Abstract:
Leguminosae includes six subfamilies, where the traditionally recognised subfamily Mimosoideae was accepted as a distinct clade included within the recircumscribed subfamily Caesalpinioideae, called informally as Mimosoid clade. The representatives of the tribes Acacieae and Ingeae can be differentiated principally in terms of the patterns of their stamens, being free in Acacieae and monadelphous in Ingeae. The floristic survey of Acacieae and Ingeae in the Environmental Protection Area Serra Branca included analysis of specimens collected from June 2011 to September 2012. The analyses were supplemented with dried collections from the following herbaria: ALCB, HRB and HUEFS. Ten species were cataloged, distributed among four genera of Ingeae: Calliandra Benth. (1 sp.), Chloroleucon (Benth.) Britton & Rose ex Record (1 sp.), Enterolobium Mart. (1 sp.), Pithecellobium Mart. (1 sp.); and one genus of Acacieae: Senegalia Raf. (6 spp.). The most representative species were Calliandra aeschynomenoides Benth. associated with sandy and stony soils and Chloroleucon foliolosum (Benth.) G.P.Lewis and Senegalia bahiensis (Benth.) Seigler & Ebinger growing on sandy-clay soils. The taxonomic treatment includes a key for the identification, descriptions, illustrations, photos, data of the geographical distribution phenological data and comments about the species.
Keywords:
Biodiversity; Caatinga; Fabaceae; Floristics; Mimosoids clade
Resumo:
Leguminosae inclui seis subfamílias, onde a tradicional subfamília Mimosoideae foi reconhecida como um clado distinto dentro da recircunscrita subfamília Caesalpinioideae, chamado de clado Mimosoida. Os representantes das tribos Acacieae e Ingeae podem ser diferenciadas principalmente pelo padrão dos estames, sendo livres em Acacieae e monoadelfos em Ingeae. O levantamento florístico de Acacieae e Ingeae na Área de Proteção Ambiental Serra Branca compreendeu análises de espécimes coletados no período de junho 2011 a setembro 2012. As análises foram complementadas com coleções herborizadas depositadas nos seguintes herbários: ALCB, HRB e HUEFS. Foram catalogadas dez espécies, distribuídas em quatro gêneros de Ingeae: Calliandra Benth. (1 sp.); Chloroleucon (Benth.) Britton & Rose ex Record (1 sp.); Enterolobium Mart. (1 sp.) e Pithecellobium Mart. (1 sp.), e um gênero de Acaciae: Senegalia Raf. (6 spp.). As espécies mais representativas foram Calliandra aeschynomenoides Benth. associada a solos arenoso-pedregosos e Chloroleucon foliolosum (Benth.) G.P.Lewis e Senegalia bahiensis (Benth.) Seigler & Ebinger a solos arenoso-argilosos. O tratamento taxonômico inclui uma chave de identificação, descrições, ilustrações, fotos, dados de distribuição geográfica, dados fenológicos e comentários sobre as espécies.
Palavras-chave:
Biodiversidade; Caatinga; Fabaceae; Florística; clado Mimosoide
Introduction
Leguminosae Juss. (Fabaceae) is considered the third largest family of Angiosperms, with approximately 760 genera and about 19,500 species (LPWG 2017LPWG, Legume Phylogeny Working Group. 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66 (1): 44-77.). In Brazil, 223 genera and 2,848 species were recorded to Leguminosae, which is one of the most representative families in the Caatinga, including 611 species (Flora do Brasil 2020Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/reflora...
). According to the new classification, its species are distributed into six subfamilies, namely Caesalpinioideae DC, Cercidoideae LPWG, Detarioideae Burmeist, Dialioideae LPWG, Duparquetioideae LPWG and Papilionoideae DC. The traditionally recognised subfamily Mimosoideae was accepted as a distinct clade included within the recircumscribed subfamily Caesalpinioideae, called informally as Mimosoid clade (LPWG 2017LPWG, Legume Phylogeny Working Group. 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66 (1): 44-77.).
Mimosoid clade have a pantropical distribution and is more common in rain forests, dry savannas, and desert areas (Richardson et al. 2001RICHARDSON, J.E., PENNINGTON, R.T., PENNINGTON, T.D. & HOLLINGSWORTH, P.H. 2001. Rapid diversification of a species-rich group of Neotropical rain forest trees. Science 293: 2242-2245., Ratter at al, 2003RATTER, J.A., BRIDGEWATER, S. & RIBEIRO, J.F. 2003. Analysis of the floristic composition of the Brazilian cerrado vegetation III: Comparison of the woody vegetation of 376 areas. Edinburgh J. Bot. 60: 57-109., Schrire et al. 2005SCHRIRE, B.D., LAVIN, M. & LEWIS, G.P. 2005. Global distribution patterns of the Leguminosae: Insights from recent phylogenies. Biol. Skr. 55: 375-422., LPWG 2017LPWG, Legume Phylogeny Working Group. 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66 (1): 44-77.) The clade includes four tribes: Acacieae Dumort., Ingeae Benth., Mimoseae Bronn and Mimozygantheae Burkart. (Lewis et al. 2005LEWIS, G.P. 2005. Tribe Acacieae. In Legumes of the world (G.P Lewis., B. Schrire, B. MacKinder & M. Lock (eds.). Royal Botanical Gardens, Kew, p.187-191.). The representatives of the tribes Acacieae and Ingeae can be differentiated principally in terms of the patterns of their stamens, being free in Acacieae and monadelphous in Ingeae (Vassal 1981VASSAL, J. 1981. Acacieae. In Advances in Legume Systematics (R.M. Polhill & P.H. Raven, eds). Royal Botanic Gardens, London, p.169-171.).
According to a newly proposed circumscription, the tribe Acacieae includes seven lineages that are treated as genera: Acacia s.s., Acaciella Britton & Rose, Mariosousa Seigler & Ebinger, Parasenegalia Seigler & Ebinger, Pseudosenegalia Seigler & Ebinger, Senegalia Raf., and Vachellia Wight & Arnott, and about 1,381 species (Orchard & Maslin 2003MASLIN, B.R., MILLER, J.T.D. & SEIGLER, D.S. 2003. Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Aust. Syst. Bot. 16: 1-18., Brown et al. 2008BROWN, G.K. 2008. Systematics of the tribe Ingeae (Leguminosae - Mimosoideae) over the past 25 years. Muelleria 26(1): 27-42., Seigler et al. 2017SEIGLER, D.S., EBINGER, J.E, RIGGINS, C.W., TERRA,V. & MILLER, J.T. 2017. Parasenegalia and Pseudosenegalia (Fabaceae): New Genera of the Mimosoideae. Novon 25(2): 180-205.). Among the currently recognized genera, only Parasenegalia, Senegalia and Vachellia are registered to Brazil (Seigler et al. 2017SEIGLER, D.S., EBINGER, J.E, RIGGINS, C.W., TERRA,V. & MILLER, J.T. 2017. Parasenegalia and Pseudosenegalia (Fabaceae): New Genera of the Mimosoideae. Novon 25(2): 180-205., Seigler & Ebinger 2018SEIGLER, D.S. & EBINGER, J.E. 2018. New Combinations in Parasenegallia and Mariosousa (Fabaceae: Mimosoideae). Phytologia 100(4): 256-259., Flora do Brasil 2020Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/reflora...
), with about 63 species of which 17 occur in the Caatinga biome (Queiroz 2009QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana., Morim & Barros 2012, Terra & Garcia 2016TERRA, V. & GARCIA, F.C.P. 2016. A new species of Senegalia (Leguminosae Mimosoideae) from the Caatinga Domain, Brazil. Phytotaxa 288 (2): 181-186., BFG 2018BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113. DOI: 10.1590/2175-7860201566411.
https://doi.org/10.1590/2175-78602015664...
, Flora do Brasil 2020Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/reflora...
).
The tribe Ingeae comprises 37 genera with 966 species (including recent described: Afrocalliandra E.R.Souza and Sanjappa E.R.Souza & M.V.Krishnaraj), with two centers of diversity: one in Central and South America, and a second in southeastern Asia and Australia (Lewis & Rico-Arce 2005LEWIS, G.P. & RICO ARCE, M. L. 2005.Tribe Ingeae. In Legumes of the world (G.P Lewis., B. Schrire, B. MacKinder & M. Lock (eds.). Royal Botanical Gardens, Kew, p.193-213., Brown et al. 2008BROWN, G.K., MURPHY, D.J., MILLER, J.T. & LADIGES, P.Y. 2008. Acacia s.s. and its relationships between tropical legume relatives, tribe Ingeae (Leguminosae: Mimosoideae). Syst. Bot. 33(4): 739-751., Souza et al. 2016SOUZA, E.R. de, KRISHNARAJ, M.V. & L.P. de QUEIROZ. 2016. Sanjappa, a new genus in the tribe Ingeae (Leguminosae: Mimosoideae) from India. Rheedea. 26(1) 1-12.). Ingeae is represented in Brazil by 15 genera with 391 species (Lima et al. 2012LIMA, H. C., QUEIROZ, L. P., MORIM, M. P., DUTRA, V. F., BORTOLUZZI, R. L. C., IGANCI, J. R. V., FORTUNATO, R. H., VAZ, A. M. S. F., SOUZA, E. R., FILARDI, F. L. R., GARCIA, F. C. P., FERNANDES, J. M., MARTINS-DA-SILVA, R. C. V., PEREZ, A. P. F., MANSANO, V. F., MIOTTO, S. T. S., LIMA, L. C. P., OLIVEIRA, M. L. A. A., FLORES, A. S., TORKE, B. M., PINTO, R. B., LEWIS, G. P., BARROS, M. J. F., SCHÜTZ, R, PENNINGTON, T., KLITGAARD, B. B. RANDO, J. G., SCALON, V. R., COSTA, L. C., SILVA, M. J., MOURA, T. M., BARROS, L. A. V., SILVA, M. C. R., QUEIROZ, R. T., SARTORI, A. L. B., CAMARGO, R. A., LIMA, I. B., COSTA, J., SOARES, M. V. B., SNAK, C., VALLS, J. F. M., SÃO-MATEUS, W., FALCÃO, M. J., CARDOSO, D. B. O. S., TOZZI, A. M. G. A., MARTINS, M. V., SOUZA, V. C., MEIRELES, J. E. & REIS, I. P. 2012. Fabaceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://www.floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB115 (last access on 22/05/2012).
http://www.floradobrasil.jbrj.gov.br/jab...
) of which 10 genera and 73 species occur in the Caatinga (Queiroz 2009QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana., Flora do Brasil 2020Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/reflora...
).
