ABSTRACT
Ceratitis capitata (medfly) is a globally important horticultural pest that can be controlled using the sterile insect technique (SIT), but the success of SIT depends on the sexual performance of mass-reared males when released into the field. We added “guarana” (Paullinia cupana) powder (derived from an Amazonian fruit that is considered to be a stimulant with aphrodisiac effects, capable of improving human physical stamina) to the diets of adult male medflies to determine whether it increased their sexual performance. The basic diet of a protein extract + sucrose (1:3) was enriched with guarana powder (3 % on a volume basis). Experiments examining sexual competitiveness were performed using lab-reared males fed with the enriched diet vs. lab-reared males fed on the basic diet (and lab-reared females fed on the basic diet), as well as lab-reared males fed with the enriched diet vs. wild males fed on the basic diet (and wild females fed on the basic diet). The results of both experiments indicated that males maintained on diets enriched with guarana powder showed higher copulation successes than males fed only with the basic diet. Guarana powder therefore contributed to the greater mating success of lab-reared males (probably because of its stimulant properties), and may represent a new and viable option for increasing SIT effectiveness.
Ceratitis capitata; Paullinia cupana; Tephritidae; artificial diet
Introduction
The Mediterranean fruit fly, Ceratitis capitata (Wiedemann), is a globally important agricultural pest in fruit orchards (Liquido et al., 1990Liquido, N.J.; Cunningham, R.T.; Nakagawa, S. 1990. Hosts plants of the Mediterranean fruit fly (Diptera: Tephritidae) on the Island of Hawaii (1949-1985 survey). Journal of Economic Entomology 83: 1863-1868.; Norrbom, 2004Norrbom, A.L. 2004. Host Plant Database for Anastrepha and Toxotrypana (Diptera: Tephritidae: Toxotrypanini). 2. The Diptera Data Dissemination Disk. CABI, Wallingford, UK. (CD-ROM).). The Sterile Insect Technique (SIT; Knipling, 1955Knipling, E.F. 1955. Possibilities of insect control or eradication through the use of sexual sterile males. Journal of Economic Entomology 48: 459-462.) has been a successful option for controlling fruit flies in many parts of the world and has a low ecological impact (Enkerlin, 2005Enkerlin, W.R. 2005. Impact of fruit fly control programmes using the sterile insect technique. p. 651-676. In: Dyck, V.A.; Hendrichs, J.; Robinson, A., eds. Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, Netherlands.; Klassen and Curtis, 2005Klassen, W.; Curtis, C.F. 2005. History of the sterile insect technique. p. 3-36. In: Dyck, V.A.; Hendrichs, J.; Robinson, A., eds. Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, Netherlands.). The success of SIT is dependent, however, on the successful mating of wild females with males from mass-rearing facilities. Matings require large energy outlays by the males, including the emission of pheromones and the performance of courtship behavior and territorial defense within a non-resource-based male mating aggregation (Whittier et al., 1994Whittier, T.S.; Nam, F.Y.; Shelly, T.E.; Kaneshiro, K.Y. 1994. Male courtship success and female discrimination in the Mediterranean fruit fly (Diptera: Tephritidae). Journal of Insect Behavior 7: 159-170.; Blay and Yuval, 1997Blay, S.; Yuval, B. 1997. Nutritional correlates of reproductive success of male Mediterranean fruit flies (Diptera: Tephritidae). Animal Behaviour 54: 59-66.; Yuval et al., 1998Yuval, B.; Kaspi, R.; Shloush, S.; Warburg, M.S. 1998. Nutritional reserves regulate male participation in Mediterranean fruit fly leks. Ecological Entomology 23: 211-215.).
