ABSTRACT
Composting is the process of natural degradation of organic matter carried out by environmental microorganisms whose metabolic activities cause the mineralization and partial humification of substances in the pile. This compost can be beneficially applied to the soil as organic fertilizer in horticulture and agriculture. The number of studies involving microbial inoculants has been growing, and they aim to improve processes such as composting. However, the behavior of these inoculants and other microorganisms during the composting process have not yet been described. In this context, this work aimed to investigate the effects of using a microbial inoculum that can improve the composting process and to follow the bacterial population dynamics throughout the process using the high-resolution melt (HRM) technique. To do so, we analysed four compost piles inoculated with Bacillus cereus, Bacillus megaterium, B. cereus + B. megaterium and a control with no inoculum. The analyses were carried out using samples collected at different stages of the process (5th to 110th days). The results showed that the bacterial inocula influenced the process of composting, altering the breakdown of cellulose and hemicelluloses and causing alterations to the temperature and nitrogen levels throughout the composting process. The use of a universal primer (rDNA 16S) allowed to follow the microbial succession during the process. However, the design of a specific primer is necessary to follow the inoculum throughout the composting process with more accuracy.
Index terms:
High Resolution Melt (HRM); bacteria; microbial ecology.
RESUMO
A compostagem é um processo de degradação natural da matéria orgânica realizado por microrganismos presentes no ambiente, levando a mineralização e humificação parcial das substâncias presentes na pilha, esse composto formado pode ser beneficamente aplicado ao solo como fertilizante orgânico na horticultura e agricultura. O número de estudos envolvendo inoculantes microbianos é crescente, os quais tem por objetivo a otimização de processos de compostagem. Contudo, o comportamento desses inoculantes e da microbiota ao longo do processo não tem sido caracterizado. Nesse contexto, este trabalho foi realizado com o objetivo de avaliar o efeito da utilização de um inóculo bacteriano que promova melhorias no processo de compostagem, bem como o de acompanhar a dinâmica populacional bacteriana ao longo de todo o processo através da técnica de High Resolution Melt (HRM). Para isso foram analisados quatro pilhas de compostagem inoculadas com Bacillus cereus, Bacillus megaterium, B. cereus + B. megaterium e o controle sem adição de inóculo. Foram realizadas análises químicas e moleculares (HRM) das amostras coletadas em diferentes períodos da compostagem (5º ao 110º dias). Os resultados mostraram que os inóculos bacterianos influenciaram no processo de compostagem com alteração na degradação de celulose, hemicelulose bem como alteração da temperatura e níveis de nitrogênio ao longo da compostagem. A utilização de um primer universal (rDNA 16S) permitiu acompanhar a sucessão bacteriana ao longo do processo, nos tratamentos. Contudo a construção de um primer específico é necessário para acompanhar de maneira mais precisa o inóculo durante o desenvolvimento da compostagem.
Termos para indexação:
High Resolution Melt (HRM); bacteria; ecologia microbiana.
INTRODUCTION
Composting is a natural decomposition process stemming from microbial succession, which results in the degradation and stabilization of organic matter. During this process, a spontaneous rise in temperature occurs, helping to eliminate pathogenic organisms, in this way making the compost safe for use as an organic fertilizer. This process occurs in two distinct phases; in the first, active degradation takes place, and in the second, maturation (humification) of the organic material occurs. The process time varies according the environment and the type and particle size of the material to be composted (Insam; Bertoldi, 2007INSAM, H.; BERTOLDI, M. Microbiology of the composting process. Compost Science and Technology, Waste Management Series, Elsevier Ltd: p. 25-48, 2007.; Zhang; Sun, 2015ZHANG, L.; SUN, X. Effects of earthworm casts and zeolite on the two-stage composting of green waste. Waste Management , 39:119-129, 2015.).
The maturity of the product at the end of composting is influenced by factors such as temperature, pH and humidity, which must be controlled throughout the process. In addition, the substrates utilized and the microbiota carrying out the process exercise a great influence on compost formation (Villar et al., 2016VILLAR, I. et al. Evolution of microbial dynamics during the maturation phase of the composting of different types of waste. Waste Management , 54:83-92, 2016.).