The Caatinga has a high rate of endemism and diversity, making a better understanding of its flora necessary for proper conservation measures (Prado 2003PRADO, D.E. 2003. As Caatingas da América do Sul. In Ecologia e conservação da Caatinga (L.M. Tabarelli & J.M.C. Silva, eds.). Universidade Federal de Pernambuco, Recife, p. 1-74.). However, Caatinga is probably the most undervalued and poorly known botanically biome (Giulietti et al. 2002GIULIETTI, A.M., HARLEY, R.M., QUEIROZ, L.P., BARBOSA, M.R.V., BOCAGE NETA, A.L., & FIGUEIREDO, M.A. 2002. Espécies endêmicas da Caatinga. In Vegetação e Flora da Caatinga (E. Sampaio, A.M. Giulietti, J. Virgínio, & C. Gamarra-Rojas (eds.). APNE/CNIP, Recife, p. 103-119.), yet is has the lowest number of conservation units and is one of the least protected in Brazil (Leal et al. 2005LEAL, I.R., SILVA, J.M.C., TABARELLI, M. & LACHER JR, T.E. 2005. Mudando o curso da conservação da biodiversidade na Caatinga do Nordeste do Brasil. Megadiversidade 1(1): 139-146.). Currently the Caatinga has 180 protected areas, which protect 9% of the biome, and 1,8% of this protected area belongs to the category of integral protection, 7,2 % to sustainable use (CNUC/MMA 2018CADASTRO NACIONAL DE UNIDADE DE CONSERVAÇÃO - CNUC/MMA. Unidades de Conservação por Bioma. Brasília. http://www.mma.gov.br/images/arquivo/80229/CNUC_JUL18%20-%20C_Bio.pdf (last access on 10/07/2018).
http://www.mma.gov.br/images/arquivo/802...
).
The Evironmental Protection Area Serra Branca, Raso da Catarina (EPASB) is located located in the municipality of Jeremoabo in Northeastern Bahia fully inserted into the "polígono das secas" (Fundação CTI/NE 2016FUNDAÇÃO CTI/NE. 2016. http://www.ctinordestedobrasil.com.br/poligono.html (last access on 19/01/2017).
http://www.ctinordestedobrasil.com.br/po...
). In the floristic surveys carried out in EPASB were cataloged 11 species to Bignoniaceae (Silva et al. 2016SILVA, L.R., SILVA CASTRO, M.M. & CONCEIÇÃO, A.S. 2016. The family Bignoniaceae in the Environmental Protection Area Serra Branca, Raso da Catarina, Jeremoabo, Bahia, Brazil. Acta Sci., Biol. Sci. 38(4): 395-409.), 10 species to Boraginaceae (Vieira et al. 2013VIEIRA, D.D., CONCEIÇÃO, A.S., MELO, J.I.M. & STAPF, M.N.S. 2013. A família Boraginaceae sensu lato na APA Serra Branca/Raso da Catarina, Bahia, Brasil. Rodriguésia 64(1): 151-168.), 23 species to Euphorbiaceae (Lopes 2012LOPES, A.A.S. 2012. Diversidade de Euphorbiaceae nas Caatingas arenosas da APA Serra Branca, Jeremoabo, Bahia, Brasil. Dissertação de mestrado, Universidade do Estado da Bahia, Paulo Afonso.), 16 species to Malvoideae-Malvaceae (Lima & Conceição 2016LIMA, J.B. & CONCEIÇÃO, A.S. 2016. Malvoideae Burnett (Malvaceae) in the Environmental Protection Area Serra Branca, Raso da Catarina, Jeremoabo, Bahia, Brazil. Biota Neotropica 16(4): e20160187. http://dx.doi. org/10.1590/1676-0611-BN-2016-0187 (last access on 19/01/2017).
http://dx.doi. org/10.1590/1676-0611-BN-...
), 11 species to Mimosa L. (Dourado et al. 2013DOURADO, D.A.O., CONCEIÇÃO, A.S. & SANTOS-SILVA, J. 2013. O gênero Mimosa L. (Leguminosae: Mimosoideae) na APA Serra Branca/Raso da Catarina, Bahia, Brasil. Biota Neotropica 13(4):http://www.biotaneotropica.org.br/v13n4/en/abstract?inventory+bn01713042013 (last access on 19/01/2018).
http://www.biotaneotropica.org.br/v13n4/...
), four species to Passifloraceae (Santos et al. 2016SANTOS, J.V, NUNES, T.S. & CONCEICÃO, A.S. 2016. A família Passifloraceae na APA Serra Branca/Raso da Catarina, Jeremoabo, Bahia, Brasil. Biotemas 29(1): 11-23.), and 21 species to Rubiaceae (Varjão et al. 2013VARJÃO, R.R., JARDIM, J.G. & CONCEICÃO, A.S. 2013. Rubiaceae Juss. de caatinga na APA Serra Branca/Raso da Catarina, Bahia, Brasil. Biota Neotropica 13(2): http://www.biotaneotropica.org.br/v13n2/en/abstract?inventory+bn00313022013 (last access on 19/01/2017).
http://www.biotaneotropica.org.br/v13n2/...
).
Studies on taxa of Mimosoid clade in areas of Caatinga in Bahia are few. Given the significant rate of endemism and diversity for the Caatinga biome, and the limited number of surveys for the clade therein, this study aimed to contribute to a better understanding of the Mimosoid clade in the EPASB, in order to contribute to knowledge about the flora of the semiarid region of Bahia, as well as to support the development of the area's management plan.
Materials and Methods
1. Study area
The Evironmental Protection Area Serra Branca, Raso da Catarina (EPASB, Figure 1) comprises 67,237 ha., located in the municipality of Jeremoabo in Northeastern Bahia fully inserted into the "polígono das secas" (Fundação CTI/NE 2016FUNDAÇÃO CTI/NE. 2016. http://www.ctinordestedobrasil.com.br/poligono.html (last access on 19/01/2017).
http://www.ctinordestedobrasil.com.br/po...
), delimited by the coordinates 09º53'15.5'' to 09º44'34.6''S and 38º49'36.1''to 38º52'20.4''W, limited to the South with the Vaza-Barris River and North to the Ecological Station Raso da Catarina (ESEC). The predominant vegetation is the sandy, very dense bushy Caatinga. The climate of the Ecoregion is semiarid, with average rainfalls of 500 mm/year and annual temperature is approximately 23ºC (Szabo et al. 2007SZABO, A.V., ROCHA, A.C.S., TOSATO, J.A. DE C. & BARROSO, W. 2007. Área de proteção ambiental (APA) Serra Branca Raso da Catarina. In As Caatingas: debates sobre a Ecorregião do Raso da Catarina (J. Marques, org.). Fonte Viva, Paulo Afonso. p. 21-40.). The soils are generally sandy deep and very fertile relief plan with sandstone formations (Velloso et al. 2002VELLOSO, A.L., SAMPAIO, E.V.S.B. & PAREYN, F.G.C. 2002. Ecorregiões propostas para o bioma Caatinga. Associação Plantas do Nordeste, Recife.).
Location of the EPA Serra Branca/Raso da Catarina, Bahia, Brazil (Varjão et al. 2013VARJÃO, R.R., JARDIM, J.G. & CONCEICÃO, A.S. 2013. Rubiaceae Juss. de caatinga na APA Serra Branca/Raso da Catarina, Bahia, Brasil. Biota Neotropica 13(2): http://www.biotaneotropica.org.br/v13n2/en/abstract?inventory+bn00313022013 (last access on 19/01/2017).
http://www.biotaneotropica.org.br/v13n2/... ).
2. Taxonomic study
The study was based on fieldwork carried in the period from June 2011 to September 2012, besides information complemented by the analysis of specimens deposited in the following herbaria: ALCB, HRB, and HUEFS, acronyms according to Thiers 2018THIERS, B. 2018 [continuously updated]. Index Herbariorum: a global directory of public herbaria and associated staff. In New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/ (last access on 19/04/2018).
http://sweetgum.nybg.org/ih/...
(continuously updated; Appendix 1
Supplementary material
The following online material is available for this article:
The Appendix 1 - List of additional material examined.
). The field collections and observations were performed during random walks exploring most of the study area. The herborization and material processing followed the methodology by Fosberg & Sachet (1965FOSBERG, F.R. & SACHET, M.H. 1965. Manual for tropical herbaria. Utrecht, Netherlands.) and Mori et al. (1989MORI, S.A., SILVA, L.A.M., LISBOA, G. & CORADIN, L. 1989. Manual de manejo do herbário fanerogâmico. Centro de Pesquisa do Cacau, Ilhéus.), where fertile material was collected with flowers and/or fruit. Observations were made about the distribution of the species and the type of soil (Tricart 1972TRICART, J. 1972. The landforms of the humid tropics, forests and savannas. Geographies for Advanced Study, Longman, London., Sampaio 1995SAMPAIO, E.V.S.B. 1995. Overview of the Brazilian caatinga. In Seasonally dry tropical forests (S.H. Bullock, H.A. Mooney & E. Medina, eds.). Cambridge University Press, Cambridge, p. 34-63.). The specimens were deposited in the herbarium of the State University of Bahia (HUNEB - Paulo Afonso Collection) and the duplicates will be sent to the main herbaria in the state of Bahia (ALCB, HRB and HUEFS).
The identifications were made based mainly on specialized bibliographies, protologues, photos of type collections and comparison of the collections in the visited herbaria. For the taxonomic descriptions, the terminologies proposed by Mesquita (1990)MESQUITA, A.L. 1990. Revisão taxonômica do gênero Enterolobium Mart. (Mimosoideae) para a região Neotropical. Dissertação de Mestrado, Universidade Federal Rural de Pernambuco, Recife., Barneby & Grimes (1996)BARNEBY, R.C. & GRIMES, J.W. 1996. Silk tree, Guanacaste, Monkey's Earring: A generic system for the Synandrous Mimosaceae of the AmericasMem. N. Y. Bot. Gard. 74(1): 1-292., Barneby & Grimes (1997)BARNEBY, R.C. & GRIMES, J.W. 1997. Silk Tree, Guanacaste, Monkey's Earring: A Generic System for the Synandrous Mimosaceae of the Americas. Pithecellobium, Cojoba e Zygia. Mem. N. Y. Bot. Gard. 74(2): 1-149., Barneby (1998)BARNEBY, R.C. 1998. Silk Tree, Guanacaste, Monkey's Earring: A Generic System for the Synandrous Mimosaceae of the Americas. Calliandra. Mem. N. Y. Bot. Gard. 74(3): 1-223., Harris & Harris (2001), Seigler et al. (2006)SEIGLER, D.S, EBINGER, J.E. & MILLER, J.T. 2006. The genus Senegalia (Fabaceae: Mimosoideae) from the New World. Phytologia 88(1): 38-93., and Melo et al. (2010)MELO, Y., CÓRDULA, E.R., MACHADO, S.R. & ALVES, R. 2010. Morfologia de nectários em Leguminosae senso lato em áreas de caatinga no Brasil. Acta Bot. Bras. 24(4): 1034-1045. were adopted. The inflorescences were measured considering principally the stamens. The taxonomic treatment includes an identification key, descriptions, illustrations, photos, data of the geographical distribution, phenological data and comments about the species.