Studies involving exposure to volatile compounds, aromatherapy with ginger oil (Morelli et al., 2010Morelli, R.; Paranhos, B.J.; Coelho, A.M.; Castro, R.; Garziera, L.; Lopes, F.; Bento, J.M.S. 2010. Exposure of sterile Mediterranean fruit fly (Diptera: Tephritidae) males to ginger root oil reduces female remating. Journal of Applied Entomology 137: 75-82.), dietary supplements (Yuval et al., 2007Yuval, B.; Levy, K.; Kaspi, R.; Taylor, P.; Shelly, T.E. 2007. Breakfast of champions or kiss of death? Survival and sexual performance of protein-fed, sterile Mediterranean fruit flies (Diptera: Tephritidae). Florida Entomologist 90: 115-122.), and/or dietary modifications during early adult life (Pereira et al., 2011Pereira, R.; Teal, P.E.A.; Conway, H.; Worley, J.; Sivinski, J. 2011. Influence of methoprene and dietary protein on maturation and sexual performance of sterile Anastrepha ludens (Diptera:Tephritidae). Journal of Applied Entomology 137: 191-199.; Shelly, 2001Shelly, T.E. 2001. Exposure to α-copaene and α-copaene-containing oils enhances mating success of male Mediterranean fruit flies (Diptera: Tephritidae). Annals of Entomological Society of America 94: 497-502.), have been tested in attempts to improve the sexual competitiveness of mass-reared males under field conditions. In experiments with wild flies, Arita and Kaneshiro (1988)Arita, L.H.; Kaneshiro, K.Y. 1988. Body size and differential mating success between males of two populations of the Mediterranean fruit fly. Pacific Science 42: 173-177. reported that C. capitata males that emerged from coffee berries copulated more frequently than males emerging from Jerusalem cherries (Solanum pseudocapsicum L.) – even though the latter insects were larger. One possible explanation for this improved male sexual competitiveness might be the stimulatory effects of caffeine in the coffee berries. The guarana (Paullinia cupana) fruit is frequently used by humans as a stimulant (much like coffee). This Amazonian vine is cultivated exclusively in Brazil, with the state of Bahia being the world's largest production region (Fraife Filho and Ramos, 2014Fraife Filho, G.A.; Ramos, J.V. Guaraná. 2014. Available at: http://www.ceplac.gov.br/radar/guarana.htm [Accessed Jan. 2014] (in Portuguese).
http://www.ceplac.gov.br/radar/guarana.h...
). The fruits contain large quantities of caffeine, and their seeds are roasted and ground to produce a powder – the most commonly commercialized product. Caffeine levels in guarana powder can be up to four times greater than those found in ground coffee (CEPLAC, 2011; Tfouni et al., 2007)Tfouni, S.A.V.; Camargo, M.C.R.; Vitorino, S.H.P.; Menegário, T.F.; Toledo, M.C.F. 2007. Contribution from powder guarana (Paullinia cupana) as a source of caffeine in the diet. Revista de Nutrição 20: 63-68. (in Portuguese, with abstract in English).. The effects attributed to the consumption of guarana powder by humans include aphrodisiac properties and increased metabolic rates (Kuri, 2008Kuri, C.M.B. 2008. The guarana industry in Brazil. International Business and Economics Research Journal 7: 87-98.; Webb, 2006)Webb, G.P. 2006. Dietary Supplements and Functional Foods. Blackwell, Oxford, UK.. As such, fruit flies fed on a diet with added guarana powder might be expected to show greater copulatory success. Flies fed on coffee berries during the larvae stage of C. capitata demonstrated greater mating success compared with cherry emerged flies (Arita and Kaneshiro, 1988)Arita, L.H.; Kaneshiro, K.Y. 1988. Body size and differential mating success between males of two populations of the Mediterranean fruit fly. Pacific Science 42: 173-177..
The present work sought to determine if the inclusion of guarana powder in the diets of lab-reared males of C. capitata would improve their sexual performance and thus potentially increase the effectiveness of SIT.
Materials and Methods
Experiment 1: The male flies used in this experiment were obtained from a line of C. capitata maintained in the laboratory for 15 years and fed as larvae on a yeast-based diet (Carvalho et al., 1998Carvalho, R.S.; Nascimento, A.S.; Matrangolo, W.J. 1998. Methodology of Exotic Parasitoid Creation Diachasmimorpha longicaudata (Hymenoptera: Braconidae) to Laboratory Studies and Field = Metodologia de Criação do Parasitóide Exótico Diachasmimorpha longicaudata (Hymenoptera: Braconidae), Visando Estudos em Laboratório e em Campo. Embrapa-CNPMF, Cruz-das-Almas, BA, Brazil (in Portuguese).). After emergence, the lab-reared males were divided into two groups and fed for five days on different diets.