Studies have been conducted addressing the use of microbial inoculants with the purpose of accelerating composting and improving the final product (Zeng et al., 2009ZENG, G. M. et al. Effect of inoculating white-rot fungus during different phases on the characteristics of humic acid. Chemosphere, 68(4):368-374, 2009.; Figueiredo et al., 2013FIGUEIREDO, V. R. et al. Microbial inoculation during composting improves productivity of sun mushroom (Agaricus subrufescens Peck). African Journal of Microbiology Research, 7(35): 4430-4434, 2013.). García et al. (2006)GARCIA, M. C. V. et al. Influence of microbial inoculation and co-composting material on the evolution of humic-like substances during composting of horticultural wastes. Process Biochemistry, 40(6):1438-1443, 2006. observed in their study that the use of bacterial inoculants (Bacillus and actinobacteria) in the composting of vegetable products increased the final humification of the compost and consequentially improved the agricultural quality of the product. Nevertheless, further information about the composting system is necessary.
Recent molecular techniques such as high-resolution melt (HRM) analysis have been used in microbiological research (Hrncirova et al., 2010HRNCIROVA, K. et al. Rapid detection and identification of mucormycetes from culture and tissue samples by use of High-Resolution Melt Analysis. Journal of Clinical Microbiology , 48(9):3392-3394, 2010.; Smith; Lu; Bremer, 2010SMITH, B. L.; LU, C. P.; BREMER, J. R. A. High-resolution melting analysis (HRMA): A highly sensitive inexpensive genotyping alternative for population studies. Molecular Ecology Resources, 10(1):193-196, 2010.; Won et al., 2010WON, H. et al. Rapid identification of bacterial pathogens in positive blood culture bottles by use of a compost maturity of agricultural wastes. Process Biochemistry , 44:396-400, 2010.; Derzelle et al., 2011DERZELLE, S. et al. Characterization of genetic diversity of Bacillus anthracisin France by using High Resolution Melt. Journal of Clinical Microbiology, 49(12):4286-4292, 2011.). There are several advantages in the use of this technique, mainly its rapid application, low cost, non-destructive analysis of DNA and wide range of applications which include distinguishing mutations to the very accurate identification of bacterial genotypes in complex processes such as composting (Derzelle et al., 2011DERZELLE, S. et al. Characterization of genetic diversity of Bacillus anthracisin France by using High Resolution Melt. Journal of Clinical Microbiology, 49(12):4286-4292, 2011.; Gabriel et al., 2012GABRIEL, E. M. et al. High resolution melting analysis for rapid detection of linezolid resistance (mediated by G2576T mutation) in Staphylococcus epidermidis. Journal of Microbiological Methods, 90(2):134-139, 2012.; Porcellato et al., 2012PORCELLATO, D. et al. Rapid lactic acid bacteria identification in dairy products by high-resolution melt analysis of DGGE bands. Letters in Applied Microbiology, 54(4):344-351, 2012.).
In this work, the effect of the utilization of two species of bacteria (Bacillus cereus and B. megaterium) inoculated both individually and in combination during the composting process with the aim of obtaining organic fertilizer was evaluated. In addition, the physical properties and chemical composition of the compost as well as bacterial succession over the process of composting was evaluated by utilizing the high-resolution melt (HRM) technique.
MATERIAL AND METHODS
Composting process and sampling and treatments
Composting piles were set up in an open yard with the aid of wooden frames with the dimensions of 1.2 × 1.2 m. Each pile, weighing 137 kg, was composed ofsugarcane bagasse (68,5 kg) and coast-cross grass (68,5 Kg) at the proportion of 1:1 in alternating layers, using the adapted formula described by Figueiredo et al. (2013FIGUEIREDO, V. R. et al. Microbial inoculation during composting improves productivity of sun mushroom (Agaricus subrufescens Peck). African Journal of Microbiology Research, 7(35): 4430-4434, 2013.). In the fourth turning, the piles were supplemented with 10% wheat meal, 2% limestone, 2% agricultural gypsum and 1.7% urea. The purpose of the supplement was to correct the pH of the compost and increase the nitrogen content available in the pile.
During the first month of composting, hand turnings were performed three times a week. However, in the second and third month, fortnightly hand turnings were performed. Temperature, pH and moisture were evaluated during each turning, with adjustments being made when necessary. The bacteria originally isolated from the compost were reactivated in nutrient broth and afterwards incubated at 45 °C for 24±2 hours before inoculation.
In the fourth turning, the bacterial inoculum was distributed uniformly on the surface of the compost. Four composting piles were established: Treatment 1: Bacillus cereus; Treatment 2: Bacillus megaterium; Treatment 3: Bacillus cereus + Bacillus megaterium; Treatment 4: control (no addition of inoculum).