Results
Ten taxa were recorded for the tribes Acacieae and Ingeae in the EPASB (Figure 2). For the Acacieae, six species of the genus Senegalia were cataloged: S. bahiensis (Benth.) Seigler & Ebinger, S. piauhiensis (Benth.) Seigler & Ebinger, S. limae (Bocage & Miotto) L.P.Queiroz, S. globosa (Bocage & Miotto) L.P.Queiroz, S. polyphylla (DC.) Britton & Rose, and S. tenuifolia (L.) Britton & Rose. For the Ingeae, four genera were recorded, with only one species each: Pithecellobium diversifolium Benth., Chloroleucon foliolosum (Benth.) G.P.Lewis, Calliandra aeschynomenoides Benth., and Enterolobium timbouva Mart.
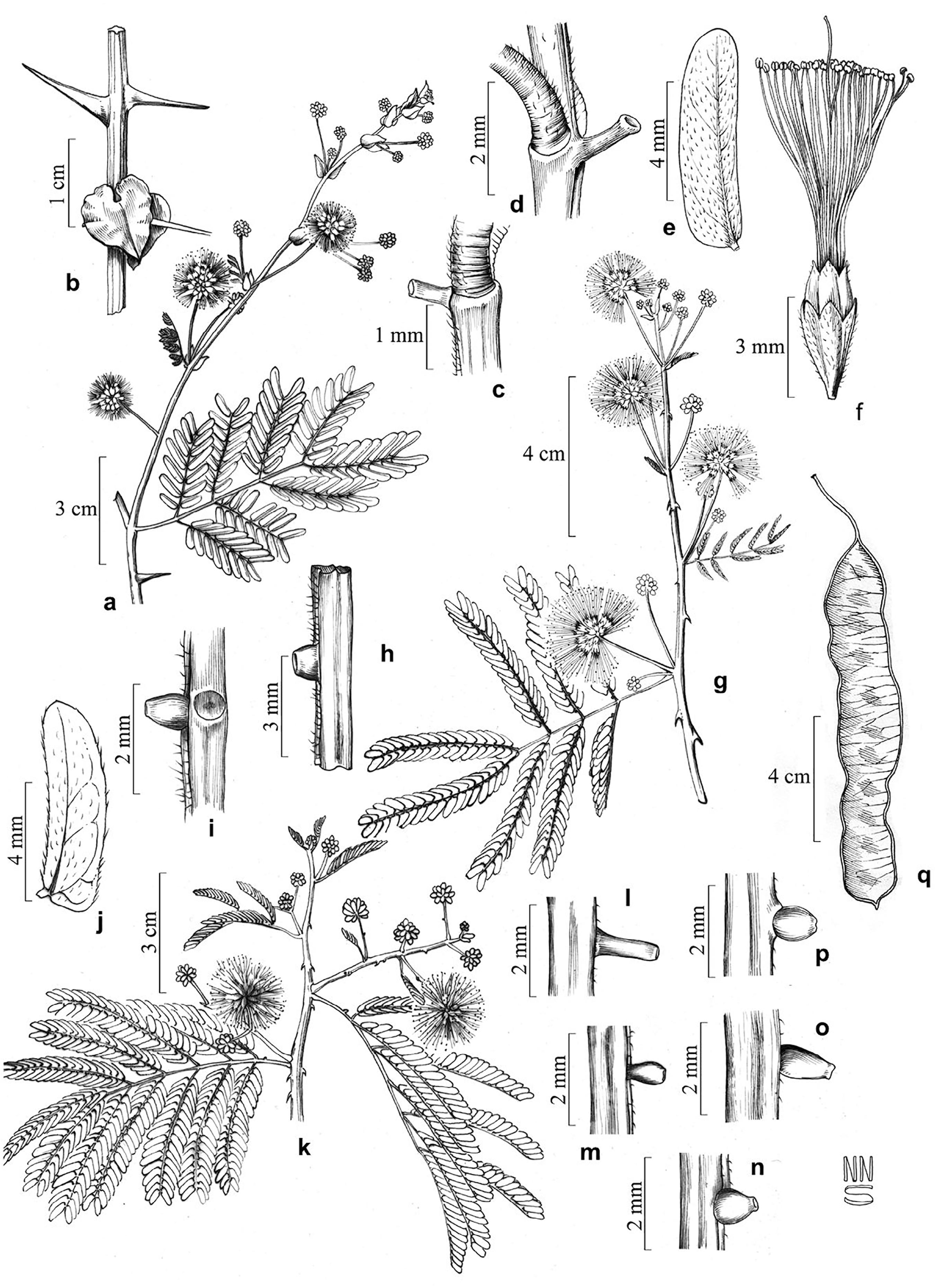
Representatives of the Tribes Acacieae and Ingeae in the EPASB. a-j. Acacieae: a-b. Senegalia bahiensis. c. Senegalia globosa. d-e. Senegalia limae. f. Senegalia piauhiensis. g-h. Senegalia polyphylla. i-j. Senegalia tenuifolia. k-q. Ingeae: k-l. Calliandra aeschynomenoides. m-n. Chloroleucon foliolosum. o. Enterolobium timbouva. p-q. Pithecellobium diversifolium.
The species in the EPASB are distributed throughout the subshrub to arboreal caatinga vegetation in well-preserved areas and in anthropogenically modified zones growing on sandy, sandy-stony, or sandy-clayey soils. The most representative species were Calliandra aeschynomenoides, Chloroleucon foliolosum, and Senegalia bahiensis. Calliandra aeschynomenoides occurs in fragments of the EPASB, associated with shrub vegetation on sandy-stony soils. Chloroleucon foliolosum and S. bahiensis are associated with shrub to arboreal caatinga vegetation occurring on sandy-clayey soils. The diagnostic morphological characters used to identify these taxa in the area were: the presence or absence of prickles, spines, the shapes of the stipules, numbers of leaflet pairs; shapes and locations of the extrafloral nectaries, inflorescence patterns, and types of fruits.
1. Tribe Acacieae Benth
Trees, small trees, or shrubs. Branches with or without prickle. Stipules not spiny, foliaceous, cordiform, oval, lanceolate or filiform. Leaves bipinnate, pinnae 2-11 pairs, leaflets 8-31 pairs. Extrafloral nectaries elevated, globose, concave, estipitate cylindrical, caliciform or conical . Inflorescences in spikes or in panicle homomorphic. Flowers sessile, stamens polystamenous, free. Fruit chartaceous, dehiscent, linear.
2. Tribe Ingeae Benth
Trees, small trees, shrubs or subshrubs. Branches with or without prickle. Stipules spiny (transformed into spines) or foliaceous, oval to lanceolate. Leaves 1-6 pairs of pinnae, leaflets 1-24 pairs. Extrafloral nectaries present or (rarely) absent (only in Calliandra Benth.). Inflorescences in homomorphic or heteromorphic glomerules; when heteromorphic, central flower differentiated with long perianth, showing greatly exserted stamens. Flowers sessile or pedicellate, stamens polystamenous, monadelphous. Fruit chartaceous or coriaceous indehiscent or dehiscent, linear, oblanceolate, linear flat to curved 90º to 360º, auriculate, linear spiraled.
Identification key for the representatives of the Acacieae and Ingeae in the EPASB
-
1. Extrafloral nectaries absent on the petiole and/or leaf rachis, flowers red-vinaceous ................................................ 1. C. aeschynomenoides
-
1'. Extrafloral nectaries present on the petiole and/or leaf rachis, flowers greenish white, green, white, light green, cream-colored.
-
2. Flowers with stamens monadelphous.
-
3. Branches with nodal paired and isolated spines; dormant buds perulate; glomerules heteromorphic ................. 2. Ch. foliolosum
-
3' Branches unamard sometimes with stipules spiny; dormant buds absent; glomerules homomorphic.
-
4. Stipules foliaceous, caducous; leaves 2-4 pairs of pinnae; petiole cylindrical, furrow absent; corolla campanulate, glabrous, apices of the lacinias pubescent; fruit indehiscent, auriculate ................................................................................ 3. E. timbouva
-
4'. Stipules spiny and persistent; leaves 1-2 pairs of pinnae; petiole sulcate; corolla infundibuliform villous, lacinias villous; fruit dehiscent, sigmoidal ............................ 4. P. diversifolium
-
-
-
2'. Flowers with stamens free.
-
5. Branches slightly angular.
-
6. Prickle straight or incurvate; stipules foliaceous, cordiform, to oval, 8-9 mm long ......................................... 5. S. bahiensis
-
6'. Prickle recurved; stipules linear, lanceolate, oval, 2.5-6 mm long.
-
7. Petiole 12-17 mm long; extrafloral nectaries cylindrical or conical; glomerules 17-22 mm diam.; flowers 11-12 mm long .................................................................. 6. S. globosa
-
7'. Petiole 3-5 mm long; extrafloral nectaries globose, concave, estipitate cylindrical, caliciform or conical; glomerules 25-30 mm diam.; flowers 16-18 mm long ..................... 7. S. limae
-
-
-
5'. Branches cylindrical.
-
8. Leaves 2-4 pairs of pinnae; extrafloral nectaries sessile elevated-concave to enclosed- concave inflorescences in spikes ......................................................................... 8. S. piauhiensis
-
8'. Leaves 6-11 pairs of pinnae, extrafloral nectaries elevated-concave to short stipitate; inflorescences in panicle.
-
9. Branches not lenticular; prickle incurvate not forming in triplets in the nodal region, midrib with a tuft of trichomes at the base of the abaxial face ...................... 9. S. polyphylla
-
9'. Branches lenticular; prickle recurved, in triplets in the nodal region, midrib with a tuft of trichomes at the base of the abaxial face absent ...................................... 10. S. tenuifolia
-
-
-
-
1. Calliandra aeschynomenoides Benth., Flora Brasiliensis 15(3): 415. 1876. Figures 2k-l; 3a-d.
a-d. Calliandra aeschynomenoides. a. flowering branch; b. leaflet, abaxial surface; c. flower; d. fruit. e-j. Chloroleucon foliolosum. e. fruiting branch showing leaves and nodal thorns; f. gems; g. glomerulus; h. peripheral flower; i. central flower; j. fruit. a-d from A. F. S. Brito 13; e-j from A. F. S. Brito 33.
Subshrub to shrub 1.2-2 m tall. Branches cylindrical, unarmed, grayish, pubescent. Tector trichomes distributed on the branches, stipules, petioles, leaf rachis, leaflets, peduncles, bracts, calyx, corolla, ovaries, and fruits. Buds not observed. Stipules persistent 3-6.5 × 1-1.5 mm, foliaceous, oval to lanceolate, striated, glabrescent. Extrafloral nectaries absent. Leaves with 1 pair of pinnae; petiole 0.5-1.5 cm long, subcylindrical, pilous; rachis 0.5-1 mm long, cylindrical, villous, unarmed; paraphylls absent; leaflets 7-14 pairs, 5-11 × 1.5-4 mm, chartaceous, discolorous, oblong-elliptic, apices acuminate, base oblique, margins entire, adaxial face glabrous, abaxial face pilous; venation campylodromous-brochidodromous, midrib exserted. Homomorphic glomerules axillary, isolated, rarely opposite, 6-9 mm diam.; peduncle 4-6 mm long, sulcate, villous; bracts 2.5-5 mm long, persistent, striated, adaxial face glabrous, abaxial face glabrescent. Flowers 15-20.5 mm long, pentamerous, polystamenous, sessile, 13-16 per inflorescence; calyx 1.5-2.0 mm long, campanulate, striated, vinaceous, pubescent; lacinias ca. 0.5 mm long, erect, striated; corolla 3-3.5 mm long, campanulate, striated, red-vinaceous, pubescent; lacinias 1-1.5 mm long, erect, striated; stamens, filaments 2-4.5 mm long, red; stamen tube 12-15 mm long, exserted; style 15-20 mm long, red; ovary 1.5-2 mm long, sessile, red-vinaceous, glabrescent. Fruits dehiscent, 3.5-6.2 × 0.4-0.5 cm, linear, apices acuminate, base acute stipitate, coriaceous, villous. Seeds 5.5-6.5 × 2.5-3 mm, green, oval to elliptic.