The first group received a diet based on yeast extract + sucrose (1:3) – the diet regularly used in maintaining the adults of these populations (Silva Neto et al., 2012Silva Neto, A.M.; Dias, V.S.; Joachim-Bravo, I.S. 2012. Reproductive behavior of Ceratitis capitata Wiedemann (Diptera: Tephritidae): effect of males size on mating success. EntomoBrasilis 5: 190-197 (in Portuguese, with abstract in English).) – and referred to hereafter as the basic diet. The second group received the basic diet but with the addition of guarana powder (3 % vol/vol). On the sixth day, 10 male flies from each dietary group were placed together with 10 five-day-old lab-reared virgin females (fed on basic diet) in a screen-mesh cage (68 × 68 × 90 cm) containing a small pitanga tree (Eugenia uniflora) approximately 60 cm tall. Each group of males was encased in tulle to prevent them moving and marked on the dorsal region of the thorax with a spot of non-toxic ink for identification. Observations of mating behavior were performed from 07h00-12h00, and each copulating pair was gently removed from the cage. The latency time for copulation (the time from the release of the individuals into the cage at the beginning of the experiment until the beginning of copulation), as well as copulation duration (the time from the insertion of the adeagus to the moment the flies separated) were recorded. The experiments were undertaken under controlled laboratory conditions (temperature 25 ± 1 °C, 70 % relative humidity, and an illumination regime of 1500 lux from a fluorescent light on a 12:12 L:D cycle). Fifteen replicates were performed.
Experiment 2: Lab-reared males fed on a diet of yeast extract + sucrose (1:3) enriched with guarana powder (3 % vol/vol) were tested for their sexual competitiveness in relation to wild males (obtained from infested Terminalia cattapa fruits) that were fed on the basic diet after emergence (without added guarana powder). The sexual competition conditions followed those used in experiment 1, with a number of modifications. Tests were conducted in field cages (230 × 150 × 230 cm) containing a pitanga (Eugenia uniflora) bush to simulate the natural environment. Twenty males from each group (previously marked with non-toxic paint) were liberated together with 20 wild females that had been fed on a basic diet. In this experiment, the wild males and wild females were 15 days old due to their longer sexual maturity; the laboratory males used were six days old, as in the previous experiment. All of the flies were fed with their respective diets until the day before initiating the mating competition tests. Five repetitions were performed. In a previous experiment, sexual competitiveness was compared for males reared in laboratory, fed with basic diet (sugar + yeast extract - 3: 1), and wild males also fed with the basic diet, in field cages. In that experiment, wild females chose to mate with significantly more wild males (at least twice more) than lab-reared males (unpaired t-test; p < 0.0001) (Aquino and Joachim-Bravo, 2014)Aquino, J.C.; Joachim-Bravo, I.S. 2014. Relevance of male size to female mate choice in Ceratitis capitata (Diptera: Tephritidae): investigations with wild and laboratory-reared flies. Journal of Insect Behavior 27: 162-176.. This experiment was used as reference for comparison of the new data we present here, since it used the same lineage flies and the same methodology and laboratory conditions. In order to test the influence of guarana-diet on the length of the male calling behavior (the first step of a sequence of behavioral courtship units, in which the male releases a pheromone drop by eversion of the anal epithelium and flaps its wings to aid dissemination), a complementary experiment was performed. Sexually mature wild males fed with or without guarana-diet (one of each group) were painted with different colors and placed in glass boxes (90 × 90 × 70 mm) for observation during 10 min to compare the duration of calling behavior between them. The experiment was conducted in the morning with 10 replicates.
Data analysis: Sexual competitiveness, mating latency, copulation duration, and calling behavior tests were compared between guarana-fed and control males, using the Student T-test, after evaluating the data for variance normality and homogeneity. The nonparametic Mann-Whitney test was used when these assumptions were not met.
Results
Experiment 1: Lab-reared C. capitata males maintained on a diet enriched with guarana powder showed greater copulatory success than lab-reared males maintained on a basic diet (T-test; p < 0.0001) (Figure 1). There were no significant differences in copulation latency between the two groups (Mann-Whitney, U = 1532, p = 0.457), but males fed with guarana demonstrated greater copulation durations (Mann-Whitney, U = 1187, p = 0.01937) (Table 1).
– Number of mated males fed on a basic diet with or without guarana powder). Bar heights represent means (+ SD = standard deviation; n = 15), (T-test; p < 0.0001). All flies used in this experiment were derived from a laboratory strain and females used were fed with basic diet without guarana.
− Latency to copula and copula duration of Ceratitis capitata males fed on diets with and without guarana powder. The data represent the medians (with ranges given) of latency to copula and copula duration. All flies used in this experiment were from a laboratory strain.