For the sampling and analyses, the turnings T2, T4, T10, T12, T13, T14, T15, T16, T17 (T = turning) corresponding to the 5th, 9th, 23rd, 40th, 54th, 68th, 82nd, 96th and 110th days of composting, respectively, were considered.
Analysis by high-resolution melt (HRM)
DNA was extracted from the compost samples and from the bacterial cultures (Bacillus cereus and Bacillus megaterium) utilized as inoculants in the treatments. The compost DNAs were extracted by utilizing a Soil DNA Isolation Kit (Norgen Biotek® Corp.) according to the recommendations of the manufacturer.
The primers 338f (ACTCCTACGGGAGGCA GCAG) and 518r (ATTACGGCGGCTGCTGG) targeting the V4 region of the 16S rDNA gene were utilized according to the protocol adapted from Fierer (2005FIERER, N. et al. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Applied Environment Microbiology, 71:4117-4120, 2005.). The reaction was carried out in a Rotor-Gene® Q cycler (Qiagen) with a thermal profile of 95 °C for 5 minutes, followed by 95 °C, for 10 seconds and 40 cycles of 30 °C for 30 seconds and 72 °C for 30 seconds. The melt conditions for the HRM analysis were as follows: 75 °C - 90 °C (0.1 °C every 2 seconds, except for the first step, which remained at 75 °C for 90 seconds). Changes in fluorescence were measured during the final heating cycle and were analyzed using Rotor-Gene® Q Series Software.
Chemical and physical analyses of the compost
The compost samples were submitted to chemical and physical analyses throughout the composting process. The total N content was determined by the Kjeldahl method, while standard procedures were utilized for the calculation of hemicellulose, cellulose and lignin according to Silva and Queiroz (2012SILVA, D. J.; QUEIROZ, A. C. Análise de Alimentos: Métodos químicos e biológicos. 3. ed. Viçosa, MG: UFV, 2012. 235 p.).
The temperature was monitored by means of a thermocouple, and the measurements were taken from the pile center before the turning process. Moisture was determined from composite samples (each one obtained from five subsamples from different points of the pile), which were oven-dried at 65 °C for 24 hours. Moisture was determined gravimetrically after oven-drying at 65 °C for 24 hours. The pH was measured (1:5 fresh samples) at each turning.
RESULTS AND DISCUSSION
The composting of organic residues is a natural process in which microorganisms will convert biodegradable organic matter into a final compost, humus, which will be able to be utilized in several sectors of agriculture (Souza et al., 2014SOUZA, T. P. et al. Analysis of thermophilic fungal populations during phase II of composting for the cultivation of Agaricus subrufescens. World Journal Microbiology Biotechnology, 30:2419-2425, 2014.). A large number of factors are responsible for the final quality and stability of the product, such as temperature, moisture, pH, nitrogen and particle size (Wong et al., 2011WONG, J. W. C. et al. Influence of different mixing ratios on in-vessel co-composting of sewage sludge with horse stable straw bedding waste: maturity and process evaluation. Waste Management & Research, 29(11):1164-1170, 2011.; Zhang et al., 2012ZHANG, Y. G. et al. Modelling of organic matter dynamic during the composting process. Waste Management , 32:19-30, 2012.). In addition, the substrates that can be utilized for the preparation of compost are varied, such as used coffee grounds, sugarcane bagasse, vegetables and pre-growing compost of mushrooms (Silva et al., 2013SILVA, D. J. et al. Composto orgânico em mangueiras (Mangifera indica L.) cultivadas no semiárido do nordeste brasileiro. Revista Brasileira de Fruticultura, 35(3): 875-882, 2013.; Shemekite et al., 2014SHEMEKITE, F. et al. Coffee husk composting: An investigation of the process using molecular and non-molecular tools. Waste Management, 34:642-652. 2014.).
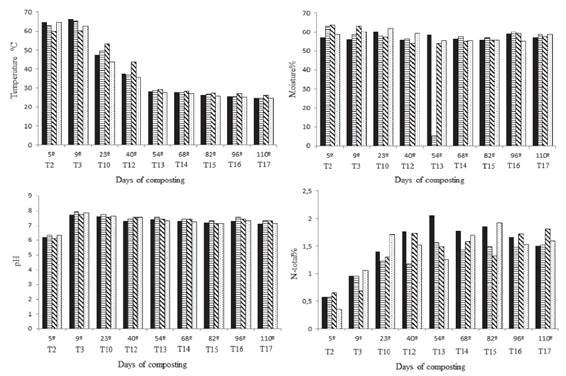
Both temperature and moisture are important factors in the composting process. Temperature was measured at the pile center throughout the process, which is the location of the highest temperature. Over the course of the experiment, the temperature ranged between 24.6 °C and 66.1 °C (Figure 1). In all the treatments, it was found that the thermophilic phase reached a peak between the 5th and 9th day of composting, with the maximum temperature of 66.1 °C observed in Treatment 1 (inoculation with B. cereus).