Materials examined: BRAZIL. BAHIA: Jeremoabo, APA Serra Branca, Baixão do Araçá, 8/V/2008, fl., M.V. Romão 133 (HUNEB); Estrada sentido ESEC Raso da Catarina, próximo ao povoado Quelés, 16/VI/ 2011, fl., A.F.S. Brito 13l (HUNEB); Estrada de acesso ao povoado Quelés, sentido ESEC Raso da Catarina, 28/VII/2009, fl., fr., A.S. Conceição 1772 (HUNEB); Fazenda Serra Branca, trilha da coleta, 19/IV/2008, fl., A.S. Conceição 1376 (HUNEB); Estrada sentido Vaca Morta, 4/XI/ 2011, fl., A.F.S. Brito 40 (HUNEB).
2. Chloroleucon foliolosum (Benth.) G.P.Lewis, Legumes of Bahia: 166.1987. Pithecellobium foliolosum Benth., London J. Bot. 3: 223.1844. Figures 2m-n; 3 e-j.
Shrub to tree 1.5-6 m tall. Branches cylindrical, flexuous, armed with nodal spines, brown to grayish, lenticular, glabrous to glabrescent. Tector trichomes distributed on the branches, petioles, leaf rachis, leaflets, and peduncles. Spines 10-13 mm long, straight, isolated or paired, glabrescent when young. Buds 1.5-2 mm long, striated, perulate, dormant. Stipules caducous, not observed. Extrafloral nectaries 0.5-1 mm long, elevated to short stipitate, caliciform to cylindrical, located on the petiole and rachis, between the last three pairs of pinnae and the distal leaflet pairs. Leaves with 5-6 pairs of pinnae, displayed on short lateral branches; petiole 1-1.5 mm long, flat, oblong, villous; rachis 2-3.5 mm long, sulcate, glabrescent; rachis segments 5-10 mm long; paraphylls absent; leaflets 13-24 pairs, 3-5.5 × 1-1.5 mm, chartaceous, discolorous, linear, falcate, apices acute, base oblique, margins entire; glabrous to pubescent; venation campylodromous-brochidodromous, midrib sub-centric. Glomerules heteromorphic, axillary, isolated or in pairs in the leaf axils, 20-25 mm diam., peduncle 11-17 mm long, sulcate, glabrous; bracts 2-3.5 mm long, caducous, slightly striated, margins pubescent. Flowers peripheral 17-18.5 mm long, pentamerous, subsessile, 10-22 per inflorescence, calyx 1.5-2 mm long, infundibuliform to campanulate, greenish white, glabrous; lacinias small ca. 1 mm long; corolla 4-5.5 mm long, infundibuliform, greenish white, glabrous; lacinias 1-1.5 mm long, erect, margins ciliate; stamens monadelphous, filaments 11-12 mm long, white; stamen tube 2-2,5 mm long (peripheral flowers), style 14-15 mm ovary 1.8-2 mm long, subsessile, glabrous. Central flowers 18-19 mm long, pentamerous, sessile, 1-2 per inflorescence; calyx 1.0-1.5 mm long, infundibuliform to campanulate, greenish white, glabrous; lacinias small 1-1.2 mm long; corolla 5-6 mm long, infundibuliform, greenish white, glabrous; lacinias 1-1.5 mm long, erect, margins ciliate; stamens enclosed within the corolla, 9-11 mm long, exserted, dilated and fimbriate at apex; style 17-17.5 mm long, white; ovary ca. 2 mm long, subsessile, glabrous. Fruits indehiscent 11-28 cm long, coriaceous, glabrous, linear, flat to curved 90º to 360º, apices acute to obtuse, base cuneate, margins thickened this, slightly undulating, valves fleshy, extending over the seeds. Seeds 5-6 mm long, greenish gray, oval.
Materials examined: BRAZIL. BAHIA: Jeremoabo, Faz. Serra Branca, estrada sentido Serra do Navio, 03/XI/2011, fl., fr., A.F.S. Brito 33 (HUNEB); 03/XI/ 2011, fl., fr., A.F.S. Brito 32 (HUNEB); 03/XI/2011, fl., fr., A.F.S. Brito 34 (HUNEB); Serra do Navio, Caminho da Judite, 03/XI/2011, fl., fr., A.F.S. Brito 29 (HUNEB); Trilha em direção ao Saco da Onça, 30/VII/2009, fl., fr., A.S. Conceição 1823 (HUNEB); Trilha sentido Serra do Navio, 31/VII/2009, fl., fr., A.S. Conceição 1881 (HUNEB); Vaca Morta, 22/VIII/2008, fl., M.V. Romão 267 (HUNEB); 17/IX/2009, fl., fr., M.V. Romão 304 (HUNEB); 17/IX/2009, fl., M.V. Romão 469 (HUNEB).
3. Enterolobium timbouva Mart., Flora 20 II. Beibl.128.1837. Figures 2o; 4a-d.
Tree 8-10 m tall. Branches cylindrical, unarmed, grayish, lenticular, glabrous. Tector trichomes distributed on the peduncles and lacinias of the calyx and corolla. Buds no observed. Stipules caducous, 0.5-1 mm long, foliaceous, linear, glabrous. Extrafloral nectaries 1-1.5 × ca. 0.5 mm, elevated, cylindrical to elliptic, located on the petiole below the basal pair of pinnae, additional nectaries below the leaflet pairs. Leaves with 2-4 pairs de pinnae; petiole 4.4-8.3 cm long, cylindrical, furrow absent, glabrous; rachis 5.8-12.8 cm long, cylindrical, costate, glabrous to glabrescent; rachis segments 13-22 mm long; paraphylls short or vestigial; 9-11 leaflet pairs, 20-25 × 8-10 mm, chartaceous, discolorous, oblong, slightly falcate, apices apiculate, base oblique, margins entire, glabrous to sericeous; venation campylodromous-brochidodromous, midrib exserted. Homomorphic glomerules united in axillary bundles, 20-25 mm diam.; peduncle 1.8-5 mm long, cylindrical, sulcate, villous; bracts c. 2 mm, caducous, oval, glabrous. Flowers 12-16 mm long, pentamerous, subsessile, c. 25 flowers per inflorescence; calyx 4.5-5 mm long, tubular to slightly campanulate, green, glabrous; lacinias 1-2 mm long, apices pubescent; corolla 7.5-8.5 mm long, campanulate, green, glabrous, lacinias 2-3 mm long, apices pubescent; stamens monadelphous, filaments 14-15 mm long, whitish; stamen tube 7-9 mm long, exserted; style 14-15.5 mm long, whitish; ovary 2-2.5 mm long, stipitate, glabrous, stipe c. 5 mm long. Fruits indehiscent 11.5-3.6 × 3.2-5.7 cm, auriculate, stipitate, blackish when mature, coriaceous, glabrous, oblong, curved. Seeds not observed.
a-d. Enterolobium timbouva. a. leaflet, abaxial surface; b. fruiting branch; c. flower; d. fruit. e-h. Pithecellobium diversifolium. e. fruiting branch; f. detail of the leaves and spiny stipules. g. leaflet, abaxial surface; h. fruit. a-d from A. F. S. Brito 38; D. D. Vieira 56; e-h from A. F. S. Brito 38.
Materials examined: BRAZIL. BAHIA: Jeremoabo, APA Serra Branca, caminho da ESEC em direção ao Povoado Quelés, 09/12/2009, fl., D.D. Vieira 56 (HUNEB); Estrada ca. 200 m do Povoado Quelés, 26/III/2012, fl., A.F.S. Brito 44 (HUNEB); Estrada principal sentido Povoado Quelés, próximo a roça com bebedouro, 16/VI/2011, fr., A.F.S. Brito 17 (HUNEB); Estrada do Tamburi, 14/V/ 2012, fr., A.F.S. Brito 55 (HUNEB).
4. Pithecellobiumdiversifolium Benth., London J. Bot.3: 201. 1844. Figures 2p-q; 4e-h.
Tree ca. 5 m tall. Branches cylindrical, flexuous, grayish, lenticular, glabrous. Spines absent. Tector trichomes distributed on the petioles, leaf rachis, leaflets, peduncles, bracts, calyx, corolla, and ovaries. Buds not observed. Stipules spiny, persistent, 5-20 mm long, glabrous. Extrafloral nectaries ca. 0.5 mm long, elevated, cylindrical, located on the rachis, between each pair of pinnae and the on the pair of leaflets. Leaves with 1-2 pairs of pinnae; petiole 2-10 mm long, sulcate, winged, pubescent; leaf rachis 2-10 mm long, cylindrical, canaliculate, pubescent; rachis segments 3-5 mm long; paraphylls absent; leaflets 1-2 pairs, 1-2 × 0.5-0.9 cm, chartaceous to subcoriaceous, discolorous, elliptic to oval, apices retuse to mucronate, base subcordate to oblique, margins entire, slightly revolute, glabrescent to villous; venation campylodromous-brochidodromous, midrib sub-centric, with a tuft of white trichomes at the base of the abaxial surface. Homomorphic glomerules in axillary bundles, ca. 20 mm long; peduncle ca. 15 mm long, costate, villous; bracts caducous, 0.5-1 mm long, spatulate, abaxial surface pubescent. Flowers 20-22 mm long, pentamerous, sessile, ca. 10 flowers per inflorescence; calyx 2-2.5 mm long, infundibuliform, greenish white; lacinias ca. 0.1 mm long; corolla 7-8 mm long, infundibuliform, greenish white, villous; lacinias 2-3 mm long, villous; stamens monadelphous, filaments 21-24 mm long, cream-colored; stamen tube 6-8 mm long, cream-colored, enclosed or slightly exserted from the corolla; style ca. 14 mm long; ovary ca. 2 mm long, stipitate, glabrous to glabrescent; stipe 1.2-2 mm long. Fruits dehiscent, 2.5-7×7-8 cm, sigmoidal, red when mature, coriaceous, glabrous, linear spiraled, short stipitate, apex acuminate, base attenuated; stipe ca. 3 mm long. Seeds 10-11 mm long, brilliant black, oblong to oval, aril red.