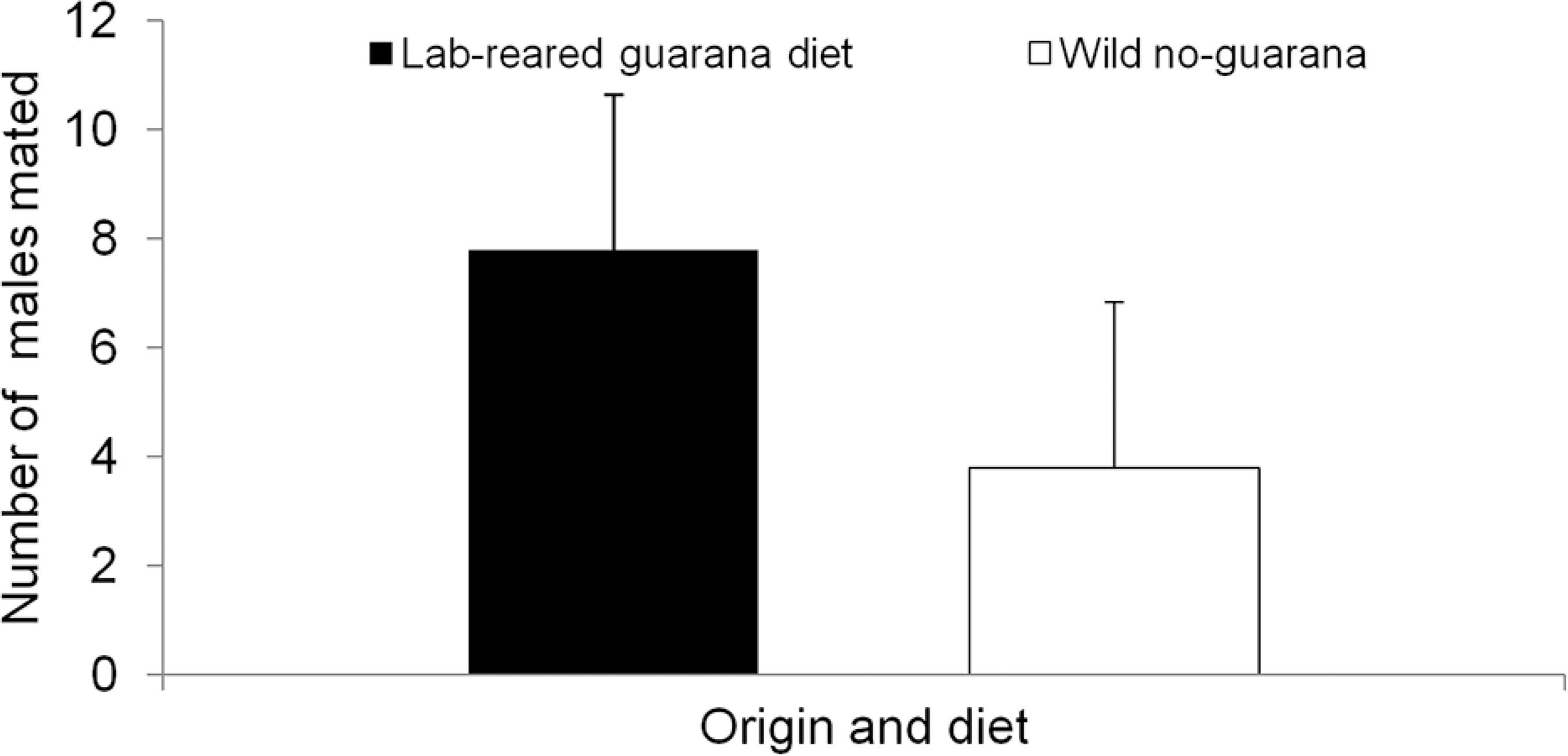
Experiment 2: In competitive experiments between lab-reared and wild males, the lab-males maintained on the guarana diet had greater mating success than wild males fed with the basic diet (T-test, t = 2.144; p < 0.05) (Figure 2). The results were based on minimum replicates necessary to allow the t-test, due to the experiment’s limitations (the experiment was conducted under field conditions with the use of a large number of wild C. capitata, which are difficult to obtain).
− Number of mated males (lab-reared males fed with guarana-diet and wild males fed with the same basic diet without guarana powder). Wild females fed with basic diet without guarana were used in these experiments. Bar heights represent means (+ SD = standard deviation; n = 5), (T-test; p < 0.05).
There were no significant differences in copulation latency time (T-test, t = 0.336, p = 0.745) or copulation duration between the groups (T-test, t = 0.968, p = 0.362) (Table 2).