Temperature, moisture, pH and nitrogen total contend during the composting process. Each turning corresponds to the respective day of composting. T = turning.
According to Miller (1992MILLER, F. C. Composting as a process based on the control of ecologically selective factors. Soil Microbial Ecology, 18(3):515-543, 1992.), after 72 hours of composting, the process has already entered a thermophilic phase, reaching temperatures above 45 °C. An important consideration is that during this process, the temperature did not reach values much above 65 °C, which are considered to harm the composting process by decreasing the microbial population (Valente et al., 2009VALENTE, B. S. et al. Fatores que afetam o desenvolvimento da compostagem de resíduos orgânicos. Archivos de Zootecnia, 58(237):59-85, 2009.). Very low temperatures are also undesirable early in composting because they delay the compost-forming process (Bernal; Alburquerque; Moral, 2009BERNAL, M. P.; ALBURQUERQUE, J. A.; MORAL, R. Composting of animal manures and chemical criteria for compost maturity assessment: A review. Bioresource Technology, 100(22): 5444-5453, 2009.), which did not occur in this experiment because all the treatments were already presenting temperatures either equal or higher than 60 °C on the fifth day of composting.
On the 23rd day, the control (Treatment 4) presented a temperature that was lower than that of the inoculated treatments. When compared with Treatment 3 (B. cereus + B. megaterium), the temperature of the control was 10 °C lower. As demonstrated in Figure 1, the compost inoculated with the two combined bacteria (Treatment 3) presented temperatures that were always superior to the other treatments and to the control from the 23rd day. Possibly the inoculated bacteria have had later activation after nitrogen supplementation on fourth turning, that is explain the temperature initial variation between treatments.
According to Gajalakshmi and Abbasi (2008GAJALAKSHMI, S.; ABBASI, S. A. Solid waste management by composting: State of the art. Critical Reviews in Environmental Science and Technology, 38(5):311-400, 2008.), the ideal moisture content for an efficient composting process lies between 50-60%. Moisture contents below these values inhibit microbial metabolism and consequently substrate degradation. The moisture was adjusted throughout the experiment, remaining between 53% and 60% (Figure 1) for all the treatments (Valente et al., 2009VALENTE, B. S. et al. Fatores que afetam o desenvolvimento da compostagem de resíduos orgânicos. Archivos de Zootecnia, 58(237):59-85, 2009.). In addition to normal water loss caused by the difference in moisture between the substrate and air, the highest temperatures during the thermophilic phase contribute to the reduction of moisture during composting (Gajalakshmi; Abbasi, 2008GAJALAKSHMI, S.; ABBASI, S. A. Solid waste management by composting: State of the art. Critical Reviews in Environmental Science and Technology, 38(5):311-400, 2008.).
The pH values found in the experiment are in agreement with the data described as ideal in the literature, being more acidic at the beginning of the process and alkaline during the subsequent steps. According to Kiehl (2002KIEHL, E. J. Manual da compostagem, maturação e qualidade do composto. Piracicaba: E. J. Kiehl, 2002. 171 p.), at the beginning of the process, in the thermophilic phase of active degradation, organic acids are formed, causing the pH to become more acidic, approximately 5.5 to 6.0. Afterwards, these organic acids will react with the released bases from the organic matter, thus raising the pH values. According to Miller (1992MILLER, F. C. Composting as a process based on the control of ecologically selective factors. Soil Microbial Ecology, 18(3):515-543, 1992.), the optimal values are between 5.5 and 7.5. Values of pH above these can cause nitrogen loss by ammonia volatilization, resulting in a disagreeable odor in the compost. In spite of this, one should take into account that to be utilized as an organic fertilizer, the compost needs to be at a pH compatible with the requirements or sensitivity of the plants. During this experiment, the compost pH was more acidic during the first five days, reaching the condition of highest alkalinity soon afterwards (Figure 1). From that point on, there was a reduction in the pH across all treatments until it stabilized close to neutrality, ranging between 7.1 and 7.3.