Materials examined: BRAZIL. BAHIA: Jeremoabo, APA Serra Branca, próximo ao rio Vaza Barris, 20/IX/2008, fl., M.V.V. Romão 337 (HUNEB); Fazenda. Serra Branca, caminho Velho em direção ao João Gomes, depois da porteira, 03/XI/2011, fl., fr. A.F.S. Brito 38 (HUNEB).
5. Senegalia bahiensis (Benth.) Seigler & Ebinger, Phytologia 88(1): 49. 2006SEIGLER, D.S, EBINGER, J.E. & MILLER, J.T. 2006. The genus Senegalia (Fabaceae: Mimosoideae) from the New World. Phytologia 88(1): 38-93.. Acacia bahiensis Benth. Trans. Linn. Soc. London 30 (3): 525. 1875. Figures 2a-b; 5a-f.
a-f. Senegalia bahiensis. a. flowering branch; b. details of the stipule and prickle; c. stipe cylindrical extrafloral nectaries; d. stipe caliciform extrafloral nectaries; e. leaflet, abaxial surface; f. flower. g-j. Senegalia globosa. g. flowering branch; h. globose extrafloral nectaries; i. conic extrafloral nectaries; j. leaflet, adaxial surface. k-q. Senegalia limae. k. flowering branch; l. stipitate cylindrical extrafloral nectaries; m. calicioide extrafloral nectaries; n-o. conic extrafloral nectarie; p. globose concave extrafloral nectaries; q. fruit. a-f from . A. F. S. Brito 48; g-j from A. F. S. Brito 96; A. S. Conceição 1765; k-q from A. F. S. Brito 91.
Shrub ca. 7 m tall. Branches angular, aculeate, brown, glabrous, glabrescent to villous; prickle 1-3.4 mm long, straight or incurvate, base thick. Tector trichomes distributed on the branches, stipules, petioles, leaf rachis, leaflets, inflorescence axis, bracts, calyx and ovaries. Buds not observed. Stipules caducous, 8-9 × 4-7 mm, foliaceous, cordiform to oval, glabrous to pubescent. Extrafloral nectaries 0.5-1.2 × ca. 0.5 mm, stipe cylindrical to caliciform, located on the petiole just below the basal pair of pinnae, or on the rachis between the basal pair of pinnae, additional nectaries between the other pairs of pinnae. Leaves with 3-6 pairs of pinnae; petiole 1-2.2 cm long, sulcate, winged, laterally constricted, glabrous to glabrescent; leaf rachis 2.4-6.4 cm long, sulcate, pubescent, armed or aculeate; rachis segments 9-12 mm long; paraphylls elliptic; leaflets 5-10 pairs, 4-11 × 2-3.5 mm, chartaceous, discolorous, oblong, flat, apices obtuse to rounded, base oblique, margins ciliate, glabrous to pubescent; venation campylodromous-brochidodromous, midrib exserted, with a tuft of trichomes at the base of the abaxial surface. Homomorphic glomerules, united in bundles located in the terminal or axillary (rarely) panicles on distal leaves, 7-9 mm diam.; peduncle 16-24 mm long, canaliculate, villous; bracts 6-10 × 3-6 mm long, persistent, oval, glabrous to pubescent at the base of the peduncle. Flowers 8-9 mm long, pentamerous, sessile, 22-35 per inflorescence; calyx 2.5-3 mm long, tubular, green, pubescent; lacinias ca. 5 mm long; corolla 4-4.5 mm long, tubular, green, glabrous, lacinias ca. 1 mm long; stamens, filaments 7-10 mm long whitish; style 11.5-12 mm long, whitish; ovary ca. 1 mm long, stipitate, tomentose; stipe 1.9-2 mm long. Fruits dehiscent, 7.5-10.1 × 1.4-2.1 cm, light brown when mature, chartaceous, glabrescent to villous, linear, apices acute, base obtuse, margins slightly undulating. Seeds not observed.
Materials examined: BRAZIL. BAHIA: Jeremoabo, Fazenda Serra Branca, Baixa dos Mandacarus, próximo ao barreiro, 27/III/2012. A.F.S. Brito 48, fl.; fr. (HUNEB); 9 km da base da APA, 09/V/2008, M.V.V. Romão 153, fr. (HUNEB); Estrada sentido Serra do Navio, 10/VII/2012, A.F.S. Brito 93, fl. (HUNEB); Dedo de Deus, 10/VII/2012, J.V. Santos 41, fl. (HUNEB). Serra do Navio, 3/XI/2011, A.F.S. Brito 30, fr. (HUNEB); Serra do Navio, próximo ao barreiro, 10/VII/2012, J.V. Santos 39, fl.; fr. (HUNEB); Idem, 27/III/2012, A.F.S. Brito 49, fl.; fr. (HUNEB); 27/III/2012, A.F.S. Brito 50 (HUNEB); Vaca Morta, 13/III/2008, A.S. Conceição 1258, fl. (HUNEB); 17/IV/2008, A.S. Conceição 1283, fl. (HUNEB).
6. Senegalia globosa (Bocage & Miotto) L.P.Queiroz, Legum. Caatinga 196. 2009QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana.. Acacia globosa Bocage & Miotto, Rodriguésia 57: 131.2006. Figures 2c; 5g-j.
Shrub ca. 2 m tall. Branches slightly angular, aculeate, cream-colored to brown, glabrous; prickle 1-3 mm long, sparse, recurved, base thick. Tector trichomes distributed on the petioles, leaf rachis, leaflets, bracts, peduncles, and calyx. Buds absent. Stipules caducous, 2.5-3.5 × 0.5-1 mm, linear to lanceolate. Extrafloral nectaries 0.5-1.5 × ca. 0.5 mm, cylindrical to conical, located on the petioles, below the basal pair of pinnae, additional nectaries along the rachis or between the last two pairs of leaflets. Leaves with 4-8 pairs of pinnae; petiole 12-17 mm long, slightly sulcate, winged, laterally constricted, sericeous; rachis 2.5-4.1 cm long, canaliculate, sericeous; rachis segments 7.5-10 mm long; paraphylls linear to oblong; leaflets 18-24 pairs, 5-8 × 1.5-3 mm, chartaceous, discolorous, linear to oblong, slightly falcate, apices obtuse, base oblique, margins ciliate; venation campylodromous-brochidodromous, midrib exserted, with tuft of sericeous trichomes at the base of the abaxial face. Homomorphic glomerules united in axillary and terminal bundles, 17-22 mm diam.; peduncle 1.4-2.3 cm long, canaliculate, pubescent; bracts ca. 1 mm long, caducous, lanceolate, pubescent, at base of peduncle. Flowers 11-12 mm long, pentamerous, polystamenous, sessile, ca. 22 per inflorescence; calyx 2-2.5 mm long, campanulate to tubular, costate, white, glabrescent; lacinias ca. 5 mm long; corolla 4-5 mm long, tubular, white, glabrous, lacinias c. 0.5 mm long, margins ciliate; stamens 11-12 mm long, whitish; style 10.5-11.5 mm long, white; ovary ca. 1 mm long, stipitate, glabrous, stipe 1.0-2.5 mm long. Fruit not observed.
Materials examined: BRAZIL. BAHIA: Jeremoabo, APA Serra Branca, Baixa dos Quelés, 11/VII/2012, fl., A.F.S. Brito 96 (HUNEB); Baixa Grande, próximo ao Povoado dos Quelés, 19/III/2009, fl., A.S. Conceição 1592 (HUNEB); Quelés, 05/VI/2012, fl., A.F.S. Brito et al. 67 (HUNEB); Quelés, trilha que dá acesso à Estação Ecológica Raso da Catarina, 19/VI/2009, fl., A.S. Conceição 1765 (HUNEB).
7. Senegalia limae (Bocage & Miotto) L.P.Queiroz, Legum. Caatinga 198. 2009QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana.. Acacia limae Bocage & Miotto, Rodriguésia 57: 134.2006. Figures 2d-e; 5k-q.
Climbing shrub ca. 3 m tall. Branches angular, aculeate, cream-colored with darker longitudinal stripes, glabrescent; prickle 1-6 mm long, recurved, base thick. Tector trichomes distributed on the branches, stipules, petioles, leaf rachis, leaflets, bracts, calyx, and fruits. Buds not observed. Stipules 5-6 × 1.5-2.0 mm long, caducous, lanceolate to oval, glabrous, margins ciliate. Extrafloral nectaries 2-3 × ca. 1 mm, elevated, globose, concave, estipitate cylindrical, caliciform or conical, located on the petioles below the basal pair of pinnae, or on the rachis between the basal pair of pinnae, additional nectaries along the rachis, between or below the pairs of pinnae. Leaves with 6-7 pairs of pinnae; petiole 1.9-3.5 cm long, sulcate, winged, with lateral constriction, glabrous to glabrescent; rachis 3.0-7.7 cm long, cylindrical, sulcate, unarmed to aculeate, glabrescent to pubescent; rachis segments 1.0-1.8 cm long; paraphylls lanceolate to filiform; leaflets 19-22 pairs, 6-8 × 2-3 mm, chartaceous, discolorous, oblong, falcate, apices obtuse to cuneiform, base oblique, margins ciliate, glabrous to glabrescent; venation campylodromous-brochidodromous, midrib exserted, with a tuft of trichomes at the base of the abaxial face. Homomorphic glomerules united in bundles held in terminal or axillary panicles, 25-30 mm diam.; peduncle 1.4-5.2 cm long, canaliculate, villous; bracts ca. 1.5 mm long, caducous, oval, glabrescent, at base of peduncle. Flowers 16-18 mm long, pentamerous, sessile, ca. 25 flowers per inflorescence; calyx 1.5-2.5 mm long, campanulate to infundibuliform, light green, pubescent; lacinias c. 5 mm long; corolla 5-7 mm long, tubular, light green, glabrous, lacinias ca. 0.5 mm long; stamens free, filaments 10-14 mm long, cream-colored; style 16-17 mm long; ovary stipitate 1.5-2 mm long, glabrous, stipe 2-3 mm long. Fruits dehiscent, 9-13.5 × 1.5-3.3 cm long, chartaceous, greenish brown when young, pubescent, linear, apices acute to cuspidate, base attenuated to obtuse, margins thick, stipitate. Seeds not observed.
Materials examined: BRAZIL. BAHIA: Jeremoabo, APA Serra Branca, Baixa dos Quelés, estrada do Tamburi, 09/VII/2012, fl., A.F.S. Brito 91 (HUNEB); Estrada secundária, Baixa, próximo a casa com caixa d'água, 17/VII/ 2011, fl., fr., A.F.S. Brito 20 (HUNEB); 17/VII/ 2011, fr., A.F.S. Brito 21 (HUNEB); Estrada do Tamburi, próximo a baixa dos Quelés, 09/VII/2012, fl., J.V. dos Santos et al. 27 (HUNEB).