− Latency time to copulation and copulation duration of wild males (fed with non-guarana diet) and lab-reared ones (fed with guarana-diet). Data represent the mean of latency time to copulation (minutes) and duration of copulation (minutes).
As regards the duration of calling behavior, males fed on the basic-diet spent more time in calling behavior (mean = 352.5 s; standard deviation - SD = 158) than males fed on the guarana-diet (mean = 62.5 s; standard deviation - SD = 55.5) (T-test; p = 0.002) (Figure 3).
– Duration of calling behavior of lab-reared male flies that as adults were provided diets either with or without 30 % guarana powder included. Bar heights represent means (+ SD = standard deviation; n = 10), (T-test; p = 0.002).
Discussion
The addition of guarana powder improved the sexual performances of lab-reared C. capitata males, as they showed greater mating success with wild females – including their preference over wild males. Previous research done by our group using the same medfly strain (wild flies fed with basic diet vs. lab-reared fed with basic diet) and about the same time (Aquino and Joachim-Bravo, 2014Aquino, J.C.; Joachim-Bravo, I.S. 2014. Relevance of male size to female mate choice in Ceratitis capitata (Diptera: Tephritidae): investigations with wild and laboratory-reared flies. Journal of Insect Behavior 27: 162-176.) had shown that wild male fruit flies tend to be more successful at mating than lab-reared males – even when the physical characteristics of the lab-reared males appeared to be more favorable. These experiments indicated that although lab-reared males demonstrated characteristics that would otherwise make them more favorable to females (such as having larger body sizes (Anderson, 1994Anderson, M. 1994. Sexual Selection. Princeton University Press, Princeton, NJ, USA.; Anjos-Duarte et al., 2010Anjos-Duarte, C.S.; Costa, A.M.; Joachim-Bravo, I.S. 2010. Sexual behaviour of the Mediterranean fruit fly (Diptera: Tephritidae): the influence of female size on mate choice. Journal of Applied Entomology 1: 1-7.)), wild males were more successful at copulations when present.
Many efforts have been made to improve the efficiency of SIT with medflies by attempting to improve the copulatory success of sterile males, including exposure to volatile compounds derived from fruits such as guava, lemons, and mangoes (Vera et al., 2010Vera, M.T.; Ruiz, M.J.; Oviedo, A.; Abraham, S.; Mendoza, M.; Segura, D.F.; Kouloussis, N.A.; Willink, E. 2010. Fruit compounds affect male sexual success in the South American fruit fly, Anastrepha fraterculus (Diptera: Tephritidae). Journal of Applied Entomology 137: 2-10.) and citrus oil (Haq et al., 2010Haq, I.; Cáceres, C.; Liedo, P.; Soriano, D.; Jessup, A.; Hendrichs, J.; Teal, P.E.A.; Robinson, A.S. 2010. Effect of methoprene application, adult food and feeding duration on male melon fly starvation survival. Journal of Applied Entomology 137: 61-68.; Kouloussis et al., 2010Kouloussis, N.A.; Katsoyannos, B.I.; Papadopoulos, N.T.; Ioannou, C.S.; Iliadis, I.V. 2010. Enhanced mating competitiveness of Ceratitis capitata males following exposure to citrus compounds. Journal of Applied Entomology 137: 30-38.); sterile C. capitata males have also been exposed to ginger oil in attempts to reduce female re-mating rates (Morelli et al., 2010Morelli, R.; Paranhos, B.J.; Coelho, A.M.; Castro, R.; Garziera, L.; Lopes, F.; Bento, J.M.S. 2010. Exposure of sterile Mediterranean fruit fly (Diptera: Tephritidae) males to ginger root oil reduces female remating. Journal of Applied Entomology 137: 75-82.). In addition to aromatherapy, providing food supplements during the adult stage has also shown promising results. Liedo et al., (2010)Liedo, P.; Orozco, D.; Cruz-López, L.; Quintero, J.L.; Becerra-Pérez, C.; Refugio Hernández, M.; Oropeza, A.; Toledo, J. 2010. Effect of post-teneral diets on the performance of sterile Anastrepha ludens and Anastrepha obliqua fruit flies. Journal of Applied Entomology 137: 49-60., for example, demonstrated that both wild and sterile A. obliqua males fed with mangoes and oranges showed greater mating success than flies fed on normal diets (yeast + sugar). Pérez-Staples et al., (2009)Pérez-Staples, D.; Weldon, C.W.; Smallridge, C.; Taylor, P.W. 2009. Pre-release feeding on yeast hydrolysate enhances sexual competitiveness of sterile male Queensland fruit flies in field cages. Entomologia Experimentalis et Applicata 131: 159-166. found that sterile males of Bactrocera tryoni (Froggatt) that fed on yeast hydrolysate for a brief period were as competitive as wild