Like temperature and moisture, the nitrogen content influences the formation and final quality of the product. Therefore, supplementation of the substrate is an important strategy that should be carried out throughout the process (Xie et al., 2015XIE, Kaizhi et al. Effects of supplementary composts on microbial communities and rice productivity in cold water paddy fields. Journal of Microbiology and Biotechnology, 25(5):569-578, 2015.). An increase in the nitrogen content during composting occurs as a function of the biomass lost as CO2, which is greater than the nitrogen loss by volatilization. In the beginning of the composting process, the nitrogen content was low in all of the treatments, approximately 0.62%. After the addition of the supplements during the fourth turning, the compost began presenting values of approximately 1% nitrogen. The nitrogen content increased until it reached a maximum peak close to 2% and afterwards fell to values of between 1.5 and 1.81%. For Treatments 1 and 2 (inoculated with B. cereus and B. megaterium, respectively), the highest nitrogen content was found on the 54th day, followed by a steady decrease until it stabilized at approximately 1.5%. For Treatment 3 (inoculated with the two combined bacteria), two peaks were found, the first one being on the 40th day, followed by a decrease until the 68th day, when the nitrogen content again began to rise, reaching a maximum value at the end of the process, at 1.81% nitrogen. These results may indicate an effect of the inoculation with the two bacteria because the control presented a lower nitrogen content at the end of the process. According Jurado et al. (2015JURADO, M. M. et al. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresource Technology , 186:15-24, 2015.) Nitrogenous fractions could be immobilized, volatilized as NH3 (during thermophilic phases) or oxidized. This may be the cause for the nitrogen differences found among all treatments.
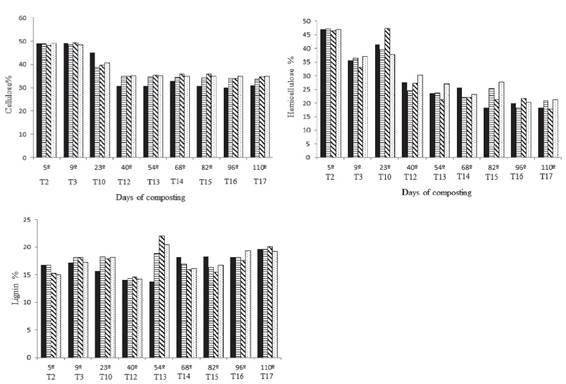
Cellulose degradation is another important step in the process of the formation of compost. Treatment 1 (B. cereus) resulted in lower levels of cellulose from the 23rd day and remained so until the end of the process, when the content of cellulose in the compost inoculated with B. cereus was 11.5% lower than that of the control (Figure 2). Therefore, it is possible that the inoculation of the compost with B. cereus increased the decomposition of that polymer. Some studies have reported the high cellulolytic potential of particular species of bacteria isolated from composting, such as those belonging to the genus Bacillus (Baharuddin; Razak; Hock, 2010BAHARUDDIN, A. S.; RAZAK, M. N. A.; HOCK, L. S. Isolation and characterization of thermophilic cellulose-producing bacteria from empty fruit bunches-palm oil mill effluent compost. American Journal of Applied Sciences, 7(7):56-62, 2010.; Lynd et al., 2012LYND, L. R. et al. Microbial cellulose utilization: fundamentals and biotechnology. Microbiology and Molecular Biology Reviews, 66(3):506-517, 2012.).
Cellulose, Hemicellulose and Lignin content on the days of composting process. Each turning corresponds to the respective day of composting (dry basis). T = turning.
The lignin content was variable, with peaks at the beginning and at the end of the composting process. The initial increase in the lignin content may be explained by the fact that as cellulose is degraded, lignin becomes more easily detected, resulting in an apparent increase. On the other hand, the highest lignin content at the final phase of the process may be explained by the reduction of cellulose and hemicellulose content, resulting in a proportional increase in lignin. The final values of lignin were practically identical (Figure 2), which was also expected because the inoculated bacteria possess no known ligninolytic activity (López-Gonzáles et al., 2015LÓPEZ-GONZÁLEZ, J. A. Dynamics of bacterial microbiota during lignocellulosic waste composting: Studies upon its structure, functionality and biodiversity. Bioresource Technology , 175:406-416, 2015.). Even in Treatment 3, the final lignin content was similar to the others. According to Jurado et al. (2015JURADO, M. M. et al. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresource Technology , 186:15-24, 2015.), lignin is one of the plant components that is the most difficult to degrade.
In the hemicellulose analysis, a final reduction of its content by approximately 55% (Figure 2) was found. The initial hemicellulose content was practically the same as that observed for cellulose, at approximately 47%, but at the end of the process, the hemicellulose content was only approximately 20%. After an initial decline observed on the ninth day of composting, an impressive increase was found on the 23rd day; however, the hemicellulose content fell again, maintaining this trend until the end of the process.