8. Senegalia piauhiensis (Benth.) Seigler & Ebinger, Phytologia 88(1):61.2006SEIGLER, D.S, EBINGER, J.E. & MILLER, J.T. 2006. The genus Senegalia (Fabaceae: Mimosoideae) from the New World. Phytologia 88(1): 38-93.. Acacia piauhiensis Benth., Trans. Linn. Soc. London 30: 523. 1875. Figures 2f; 6a-c.
a-c. Senegalia piauhiensis. a. flowering branch; b. enclosed concave extrafloral nectaries; c. spiciform inflorescence. d-f. Senegalia polyphylla. d. flowering branch; e. detail of the prickle; f. leaflet, abaxial surface. g-i. Senegalia tenuifolia. g. flowering branch; h. detail of the prickle; i. showing aculeate rachis leaf. a-c from A. F. S. Brito 50; A. S. Conceição 1337; d-f from A. F. S. Brito 56; g-I from A. F. S. Brito 69.
Small to large trees 6-12 m tall. Branches cylindrical, unarmed to rarely aculeate, brown with lighter colored longitudinal stripes, glabrous; prickle ca. 1 mm long, recurved. Tector trichomes distributed on the leaf rachis, petioles, leaflets, inflorescence axis, calyx, and corolla. Buds observed. Stipules caducous, not observed. Extrafloral nectaries 2.1-3 × c. 1 mm, elevated-concave to enclosed-concave, in different positions along the petiole. Leaves with 2-4 pairs of pinnae; petiole 2-3 mm long, flat, furrow absent, constrictions absent, glabrescent to pubescent; rachis 4.9-6.6 mm long, unarmed; rachis segments 12-20 mm long; paraphylls linear; leaflets 9-25 pairs, 6-13 × 2.5-3.5 mm, chartaceous, discolorous, linear to oblong, falcate, apices rounded to cuneiform, base oblique, margins ciliate, sericeous; venation campylodromous-brochidodromous, midrib exserted, tuft of trichomes at base of central nerve, absent on abaxial face. Spikes arranged in bundles on terminal and axillary pseudo-racemes, 8.6-10.2 x 1.6-1.9 cm long; peduncle 0.5-1.0 cm long, cylindrical, glabrous; bracts ca. 2.5 mm long, oval. Flowers 9-19 mm long, pentamerous, polystamenous, sessile, 38-78 per inflorescence; calyx 2-3 mm long, tubular, costate, white, pubescent; lacinias 0.5-1 mm long; corolla 3.5-4 mm long, tubular, whitish, glabrescent; lacinias 1-1.5 mm long; stamens 8-9 mm long, whitish; style 6-7 mm long, white; ovary stipitate 1-1.5 mm long, glabrous; stipe 1-1.5 mm long. Fruit not observed.
Materials examined: BRAZIL. BAHIA: Jeremoabo, Fazenda Serra Branca, Baixa do Mandacaru Grande, ca. 6 Km da base da Vaca Morta, 18/ IV/ 2008, fl., A.S. Conceição 1337 (HUNEB); Vaca Morta, trilha do Coleta, 28/III/2012, fl., A.F.S. Brito 52 (HUNEB).
9. Senegalia polyphylla (DC.) Britton & Rose in Britto & Killip, Ann. New York Acad. Sci. 35(3): 142.1936.
Acacia glomerosa Benth., J. Bot. (Hooker) 1:521. 1842. Senegalia glomerulosa (Benth.) Britton & Rose, N. Amer. Flowers 23:116.1928. Figures 2g-h; 6d-f.
Small tree to shrub de 2-4 m tall. Branches cylindrical, unarmed to aculeate, dark green with lighter-colored longitudinal stripes, lenticels absent, pubescent to glabrescent; prickle 2-3 mm long, straight to incurvate with thick bases, not as triplets in the nodal region. Tector trichomes distributed on the branches, stipules, petioles, leaf rachis, leaflets, inflorescence axis, peduncles, calyx, corolla, and ovary. Buds not observed. Stipules caducous, ca. 6 × 2 mm, linear, striated, glabrescent. Extrafloral nectaries ca. 0.5 × 1-1.2 mm, elevated-concave, located on the petiole, additional nectaries between the last pair of pinnae. Leaves with 6-11 pairs of pinnae; petiole 1.7-3.2 mm long, sulcate, winged, with lateral constrictions, pubescent; rachis 7.2-9.4 mm long, costate, unarmed or sparsely aculeate, pubescent; rachis segments 8-14 mm long; paraphylls linear; leaflets 18-31 pairs, 9-10 × 2-3 mm, chartaceous, discolorous, linear to oblong, falcate, apices acute to acuminate, base oblique, margins ciliate, sericeous; venation campylodromous-brochidodromous, midrib exserted with a tuft of trichomes at the base of the abaxial face. Homomorphic glomerules united in bundles, arranged in terminal and axillary panicles, 8-11 mm diam.; peduncle 0.5-1.8 cm long, angular, pubescent; bracts oval ca. 2 mm long. Flowers 6-7 mm long, pentamerous, polystamenous, sessile, 13-15 per inflorescence; calyx 1.5-2 mm long, campanulate, cream-colored, sericeous; lacinias ca. 0.5 mm long; corolla 3-4.5 mm long, tubular, cream-colored, sericeous; lacinias ca. 0.5 mm long; stamens free, filaments 6-7 mm long, cream-colored; style 3.5-5 mm long, cream-colored; ovary stipitate 1-1.5 mm long, pubescent, stipe 2.5-3 mm long. Fruit not seen.
Materials examined: BRAZIL. BAHIA: Jeremoabo, APA Serra Branca, estrada principal sentido Povoado Quelés, próximo à porteira, 16/VI/2011, fl., A.F.S. Brito 19 (HUNEB); Estrada do Tamburí, 14/V/2012, fl., A.F.S. Brito 56 (HUNEB); Baixa dos Quelés, 14/V/2012, fl., A.F.S. Brito 60 (HUNEB); 05/VI/2012, fl., A.F.S. Brito et al. 68 (HUNEB).
10. Senegaliatenuifolia (L.) Britton & Rose, N. Amer. Flowers 23 (2):128.1928. Mimosa tenuifolia L., Sp. Pl.: 523. 1753. Figures 2i-j; 6g-i.
Shrub ca. 3 m tall. Branches cylindrical, aculeate, brownish- vinaceous, lenticellate, with lighter colored longitudinal stripes, glabrescent to pubescent; prickle 2-7 mm long, straight to recurved, base thick, in triplets in the nodal region. Tector trichomes distributed on the branches, leaf rachis, leaflets, inflorescence axis, calyx, corolla, ovary, and fruits. Buds not observed. Stipules caducous, 2-8 mm long, lanceolate to linear. Extrafloral nectaries 0.5-1 × ca. 1 mm, elevated-concave to short stipitate, located on the petiole near the pulvinus, additional nectaries between the last pair of pinnae. Leaves with 5-9 pairs of pinnae; petiole 1.9-3.5 cm long, flat, slightly sinuous, furrows absent, constrictions absent, pubescent; rachis 3.5-7.9 cm long, cylindrical, sulcate, pubescent, aculeate; rachis segments 8-14 mm long; paraphylls filiform; 18-29 leaflet pairs, 5-11 × 2-3.5 mm, chartaceous, discolorous, linear, falcate, apices acute to acuminate, base oblique, margins ciliates, sericeous; venation campylodromous-brochidodromous, midrib exserted, tuft of trichomes at the base of the nerve on the abaxial face absent. Homomorphic glomerules united in bundles arranged in terminal panicles, 10-12 mm diam.; peduncle 4-7 mm long, canaliculate, tomentose, caducous bracts not seen. Flowers 5-7.5 mm long, pentamerous, polystamenous, sessile, 11-13 per inflorescence; calyx campanulate, 1.5-2 mm long, cream-colored, pubescent; lacinias ca. 0.5 mm long; corolla campanulate to tubular, 3-4 mm long, cream-colored, pubescent; lacinias ca. 0.5 mm long; stamens free, filaments 6-7 mm long, whitish; style 3.5-4 mm long, white; ovary stipitate, 1.1-1.5 mm long, tomentose; stipe 1-1.5 mm long. Fruits not observed.
Materials examined: BRAZIL. BAHIA: Jeremoabo, APA Serra Branca, Baixa dos Quelés, 14/V/2012, A.F.S. Brito 69, fl. (HUNEB).
Discussion
Calliandra aeschynomenoides is endemic to Brazil, Caatinga region, with records from the states of Pernambuco and Bahia (Queiroz 2009QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana., Flora do Brasil 2020Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/reflora...
). It differs from the other species by having unarmed branches, extrafloral nectaries absent on the petiole and leaf rachis, leaves with one pair of pinna, red flowers with long stamen tubes exserted from the corolla. It was collected with flowers in June and July, with fruit in April and October.
Chloroleucon foliolosum occurs in Argentina and Bolivia (Barneby & Grimes 1996BARNEBY, R.C. & GRIMES, J.W. 1996. Silk tree, Guanacaste, Monkey's Earring: A generic system for the Synandrous Mimosaceae of the AmericasMem. N. Y. Bot. Gard. 74(1): 1-292.) in all regions of Brazil, except the southern region (Souza 2018SOUZA, E.R. 2018. Chloroleucon in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB82941 (last access on 03/05/2018).
http://floradobrasil.jbrj.gov.br/reflora...
). It can be characterized by having flexuous branches, isolated spines or paired at the nodes, dormant buds scaly, heteromorphic glomerules, with differentiated central flowers, with long perianth, and stamen tubes exserted from the corolla. The fruit is characteristic of the species, generally dehiscent, linear, curved 90°-360°, with valves extending over the seeds. The flowers were observed in June, July and October the fruits were observed in July and October.
Enterolobium timbouva occurs in South America, with records from Brazil, Colombia, and Paraguay (Mesquita 1990MESQUITA, A.L. 1990. Revisão taxonômica do gênero Enterolobium Mart. (Mimosoideae) para a região Neotropical. Dissertação de Mestrado, Universidade Federal Rural de Pernambuco, Recife.); occurs in northern, northeastern, midwest, and southeastern Brazil in the phytogeographical domains of Caatinga, Atlantic Forest and Amazon Forest (Mesquita et al. 2018MESQUITA, A.L.; MORIM, M.P.; BONADEU, F. Enterolobium in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB83158 (last access on 22/05/2018).
http://floradobrasil.jbrj.gov.br/reflora...
). It was collected in anthropized areas, growing on sandy soils in pastures and agricultural plots. It was collected with flowers in the month of December and fruits in March and May. The species can be distinguished from the other species occurring in EPASB by having an arboreal habit, 8-10 m tall, lenticular branches, leaves with 2-4 pairs of pinnae; oval petiole, and auriculate fruit, blackish when mature. It was collected with flowers in the month of December and fruits in March and May.
Pithecellobium diversifolium is endemic to Brazil and restricted to the areas of dryland caatinga of the Northeastern region (Iganci 2015IGANCI, J.R.V. 2015 Pithecellobium in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB83607 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/jabot/f...
). This species can be recognized by presence of leaves with 1-2 pairs of pinnae, elliptic to oval leaflets, spiny stipules (stipules transformed into spines), villous corolla, and sigmoidal fruits in spirals. The seeds of this species are also important in their identification as they are shiny and black with a red aril having a sweet taste. This species is easily recognized in the field due to the morphology of the leaflets, and the spiraled fruit when young are green and ripe are red. The flowers and fruits were observed in October.