males. Other workers have shown that the application of methoprene as well as greater access to proteins in adult diets could increase the mating success of the males of some species of Tephritidae; these fruit flies also attained sexual maturity earlier (Gómez et al., 2011Gómez, Y.; Teal, P.E.A.; Pereira, R. 2011. Enhancing efficacy of Mexican fruit fly SIT programmes by large-scale incorporation of methoprene into pre-release diet. Journal of Applied Entomology 137: 252-259.; Haq et al., 2010Haq, I.; Cáceres, C.; Liedo, P.; Soriano, D.; Jessup, A.; Hendrichs, J.; Teal, P.E.A.; Robinson, A.S. 2010. Effect of methoprene application, adult food and feeding duration on male melon fly starvation survival. Journal of Applied Entomology 137: 61-68.; Haq and Hendrichs, 2011Haq, I.; Hendrichs, J. 2011. Pre-release feeding on hydrolysed yeast and methoprene treatment enhances male Bactrocera cucurbitae Coquillett (Diptera: Tephritidae) longevity. Journal of Applied Entomology 137: 99-102.; Pereira et al., 2011Pereira, R.; Teal, P.E.A.; Conway, H.; Worley, J.; Sivinski, J. 2011. Influence of methoprene and dietary protein on maturation and sexual performance of sterile Anastrepha ludens (Diptera:Tephritidae). Journal of Applied Entomology 137: 191-199.; Teal et al., 2011Teal, P.E.A.; Pereira, R.; Segura, D.F.; Haq, I.; Gómez-Simuta, Y.; Robinson, A.S.; Hendrichs, J. 2011. Methoprene and protein supplements accelerate reproductive development and improve mating success of male tephritid flies. Journal of Applied Entomology 137: 91-98.). It was demonstrated that Bactrocera tryoni males maintained on hydrolyzed yeast and sugar showed benefits such as: rapid development, longer copulation times, increasing probability of mating and spermatozoa-stocking by the females, sexual inhibition of females once they had copulated, and increased longevity (Pérez-Staples et al., 2007Pérez-Staples, D.; Prabhu, V.; Taylor, P.W. 2007. Post-teneral protein feeding enhances sexual performance of Queensland fruit flies. Physiological Entomology 32: 225-232.; Vijaysegaran et al., 2002Vijaysegaran, S.; Walter, G.H.; Drew, R.A.I. 2002. Influence of adult diet on the development of the reproductive system and mating ability of Queensland fruit fly Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Journal of Tropical Agriculture and Food Science 30: 119-136.; Weldon and Taylor, 2011Weldon, C.W.; Taylor, P.W. 2011. Sexual development of wild and mass-reared male Queensland fruit flies in response to natural food sources. Entomologia Experimentalis et Applicata 139: 17-24.) .
The reasons for the success of added guarana to the diets of lab-reared males still need to be more closely examined from the physiological and behavioral points of view. The results suggest that the addition of guarana powder to the males’ diet should contribute to their mating success but not by dint of duration of calling behavior. Another interesting result that must be analyzed in further studies concerned the greater duration of copula in guarana-fed males. Although these results cannot be fully explained yet, it is conceivable that the high concentration of caffeine in guarana powder contributed to the males' mating success. One hypothesis is that males fed on a guarana diet could be more agile to “fight” in lek and could have greater ability to reach the most interesting points of the tree. The data about mating success obtained here were similar to the results obtained by Arita and Kaneshiro (1985)Arita, L.H.; Kaneshiro, K.Y. 1985. The dynamics of the lek system and mating success in males of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann). Proceedings of the Hawaiian Entomological Society 25: 39-48., who reported the mating success of males obtained from coffee berries (also containing high caffeine levels), although the fruits had been provided during the larval stage in their experiments instead of a food supplement for adults, as reported in the present study. The authors suggested that coffee-fed males could achieve the best positions in lek and could be more chosen by females. Further studies concerning courtship behavior will be performed to investigate the reasons for the mating success of males fed on the guarana diet.
The sexual competitiveness of sterile, mass-produced C. capitata fruit flies was improved by adding natural stimulants to their diets, and this utilization of guarana powder may represent a new and viable option for increasing SIT effectiveness for medfly control in Brazil – and a viable alternative to expensive imported products such as methoprene.