From the ninth day of composting, corresponding to the thermophilic phase, a trend of the control presenting a higher content of hemicellulose was found, suggesting an effect of the inoculation of the compost with the bacteria B. cereus and B. megaterium. Nevertheless, these differences decreased from the 68th day of composting, when the hemicellulose began to become stable. In spite of this, the hemicellulose contents in Treatments 1 and 3 were still observed to be 14.7 and 16.9% lower, respectively, when compared with the control at the end of the process.
Molecular analysis during composting
The profiles among the different treatments within each turning were evaluated by HRM analysis with the purpose of observing the likely influences of the inocula on bacterial succession over the composting process (Figure 3).
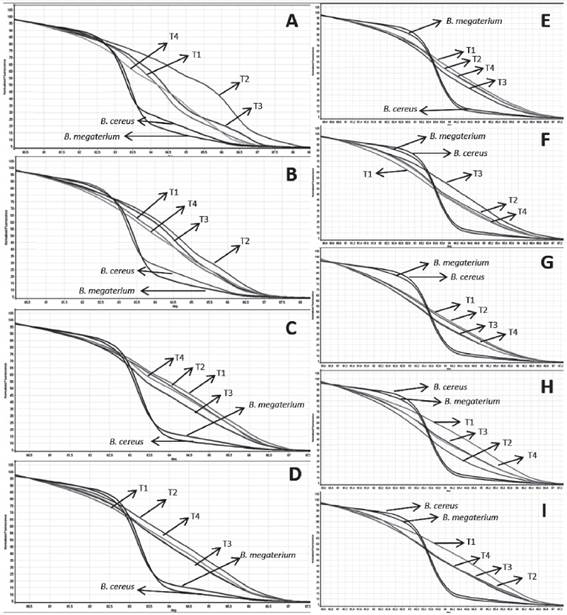
Melting curves of the different treatments at each turning. A) R2 - 5th day B) R4 - 9th day C) R10 - 23rd day D) R12 - 40th day E) R13 - 54th day F) R14 - 68thday G) R15-82th day H) R16 - 96thday I) R17 - 110th day. The plots also detail the controls, B. cereus and B. megaterium. R = turning, T = treatment.
From the analysis of the melting curve, the profile of bacterial succession over the composting process can be observed. It was found that at the beginning of composting until the ninth day (Figure 3), the treatments presented different profiles of fluorescence peaks, indicating that there was a dominance of certain bacteria species within the general bacterial community during that period of the process. The inocula were detected with the melting curve, although to lesser extent on the 23rd day (T10). One of those peaks appeared for the first time on the fifth day of composting in the control, alternating afterwards between one treatment and another. On the 23rd day (T10) the peaks associated with Treatments 3 and 4 were quite obvious in the region belonging to the inocula. The species of bacteria utilized as inocula are thermophiles, being plentiful in that phase of the process and together with fungi are responsible for cellulose degradation (Baharuddin; Razak; Hock, 2010BAHARUDDIN, A. S.; RAZAK, M. N. A.; HOCK, L. S. Isolation and characterization of thermophilic cellulose-producing bacteria from empty fruit bunches-palm oil mill effluent compost. American Journal of Applied Sciences, 7(7):56-62, 2010.; Lynd et al., 2012LYND, L. R. et al. Microbial cellulose utilization: fundamentals and biotechnology. Microbiology and Molecular Biology Reviews, 66(3):506-517, 2012.).Bacterias do genero Bacillus apresentam alta capacidade metabólica de degradar substâncias recalcitrantes como lignina e celulose, however. During the process, environmental conditions are mostly responsible for microbial fluctuations, and composting microbiota depends on its own competitive effectiveness and its survival capacity. On each composting process there are microbial groups frequently detected in moderate or high counts at all or many stages These groups constitute the composting resident microbiota (Lopes-González et al., 2014LÓPEZ-GONZÁLEZ, J. A. Dynamics of bacterial microbiota during lignocellulosic waste composting: Studies upon its structure, functionality and biodiversity. Bioresource Technology , 175:406-416, 2015.). The presence of Bacillus in all treatments suggests that it is a part of composting resident microbiota.