Senegalia bahiensis is endemic to Brazil, distributed in the northeastern (Alagoas, Bahia, Paraíba, Pernambuco, Piauí, Rio Grande do Norte, and Sergipe) and southeastern (Minas Gerais and Rio de Janeiro) regions of that country (Ribeiro 2012RIBEIRO, P.G. 2012. Flora da Bahia: Família Leguminosae, Subfamília Mimosoideae - Tribo Acacieae & Tribo Mimoseae. Dissertação de Mestrado, Universidade Estadual de Feira de Santana, Feira de Santana., Flora do Brasil 2020Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/reflora...
). It can be recognized in the EPASB by having angular branches, prickle generally straight, stipules cordiform foliaceous to oval, and stipitate extrafloral nectaries cylindrical to calicioid. The specimens were collected with flowers in May and October, and with fruits in March.
Senegalia globosa is endemic to Brazil and endemic from Bahia State in caatinga vegetation (Queiroz 2009QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana., Flora do Brasil 2020Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/reflora...
). This species demonstrates morphological variability the numbers of pairs of leaflets and the shape of its extrafloral nectaries, globose or conical, distributed below the basal pina pair or in the median region of the petiole, with or without additional nectar distributed along the rach or between last pairs of leaflets. It is characterized by having slightly angular branches, recurved prickle, stipules linear to lanceolate, and relatively large glomerules (17-22 cm diameter). The flowers were observed in July.
Senegalia limae is endemic to Brazil, essentially native from caatinga vegetation, and has been recorded in northeastern Brazil in the states of Pernambuco and Bahia, in the southeastern region of that country in Minas Gerais State (Queiroz 2009QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana., Flora do Brasil 2020). Ribeiro (2012)RIBEIRO, P.G. 2012. Flora da Bahia: Família Leguminosae, Subfamília Mimosoideae - Tribo Acacieae & Tribo Mimoseae. Dissertação de Mestrado, Universidade Estadual de Feira de Santana, Feira de Santana. noted that many collected specimens have been identified as S. limae, Acacia limae although analyses of the type specimen and additional material indicated them to be S. lasiophylla. (Benth.) Seigler & Ebinger (Flora do Brasil 2020Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/reflora...
). We prefer to maintain the older classification in this work as Ribeiro (2012)RIBEIRO, P.G. 2012. Flora da Bahia: Família Leguminosae, Subfamília Mimosoideae - Tribo Acacieae & Tribo Mimoseae. Dissertação de Mestrado, Universidade Estadual de Feira de Santana, Feira de Santana. has not yet been formally published. This species demonstrates wide variations in the shapes and positions of its extrafloral nectaries, elevated, globose, concave, estipitate cylindrical, caliciform or conical. It can be recognized by its climbing habit, stipules lanceolate to oval, relatively large glomerules (25-30 mm in diameter), and flowers 16-18 mm long. The flowers were observed in July. Senegalia limae can sometimes be confused with S. globosa because both have leaves with similar numbers of pinnae. The two species can be easily distinguished, however, because S. limae is a climbing plant (vs. a shrub) with glomerules 25-30 mm in diameter (vs. glomerules 18-24 mm in diameter in S. globosa).
Senegalia piauhiensis endemic to Brazil, found in caatinga vegetation in northeastern Brazil, occurring in the states of Piauí, Ceará, Pernambuco, Bahia, Alagoas, and Sergipe (Queiroz 2009QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana., Flora do Brasil 2020Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
http://floradobrasil.jbrj.gov.br/reflora...
). This species is characterized by having leaves with 2-4 pairs of pinnae, elevated-concave to enclosed-concave nectaries along the petiole and, principally, by its inflorescence in spikes, 8.6-10.2 cm long. Senegalia piauhiensis differs from the other congeneric species occurring in the area by displaying its inflorescence in spikes (vs. glomerules). It was collected with flowers in March and April.
Senegalia polyphylla is widely distributed in the Americas (Rico-Arce 2007RICO-ARCE, M. L. de L. 2007. American Species of Acacia. A Checklist and Synopsis of American species of Acacia (Leguminosae: Mimosoideae). Conabio, México.). It occurs in the northern, northeastern, and central-western regions of Brazil in caatinga, cerrado, amazon forest and atlantic forest, and in the seasonally flooded "pantanal" region (Morim & Barros 2012). It is characterized by having cylindrical branches, not lenticular, incurvate prickle, not in triplets in the nodal region, midrib with a tufted of trichomes at the base of the abaxial face, glomerules 8-11 mm in diameter, and cream-colored flowers. It was collected with flowers in May and June.
Senegalia tenuifolia is distributed in South America, with records for Argentina, Brazil, and Paraguay (Rico-Arce 2007RICO-ARCE, M. L. de L. 2007. American Species of Acacia. A Checklist and Synopsis of American species of Acacia (Leguminosae: Mimosoideae). Conabio, México.). According to Ribeiro (2012)RIBEIRO, P.G. 2012. Flora da Bahia: Família Leguminosae, Subfamília Mimosoideae - Tribo Acacieae & Tribo Mimoseae. Dissertação de Mestrado, Universidade Estadual de Feira de Santana, Feira de Santana., this species has been recorded to northern (in the states of Acre, Amazonas, and Pará), northeastern (Bahia, Paraíba and Pernambuco), central-western (Goiás and Mato Grosso), and southeastern (Minas Gerais, Rio de Janeiro and São Paulo) regions of Brazil. According to Queiroz (2009)QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana., this taxon occurs principally in seasonal forests and rainforests in northern Brazil, and occasionally in caatinga vegetation, especially arboreal caatinga. It was registered in arboreal shrub vegetation in the EPASB, growing on sandy soils. Senegalia tenuifolia can be recognized in the field by having branches cylindrical, lenticular, armed with recurved prickle in triplets in the nodal region, and by having small glomerules, 10-12 mm in diameter. It can be confused with S. polyphylla in the study area as both produce inflorescences in small glomerules with cream-colored flowers. However, S. tenuifolia can be easily distinguished by having lenticular branches (vs. branches not lenticular), recurved prickle, in triplets in the nodal region (vs. incurvate prickle not in triplets). Flowering was observed in May.
The morphological characters for recognition of the Acacieae and Ingeae tribes were: arrangement of prickles on the branches, presence or absence of spines or prickles, shape of stipules, shape and position of extrafloral nectars, number of leaflet pairs, inflorescence pattern and types of fruits.
With the study carried out at the EPASB, we can infer the environmental, taxonomic, and systematic relevance of the identified groups, mainly the degree of endemism of the Brazil for the species recorded. Among the species studied, six are registered as endemic to Brazil. The Acacieae Tribe is represented by S. bahiensis, S. globosa, S. limae, e S. piauhinesis. The Ingeae Tribe is represented by Calliandra aeschynomenoides and Pithecellobium diversifolium. These species also present distribution to the Caatinga domain (sensu stricto). Of these, only S. globosa was registered as endemic to the Bahia states.
Acknowledgments
Thanks to the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB, PET # 0023/2007) and to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Proc. # 552589/2011-0) for financial support. To Companhia Hidrelétrica do São Francisco (CHESF) for their support during field work and Coordenação de aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship to the first author and postdoctoral grant to the third author (PNPD nº 02697/09-2). To Pétala Gomes Ribeiro by confirmation of the identities of part of the species. The first author thanks the curators and technicians of the herbaria that were visited for their readiness during the consultation of the collections and Natanael Santos for the botanical illustrations.
References
- BARNEBY, R.C. & GRIMES, J.W. 1996. Silk tree, Guanacaste, Monkey's Earring: A generic system for the Synandrous Mimosaceae of the AmericasMem. N. Y. Bot. Gard. 74(1): 1-292.
- BARNEBY, R.C. & GRIMES, J.W. 1997. Silk Tree, Guanacaste, Monkey's Earring: A Generic System for the Synandrous Mimosaceae of the Americas. Pithecellobium, Cojoba e Zygia. Mem. N. Y. Bot. Gard. 74(2): 1-149.
- BARNEBY, R.C. 1998. Silk Tree, Guanacaste, Monkey's Earring: A Generic System for the Synandrous Mimosaceae of the Americas. Calliandra Mem. N. Y. Bot. Gard. 74(3): 1-223.
- BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113. DOI: 10.1590/2175-7860201566411.
» https://doi.org/10.1590/2175-7860201566411 - BROWN, G.K. 2008. Systematics of the tribe Ingeae (Leguminosae - Mimosoideae) over the past 25 years. Muelleria 26(1): 27-42.
- BROWN, G.K., MURPHY, D.J., MILLER, J.T. & LADIGES, P.Y. 2008. Acacia s.s and its relationships between tropical legume relatives, tribe Ingeae (Leguminosae: Mimosoideae). Syst. Bot. 33(4): 739-751.
- CADASTRO NACIONAL DE UNIDADE DE CONSERVAÇÃO - CNUC/MMA. Unidades de Conservação por Bioma. Brasília. http://www.mma.gov.br/images/arquivo/80229/CNUC_JUL18%20-%20C_Bio.pdf (last access on 10/07/2018).
» http://www.mma.gov.br/images/arquivo/80229/CNUC_JUL18%20-%20C_Bio.pdf - Calliandra in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB18171 (last access on 17/08/2018).
» http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB18171 - DOURADO, D.A.O., CONCEIÇÃO, A.S. & SANTOS-SILVA, J. 2013. O gênero Mimosa L. (Leguminosae: Mimosoideae) na APA Serra Branca/Raso da Catarina, Bahia, Brasil. Biota Neotropica 13(4):http://www.biotaneotropica.org.br/v13n4/en/abstract?inventory+bn01713042013 (last access on 19/01/2018).
» http://www.biotaneotropica.org.br/v13n4/en/abstract?inventory+bn01713042013 - Fabaceae in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 (last access on 17/08/2015).
» http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB103016 - FOSBERG, F.R. & SACHET, M.H. 1965. Manual for tropical herbaria. Utrecht, Netherlands.
- FUNDAÇÃO CTI/NE. 2016. http://www.ctinordestedobrasil.com.br/poligono.html (last access on 19/01/2017).
» http://www.ctinordestedobrasil.com.br/poligono.html - GIULIETTI, A.M., HARLEY, R.M., QUEIROZ, L.P., BARBOSA, M.R.V., BOCAGE NETA, A.L., & FIGUEIREDO, M.A. 2002. Espécies endêmicas da Caatinga. In Vegetação e Flora da Caatinga (E. Sampaio, A.M. Giulietti, J. Virgínio, & C. Gamarra-Rojas (eds.). APNE/CNIP, Recife, p. 103-119.
- HARRIS, J.G. & HARRIS, M.W. 1994. Plant identification terminology: an illustrated glossary. 2nd ed. Spring Lake Publishing, Spring Lake.