References
- Anderson, M. 1994. Sexual Selection. Princeton University Press, Princeton, NJ, USA.
- Anjos-Duarte, C.S.; Costa, A.M.; Joachim-Bravo, I.S. 2010. Sexual behaviour of the Mediterranean fruit fly (Diptera: Tephritidae): the influence of female size on mate choice. Journal of Applied Entomology 1: 1-7.
- Arita, L.H.; Kaneshiro, K.Y. 1985. The dynamics of the lek system and mating success in males of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann). Proceedings of the Hawaiian Entomological Society 25: 39-48.
- Arita, L.H.; Kaneshiro, K.Y. 1988. Body size and differential mating success between males of two populations of the Mediterranean fruit fly. Pacific Science 42: 173-177.
- Aquino, J.C.; Joachim-Bravo, I.S. 2014. Relevance of male size to female mate choice in Ceratitis capitata (Diptera: Tephritidae): investigations with wild and laboratory-reared flies. Journal of Insect Behavior 27: 162-176.
- Blay, S.; Yuval, B. 1997. Nutritional correlates of reproductive success of male Mediterranean fruit flies (Diptera: Tephritidae). Animal Behaviour 54: 59-66.
- Carvalho, R.S.; Nascimento, A.S.; Matrangolo, W.J. 1998. Methodology of Exotic Parasitoid Creation Diachasmimorpha longicaudata (Hymenoptera: Braconidae) to Laboratory Studies and Field = Metodologia de Criação do Parasitóide Exótico Diachasmimorpha longicaudata (Hymenoptera: Braconidae), Visando Estudos em Laboratório e em Campo. Embrapa-CNPMF, Cruz-das-Almas, BA, Brazil (in Portuguese).
- Enkerlin, W.R. 2005. Impact of fruit fly control programmes using the sterile insect technique. p. 651-676. In: Dyck, V.A.; Hendrichs, J.; Robinson, A., eds. Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, Netherlands.
- Fraife Filho, G.A.; Ramos, J.V. Guaraná. 2014. Available at: http://www.ceplac.gov.br/radar/guarana.htm [Accessed Jan. 2014] (in Portuguese).
» http://www.ceplac.gov.br/radar/guarana.htm - Gómez, Y.; Teal, P.E.A.; Pereira, R. 2011. Enhancing efficacy of Mexican fruit fly SIT programmes by large-scale incorporation of methoprene into pre-release diet. Journal of Applied Entomology 137: 252-259.
- Haq, I.; Cáceres, C.; Liedo, P.; Soriano, D.; Jessup, A.; Hendrichs, J.; Teal, P.E.A.; Robinson, A.S. 2010. Effect of methoprene application, adult food and feeding duration on male melon fly starvation survival. Journal of Applied Entomology 137: 61-68.
- Haq, I.; Hendrichs, J. 2011. Pre-release feeding on hydrolysed yeast and methoprene treatment enhances male Bactrocera cucurbitae Coquillett (Diptera: Tephritidae) longevity. Journal of Applied Entomology 137: 99-102.
- Klassen, W.; Curtis, C.F. 2005. History of the sterile insect technique. p. 3-36. In: Dyck, V.A.; Hendrichs, J.; Robinson, A., eds. Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, Netherlands.
- Knipling, E.F. 1955. Possibilities of insect control or eradication through the use of sexual sterile males. Journal of Economic Entomology 48: 459-462.
- Kouloussis, N.A.; Katsoyannos, B.I.; Papadopoulos, N.T.; Ioannou, C.S.; Iliadis, I.V. 2010. Enhanced mating competitiveness of Ceratitis capitata males following exposure to citrus compounds. Journal of Applied Entomology 137: 30-38.
- Kuri, C.M.B. 2008. The guarana industry in Brazil. International Business and Economics Research Journal 7: 87-98.
- Liedo, P.; Orozco, D.; Cruz-López, L.; Quintero, J.L.; Becerra-Pérez, C.; Refugio Hernández, M.; Oropeza, A.; Toledo, J. 2010. Effect of post-teneral diets on the performance of sterile Anastrepha ludens and Anastrepha obliqua fruit flies. Journal of Applied Entomology 137: 49-60.
- Liquido, N.J.; Cunningham, R.T.; Nakagawa, S. 1990. Hosts plants of the Mediterranean fruit fly (Diptera: Tephritidae) on the Island of Hawaii (1949-1985 survey). Journal of Economic Entomology 83: 1863-1868.