Like the melting curve, the profiles of the normalization curve of the treatments also presented the greatest differences at the beginning of composting (Figure 3). On the 40th day (T12) (Figure 4), the dissociation curves of Treatments 1 and 3 overlapped each other, indicating similarities in the prokaryote community at that moment of composting. On the 54th day (T13), the curves of Treatments 2 and 4 (control) overlapped each other, showing that, independent of the addition of the B. cereus inoculum, the bacterial populations of those treatments were similar during that phase of the process. On the 82nd day (T14) (Figure 4), the treatments were divergent, presenting no similarities.
Normalized curves of the different treatments at each turning. A) R2 - 5th day B) R4 - 9th day C) R10 - 23rd day D) R12 - 40th day E) R13 - 54th day F) R14 - 68th day G) R15-82nd day H) R16 - 96th day I) R17 - 110th. The plots also detail the controls, B. cereus and B. megaterium. R = turning, T = treatment.
In the last three analyses, the 82nd day (T15), the 96th day (T16) and the 110th day (T17) (Figure 4), the dissociation curves of Treatments 3 (B. cereus + B. megaterium) and 4 (control) overlapped each other, showing that regardless of inoculation, the bacterial community between those treatments are very similar.
For this experiment, the universal primer was utilized with the purpose of identifying the fullest bacterial community across the composting process to evaluate the complete process of composting succession. These primers anneal in highly conserved regions of the bacterial genome, and although the inoculum was detected on the 23rd day, other species of bacteria different from those used as inocula may have been more abundant over the course of the experiment, overlapping the inoculated species and hindering their identification in the other periods of composting by the HRM technique.
The utilization of the HRM technique allowed prokaryotic succession to be followed throughout the entire composting process as well as the determination of similarities among treatments over the course of composting and in the formation of the final product. However, because the primer accesses a conserved region common to most prokaryotes, this made the identification of inoculum in the treatment samples difficult. Although many studies have evaluated HRM profiles using the profiles of curves established from reference samples, thus making the identification of species easier (Hardick et al., 2012HARDICK, H. et al. Identification of bacterial pathogens in ascitic fluids from patients with suspected spontaneous bacterial peritonitis by use of broad-range PCR (16S PCR) coupled with High-Resolution Melt Analysis. Journal of Clinical Microbiology , 50(7):2428-2432, 2012.; Smith; Lu; Bremer, 2010SMITH, B. L.; LU, C. P.; BREMER, J. R. A. High-resolution melting analysis (HRMA): A highly sensitive inexpensive genotyping alternative for population studies. Molecular Ecology Resources, 10(1):193-196, 2010.; Won et al., 2010WON, H. et al. Rapid identification of bacterial pathogens in positive blood culture bottles by use of a compost maturity of agricultural wastes. Process Biochemistry , 44:396-400, 2010.). The use of pure DNA of B. cereus and B. megaterium as a standard along with the utilization of a primer specific for the two species utilized as inocula proved necessary.
CONCLUSIONS
The addition of inoculum showed a positive influence on the values of temperature and on the degradation of both cellulose and hemicellulose during the thermophilic period of the composting process. Through the HRM analysis, it was possible to follow bacterial succession over the process of composting using the melting curve to analyze the similarities and differences among the treatments through the normalization curve. In the thermophilic phase, the addition of B. cereus and B. megaterium inocula was found to influence the bacterial community of the compost.
REFERENCES
- BAHARUDDIN, A. S.; RAZAK, M. N. A.; HOCK, L. S. Isolation and characterization of thermophilic cellulose-producing bacteria from empty fruit bunches-palm oil mill effluent compost. American Journal of Applied Sciences, 7(7):56-62, 2010.
- BERNAL, M. P.; ALBURQUERQUE, J. A.; MORAL, R. Composting of animal manures and chemical criteria for compost maturity assessment: A review. Bioresource Technology, 100(22): 5444-5453, 2009.
- DERZELLE, S. et al. Characterization of genetic diversity of Bacillus anthracisin France by using High Resolution Melt. Journal of Clinical Microbiology, 49(12):4286-4292, 2011.
- FIERER, N. et al. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Applied Environment Microbiology, 71:4117-4120, 2005.
- FIGUEIREDO, V. R. et al. Microbial inoculation during composting improves productivity of sun mushroom (Agaricus subrufescens Peck). African Journal of Microbiology Research, 7(35): 4430-4434, 2013.
- GABRIEL, E. M. et al. High resolution melting analysis for rapid detection of linezolid resistance (mediated by G2576T mutation) in Staphylococcus epidermidis Journal of Microbiological Methods, 90(2):134-139, 2012.
- GAJALAKSHMI, S.; ABBASI, S. A. Solid waste management by composting: State of the art. Critical Reviews in Environmental Science and Technology, 38(5):311-400, 2008.