- IGANCI, J.R.V. 2015 Pithecellobium in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB83607 (last access on 17/08/2015).
» http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB83607 - LEAL, I.R., SILVA, J.M.C., TABARELLI, M. & LACHER JR, T.E. 2005. Mudando o curso da conservação da biodiversidade na Caatinga do Nordeste do Brasil. Megadiversidade 1(1): 139-146.
- LEWIS, G.P. 2005. Tribe Acacieae. In Legumes of the world (G.P Lewis., B. Schrire, B. MacKinder & M. Lock (eds.). Royal Botanical Gardens, Kew, p.187-191.
- LEWIS, G.P. & RICO ARCE, M. L. 2005.Tribe Ingeae. In Legumes of the world (G.P Lewis., B. Schrire, B. MacKinder & M. Lock (eds.). Royal Botanical Gardens, Kew, p.193-213.
- LIMA, J.B. & CONCEIÇÃO, A.S. 2016. Malvoideae Burnett (Malvaceae) in the Environmental Protection Area Serra Branca, Raso da Catarina, Jeremoabo, Bahia, Brazil. Biota Neotropica 16(4): e20160187. http://dx.doi. org/10.1590/1676-0611-BN-2016-0187 (last access on 19/01/2017).
» http://dx.doi. org/10.1590/1676-0611-BN-2016-0187 - LIMA, H. C., QUEIROZ, L. P., MORIM, M. P., DUTRA, V. F., BORTOLUZZI, R. L. C., IGANCI, J. R. V., FORTUNATO, R. H., VAZ, A. M. S. F., SOUZA, E. R., FILARDI, F. L. R., GARCIA, F. C. P., FERNANDES, J. M., MARTINS-DA-SILVA, R. C. V., PEREZ, A. P. F., MANSANO, V. F., MIOTTO, S. T. S., LIMA, L. C. P., OLIVEIRA, M. L. A. A., FLORES, A. S., TORKE, B. M., PINTO, R. B., LEWIS, G. P., BARROS, M. J. F., SCHÜTZ, R, PENNINGTON, T., KLITGAARD, B. B. RANDO, J. G., SCALON, V. R., COSTA, L. C., SILVA, M. J., MOURA, T. M., BARROS, L. A. V., SILVA, M. C. R., QUEIROZ, R. T., SARTORI, A. L. B., CAMARGO, R. A., LIMA, I. B., COSTA, J., SOARES, M. V. B., SNAK, C., VALLS, J. F. M., SÃO-MATEUS, W., FALCÃO, M. J., CARDOSO, D. B. O. S., TOZZI, A. M. G. A., MARTINS, M. V., SOUZA, V. C., MEIRELES, J. E. & REIS, I. P. 2012. Fabaceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. http://www.floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB115 (last access on 22/05/2012).
» http://www.floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB115 - LOPES, A.A.S. 2012. Diversidade de Euphorbiaceae nas Caatingas arenosas da APA Serra Branca, Jeremoabo, Bahia, Brasil. Dissertação de mestrado, Universidade do Estado da Bahia, Paulo Afonso.
- LPWG, Legume Phylogeny Working Group. 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66 (1): 44-77.
- LUCKOW, M., MILLER J.T., MURPHY, D.J. & LIVSHULTZ, T. 2003. A phylogenetic analysis of the Mimosoideae (Leguminosae) based on chloroplast DNA sequence data. In Advances in legume systematic(B. B. Klitgaard & A. Bruneau, eds). Royal Botanical Garden, London, p.197-220.
- MASLIN, B.R., MILLER, J.T.D. & SEIGLER, D.S. 2003. Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Aust. Syst. Bot. 16: 1-18.
- MELO, Y., CÓRDULA, E.R., MACHADO, S.R. & ALVES, R. 2010. Morfologia de nectários em Leguminosae senso lato em áreas de caatinga no Brasil. Acta Bot. Bras. 24(4): 1034-1045.
- MESQUITA, A.L. 1990. Revisão taxonômica do gênero Enterolobium Mart. (Mimosoideae) para a região Neotropical. Dissertação de Mestrado, Universidade Federal Rural de Pernambuco, Recife.
- MESQUITA, A.L.; MORIM, M.P.; BONADEU, F. Enterolobium in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB83158 (last access on 22/05/2018).
» http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB83158 - MILLER, J.T. & BAYER, R.J. 2001. Molecular phylogenetics of Acacia (Fabaceae: Mimosoideae) based on the chloroplast matK coding sequence and flanking trnK intron spacer regions. Am. J. Bot. 88: 697-705.
- MILLER, J.T. & R.J.BAYER. 2003. Molecular phylogenetics of Acacia subgenera Acacia and Aculeiferum (Fabaceae: Mimosoideae), based on the chloroplast matK coding sequence and flanking trnK intron spacer regions. Aust. Syst. Bot. 16: 27- 33.
- MORI, S.A., SILVA, L.A.M., LISBOA, G. & CORADIN, L. 1989. Manual de manejo do herbário fanerogâmico. Centro de Pesquisa do Cacau, Ilhéus.
- ORCHARD, A.E. & MASLIN, B.R. 2003. Proposal to conserve the name Acacia (Leguminosae: Mimosoideae) with a conserved type. Taxon 52: 362-363.
- PRADO, D.E. 2003. As Caatingas da América do Sul. In Ecologia e conservação da Caatinga (L.M. Tabarelli & J.M.C. Silva, eds.). Universidade Federal de Pernambuco, Recife, p. 1-74.
- QUEIROZ, L.P. 2009. Leguminosas da caatinga. Universidade do Estadual de Feira de Santana, Feira de Santana.
- RATTER, J.A., BRIDGEWATER, S. & RIBEIRO, J.F. 2003. Analysis of the floristic composition of the Brazilian cerrado vegetation III: Comparison of the woody vegetation of 376 areas. Edinburgh J. Bot. 60: 57-109.
- RIBEIRO, P.G. 2012. Flora da Bahia: Família Leguminosae, Subfamília Mimosoideae - Tribo Acacieae & Tribo Mimoseae. Dissertação de Mestrado, Universidade Estadual de Feira de Santana, Feira de Santana.
- RICHARDSON, J.E., PENNINGTON, R.T., PENNINGTON, T.D. & HOLLINGSWORTH, P.H. 2001. Rapid diversification of a species-rich group of Neotropical rain forest trees. Science 293: 2242-2245.
- RICO-ARCE, M. L. de L. 2007. American Species of Acacia A Checklist and Synopsis of American species of Acacia (Leguminosae: Mimosoideae). Conabio, México.
- SAMPAIO, E.V.S.B. 1995. Overview of the Brazilian caatinga. In Seasonally dry tropical forests (S.H. Bullock, H.A. Mooney & E. Medina, eds.). Cambridge University Press, Cambridge, p. 34-63.
- SANTOS, J.V, NUNES, T.S. & CONCEICÃO, A.S. 2016. A família Passifloraceae na APA Serra Branca/Raso da Catarina, Jeremoabo, Bahia, Brasil. Biotemas 29(1): 11-23.
- SEIGLER, D.S, EBINGER, J.E. & MILLER, J.T. 2006. The genus Senegalia (Fabaceae: Mimosoideae) from the New World. Phytologia 88(1): 38-93.
- SCHRIRE, B.D., LAVIN, M. & LEWIS, G.P. 2005. Global distribution patterns of the Leguminosae: Insights from recent phylogenies. Biol. Skr. 55: 375-422.
- SEIGLER, D.S. & EBINGER, J.E. 2018. New Combinations in Parasenegallia and Mariosousa (Fabaceae: Mimosoideae). Phytologia 100(4): 256-259.
- SEIGLER, D.S., EBINGER, J.E, RIGGINS, C.W., TERRA,V. & MILLER, J.T. 2017. Parasenegalia and Pseudosenegalia (Fabaceae): New Genera of the Mimosoideae. Novon 25(2): 180-205.
- Senegalia in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB100997(last access on 17/08/2018).
» http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB100997 - SILVA, L.R., SILVA CASTRO, M.M. & CONCEIÇÃO, A.S. 2016. The family Bignoniaceae in the Environmental Protection Area Serra Branca, Raso da Catarina, Jeremoabo, Bahia, Brazil. Acta Sci., Biol. Sci. 38(4): 395-409.
- SOUZA, E.R. 2018. Chloroleucon in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB82941 (last access on 03/05/2018).
» http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB82941 - SOUZA, E.R. de, KRISHNARAJ, M.V. & L.P. de QUEIROZ. 2016. Sanjappa, a new genus in the tribe Ingeae (Leguminosae: Mimosoideae) from India. Rheedea. 26(1) 1-12.
- SZABO, A.V., ROCHA, A.C.S., TOSATO, J.A. DE C. & BARROSO, W. 2007. Área de proteção ambiental (APA) Serra Branca Raso da Catarina. In As Caatingas: debates sobre a Ecorregião do Raso da Catarina (J. Marques, org.). Fonte Viva, Paulo Afonso. p. 21-40.
- TERRA, V. & GARCIA, F.C.P. 2016. A new species of Senegalia (Leguminosae Mimosoideae) from the Caatinga Domain, Brazil. Phytotaxa 288 (2): 181-186.
- THIERS, B. 2018 [continuously updated]. Index Herbariorum: a global directory of public herbaria and associated staff. In New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/ (last access on 19/04/2018).
» http://sweetgum.nybg.org/ih/ - TRICART, J. 1972. The landforms of the humid tropics, forests and savannas. Geographies for Advanced Study, Longman, London.
- VARJÃO, R.R., JARDIM, J.G. & CONCEICÃO, A.S. 2013. Rubiaceae Juss. de caatinga na APA Serra Branca/Raso da Catarina, Bahia, Brasil. Biota Neotropica 13(2): http://www.biotaneotropica.org.br/v13n2/en/abstract?inventory+bn00313022013 (last access on 19/01/2017).
» http://www.biotaneotropica.org.br/v13n2/en/abstract?inventory+bn00313022013 - VASSAL, J. 1981. Acacieae. In Advances in Legume Systematics (R.M. Polhill & P.H. Raven, eds). Royal Botanic Gardens, London, p.169-171.
- VELLOSO, A.L., SAMPAIO, E.V.S.B. & PAREYN, F.G.C. 2002. Ecorregiões propostas para o bioma Caatinga. Associação Plantas do Nordeste, Recife.
- VIEIRA, D.D., CONCEIÇÃO, A.S., MELO, J.I.M. & STAPF, M.N.S. 2013. A família Boraginaceae sensu lato na APA Serra Branca/Raso da Catarina, Bahia, Brasil. Rodriguésia 64(1): 151-168.
Supplementary material
The following online material is available for this article:
The Appendix 1 - List of additional material examined.
https://minio.scielo.br/documentstore/1676-0611/wmDs5WScrYFcBnqsnKbJSxR/42c7c784292935f9c11076ac232f0b39cb3a48f0.pdfPublication Dates
-
Publication in this collection
15 July 2019 -
Date of issue
2019
History
-
Received
31 May 2018 -
Reviewed
10 June 2019 -
Accepted
11 June 2019