAS 2007; 104(14): 5925-5930. 10.1073/pnas.0608361104
» https://doi.org/10.1073/pnas.0608361104 - Kronka FJN, Nalon MA, Matsukuma CK. Inventário florestal da vegetação natural do Estado de São Paulo. São Paulo: Secretaria do Meio Ambiente; 2005.
- LPWG - Legume Phylogeny Working Group. Legume phylogeny and classification in the 21st century: progress, prospects and lessons for other species-rich clades. Taxon 2013; 62: 217-248. 10.5167/uzh-78167
» https://doi.org/10.5167/uzh-78167 - Matthes LAF, Leitão Filho HF, Martins FR. Bosque dos Jequitibás (Campinas, SP): composição florística e estrutura fitossociológica do estrato arbóreo. In: Congresso da Sociedade Botânica de São Paulo; 1988; Botucatu. Botucatu: SBSP; 1988. p. 55-76.
- McCune B, Mefford MJ. PC-ORD: multivariate analysis of ecological data. Versão 5. [software]. Gleneden Beach, OR, USA; 1999.
- Medeiros AS, Pereira MG, Braz DM. Estrutura e conservação de um trecho de floresta estacional em Piraí, RJ. Floresta e Ambiente 2016; 23(3): 330-339. 10.1590/2179-8087.106214
» https://doi.org/10.1590/2179-8087.106214 - Mello MHA, Pedro MJ Jr, Ortolani AA, Alfonsi RR. Chuva e temperatura: cem anos de observações em Campinas. Boletim Técnico 1994; 154.
- Mittermeier RA, Gil PR, Hoffman M. Hotspots revisited: Earth’s biologically richest and most endangered terrestrial ecoregions. Washington, DC: Conservation International; 2005.
- Oliveira-Filho A, Fontes MAL. Patterns of floristic differentiation among Atlantic Forests in Southeastern Brazil and the influence of climate. Biotropica 2000; 32: 793-810. 10.1111/j.1744-7429.2000.tb00619.x
» https://doi.org/10.1111/j.1744-7429.2000.tb00619.x - Pennington RT, Lavin M, Oliveira-Filho A. Woody plant diversity, evolution, and ecology in the tropics: perspectives from seasonally dry tropical forests. Annual Review of Ecology, Evolution and Systematics 2009; 40(4): 37-57. 10.1146/annurev.ecolsys.110308.120327
» https://doi.org/10.1146/annurev.ecolsys.110308.120327 - Perigo CV, Torres RB, Bernacci LC, Guimarães EF, Haber LL, Facanali R et al. The chemical composition and antibacterial activity of eleven Piper species from distinct rainforest areas in Southeastern Brazil. Industrial Crops and Products 2016; 94: 528-539. 10.1016/j.insoeop.2016.09.028
» https://doi.org/10.1016/j.insoeop.2016.09.028 - Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM. The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation 2009; 142(6): 1141-1153. 10.1016/j.biocon.2009.02.021
» https://doi.org/10.1016/j.biocon.2009.02.021 - Rodrigues RR. A sucessão florestal. In: Morellato PC, Leitão Filho HF, editors. Ecologia e preservação de uma floresta tropical urbana: Reserva de Santa Genebra. Campinas: Editora Unicamp; 1995. p. 30-35.
- Rolim GS, Camargo MBP, Lima DG, Moraes JFL. Classificação climática de Köppen e de Thornthwaite e sua aplicabilidade na determinação de zonas agroclimáticas para o estado de São Paulo. Bragantia 2007; 66(4): 711-720. 10.1590/S0006-87052007000400022
» https://doi.org/10.1590/S0006-87052007000400022 - Santos K, Kinoshita L, Santos FAM. Tree species composition and similarity in semideciduous forest fragments of southeastern Brazil. Biological Conservation 2007; 135: 268-277. 10.1016/j.biocon.2006.10.027
» https://doi.org/10.1016/j.biocon.2006.10.027 - Shepherd GJ. Manual do FITOPAC 2.0. Campinas: Editora Unicamp; 2008.
- Souza ACO, Torres RB, Bernacci LC, Jung-Mendaçolli SL. Espécies da flora nativa nas estações experimentais da Agência Paulista de Tecnologia dos Agronegócios, Instituto Agronômico de Campinas, estado de São Paulo, Brasil. Hoehnea 2015; 42(1): 59-92. 10.1590/2236-8906-29/2014
» https://doi.org/10.1590/2236-8906-29/2014 - Tabarelli M, Mantovani W. Ocupação de clareiras naturais na Serra da Cantareira - SP. Naturalia 1997; 22: 89-102.
- Torres RB, Adami SF, Coelho RM, editors. Atlas socioambiental da bacia do Ribeirão das Anhumas. Campinas: Pontes, 2014.
- Veiga RFA, Vieira MJFR, Costa AA, Barbosa W, Tombolato AFC, Torres RB et al. A educação ambiental no IAC. O Agronômico 2006; 58(1-2): 5-10.
Publication Dates
-
Publication in this collection
08 May 2020 -
Date of issue
2020
History
-
Received
03 Oct 2017 -
Accepted
31 July 2018