- HARDICK, H. et al. Identification of bacterial pathogens in ascitic fluids from patients with suspected spontaneous bacterial peritonitis by use of broad-range PCR (16S PCR) coupled with High-Resolution Melt Analysis. Journal of Clinical Microbiology , 50(7):2428-2432, 2012.
- GARCIA, M. C. V. et al. Influence of microbial inoculation and co-composting material on the evolution of humic-like substances during composting of horticultural wastes. Process Biochemistry, 40(6):1438-1443, 2006.
- HRNCIROVA, K. et al. Rapid detection and identification of mucormycetes from culture and tissue samples by use of High-Resolution Melt Analysis. Journal of Clinical Microbiology , 48(9):3392-3394, 2010.
- INSAM, H.; BERTOLDI, M. Microbiology of the composting process. Compost Science and Technology, Waste Management Series, Elsevier Ltd: p. 25-48, 2007.
- JURADO, M. M. et al. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresource Technology , 186:15-24, 2015.
- KIEHL, E. J. Manual da compostagem, maturação e qualidade do composto. Piracicaba: E. J. Kiehl, 2002. 171 p.
- LÓPEZ-GONZÁLEZ, J. A. Dynamics of bacterial microbiota during lignocellulosic waste composting: Studies upon its structure, functionality and biodiversity. Bioresource Technology , 175:406-416, 2015.
- LYND, L. R. et al. Microbial cellulose utilization: fundamentals and biotechnology. Microbiology and Molecular Biology Reviews, 66(3):506-517, 2012.
- MILLER, F. C. Composting as a process based on the control of ecologically selective factors. Soil Microbial Ecology, 18(3):515-543, 1992.
- PORCELLATO, D. et al. Rapid lactic acid bacteria identification in dairy products by high-resolution melt analysis of DGGE bands. Letters in Applied Microbiology, 54(4):344-351, 2012.
- SHEMEKITE, F. et al. Coffee husk composting: An investigation of the process using molecular and non-molecular tools. Waste Management, 34:642-652. 2014.
- SILVA, D. J.; QUEIROZ, A. C. Análise de Alimentos: Métodos químicos e biológicos. 3. ed. Viçosa, MG: UFV, 2012. 235 p.
- SILVA, D. J. et al. Composto orgânico em mangueiras (Mangifera indica L.) cultivadas no semiárido do nordeste brasileiro. Revista Brasileira de Fruticultura, 35(3): 875-882, 2013.
- SMITH, B. L.; LU, C. P.; BREMER, J. R. A. High-resolution melting analysis (HRMA): A highly sensitive inexpensive genotyping alternative for population studies. Molecular Ecology Resources, 10(1):193-196, 2010.
- SOUZA, T. P. et al. Analysis of thermophilic fungal populations during phase II of composting for the cultivation of Agaricus subrufescens World Journal Microbiology Biotechnology, 30:2419-2425, 2014.
- VALENTE, B. S. et al. Fatores que afetam o desenvolvimento da compostagem de resíduos orgânicos. Archivos de Zootecnia, 58(237):59-85, 2009.
- VILLAR, I. et al. Evolution of microbial dynamics during the maturation phase of the composting of different types of waste. Waste Management , 54:83-92, 2016.
- XIE, Kaizhi et al. Effects of supplementary composts on microbial communities and rice productivity in cold water paddy fields. Journal of Microbiology and Biotechnology, 25(5):569-578, 2015.
- WON, H. et al. Rapid identification of bacterial pathogens in positive blood culture bottles by use of a compost maturity of agricultural wastes. Process Biochemistry , 44:396-400, 2010.
- WONG, J. W. C. et al. Influence of different mixing ratios on in-vessel co-composting of sewage sludge with horse stable straw bedding waste: maturity and process evaluation. Waste Management & Research, 29(11):1164-1170, 2011.
- ZHANG, Y. G. et al. Modelling of organic matter dynamic during the composting process. Waste Management , 32:19-30, 2012.
- ZHANG, L.; SUN, X. Effects of earthworm casts and zeolite on the two-stage composting of green waste. Waste Management , 39:119-129, 2015.
- ZENG, G. M. et al. Effect of inoculating white-rot fungus during different phases on the characteristics of humic acid. Chemosphere, 68(4):368-374, 2009.
Publication Dates
-
Publication in this collection
Mar-Apr 2017
History
-
Received
28 Sept 2016 -
Accepted
28 Nov 2016