- Morelli, R.; Paranhos, B.J.; Coelho, A.M.; Castro, R.; Garziera, L.; Lopes, F.; Bento, J.M.S. 2010. Exposure of sterile Mediterranean fruit fly (Diptera: Tephritidae) males to ginger root oil reduces female remating. Journal of Applied Entomology 137: 75-82.
- Norrbom, A.L. 2004. Host Plant Database for Anastrepha and Toxotrypana (Diptera: Tephritidae: Toxotrypanini). 2. The Diptera Data Dissemination Disk. CABI, Wallingford, UK. (CD-ROM).
- Pereira, R.; Teal, P.E.A.; Conway, H.; Worley, J.; Sivinski, J. 2011. Influence of methoprene and dietary protein on maturation and sexual performance of sterile Anastrepha ludens (Diptera:Tephritidae). Journal of Applied Entomology 137: 191-199.
- Pérez-Staples, D.; Weldon, C.W.; Smallridge, C.; Taylor, P.W. 2009. Pre-release feeding on yeast hydrolysate enhances sexual competitiveness of sterile male Queensland fruit flies in field cages. Entomologia Experimentalis et Applicata 131: 159-166.
- Pérez-Staples, D.; Prabhu, V.; Taylor, P.W. 2007. Post-teneral protein feeding enhances sexual performance of Queensland fruit flies. Physiological Entomology 32: 225-232.
- Shelly, T.E. 2001. Exposure to α-copaene and α-copaene-containing oils enhances mating success of male Mediterranean fruit flies (Diptera: Tephritidae). Annals of Entomological Society of America 94: 497-502.
- Silva Neto, A.M.; Dias, V.S.; Joachim-Bravo, I.S. 2012. Reproductive behavior of Ceratitis capitata Wiedemann (Diptera: Tephritidae): effect of males size on mating success. EntomoBrasilis 5: 190-197 (in Portuguese, with abstract in English).
- Teal, P.E.A.; Pereira, R.; Segura, D.F.; Haq, I.; Gómez-Simuta, Y.; Robinson, A.S.; Hendrichs, J. 2011. Methoprene and protein supplements accelerate reproductive development and improve mating success of male tephritid flies. Journal of Applied Entomology 137: 91-98.
- Tfouni, S.A.V.; Camargo, M.C.R.; Vitorino, S.H.P.; Menegário, T.F.; Toledo, M.C.F. 2007. Contribution from powder guarana (Paullinia cupana) as a source of caffeine in the diet. Revista de Nutrição 20: 63-68. (in Portuguese, with abstract in English).
- Vera, M.T.; Ruiz, M.J.; Oviedo, A.; Abraham, S.; Mendoza, M.; Segura, D.F.; Kouloussis, N.A.; Willink, E. 2010. Fruit compounds affect male sexual success in the South American fruit fly, Anastrepha fraterculus (Diptera: Tephritidae). Journal of Applied Entomology 137: 2-10.
- Vijaysegaran, S.; Walter, G.H.; Drew, R.A.I. 2002. Influence of adult diet on the development of the reproductive system and mating ability of Queensland fruit fly Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Journal of Tropical Agriculture and Food Science 30: 119-136.
- Webb, G.P. 2006. Dietary Supplements and Functional Foods. Blackwell, Oxford, UK.
- Weldon, C.W.; Taylor, P.W. 2011. Sexual development of wild and mass-reared male Queensland fruit flies in response to natural food sources. Entomologia Experimentalis et Applicata 139: 17-24.
- Whittier, T.S.; Nam, F.Y.; Shelly, T.E.; Kaneshiro, K.Y. 1994. Male courtship success and female discrimination in the Mediterranean fruit fly (Diptera: Tephritidae). Journal of Insect Behavior 7: 159-170.
- Yuval, B.; Kaspi, R.; Shloush, S.; Warburg, M.S. 1998. Nutritional reserves regulate male participation in Mediterranean fruit fly leks. Ecological Entomology 23: 211-215.
- Yuval, B.; Levy, K.; Kaspi, R.; Taylor, P.; Shelly, T.E. 2007. Breakfast of champions or kiss of death? Survival and sexual performance of protein-fed, sterile Mediterranean fruit flies (Diptera: Tephritidae). Florida Entomologist 90: 115-122.
Edited by
Publication Dates
-
Publication in this collection
May-Jun 2016
History
-
Received
27 Apr 2014 -
Accepted
08 Oct 2015