Abstracts
The aim of the present investigation was to evaluate the angiogenesis on dorsal cutaneous wounds in a rodent model treated with λ660 nm laser light. New vessel formation is a multistep process involving vessel sprouting, endothelial cell migration, proliferation and tube formation. Although several in vivo studies have shown that laser phototherapy influences tissue repair, a fully understanding of angiogenesis mechanisms are not yet known. Twenty-four young adult male Wistar rats weighing between 200 and 250 g were used. Under general anesthesia, one excisional wound was created on the dorsum of each animal and they were randomly distributed into two groups: one control and one treated with laser (λ660 nm, 16 mW, 10 J/cm2). Each group was subdivided into three subgroups according to the animal death timing (2, 4 and 6 days). Laser irradiation started immediately after surgery and was repeated every other day during the experiment and marked with Sirius Red, specific for collagen, and immunomarked with anti-TGF-β and anti-von Willebrand factor. Marked sections underwent histological analysis by light microscopy and the mean area of the wound of each animal was calculated and analyzed by ANOVA and Tukey's test (α=0.05). Although at some death periods, collagen expression and number of blood vessels on irradiated animals were higher than in the control ones, no significant differences were found at any time in relation to TGF-β expression (p>0.05). It was concluded that laser treatment (λ660 nm) contributed to increase angiogenesis.
angiogenesis; TGF-β; laser; repair
O objetivo do trabalho foi avaliar a angiogênese em feridas cutâneas no dorso de ratos tratados com o laser de λ660 nm. A neovascularização é um processo que envolve o aparecimento vascular, a migração das células endoteliais, a proliferação e a formação tubular. Embora diversos estudos in vivo demonstrem que a fototerapia laser influencia no reparo tecidual, uma compreensão completa dos mecanismos da angiogênese ainda não é conhecida. Foram utilizados 24 ratos Wistar novos, machos e adultos pesando entre 200 e 250 g. Uma ferida excisional foi criada no dorso de cada animal sob anestesia geral e os animais foram distribuídos aleatoriamente em dois grupos: G0 (controle) e G1 (laser λ660 nm, 16 mW, 10 J/cm2). Cada grupo foi subdividido em três subgrupos de acordo com o sincronismo da morte dos animais (2, 4 e 6 dias). A irradiação laser foi iniciada imediatamente após a cirurgia, sendo repetida diariamente durante a experiência, avaliada por meio de vermelho de Sirius, específico para o colágeno e avaliação imunológica com anti-TGF-β e o Fator anti-von Willebrand. As seções marcadas foram submetidas à análise histológica no foto-microscópio, onde a área média de cada subgrupo foi calculada e analisada usando o teste ANOVA e de Tukey (α=0,05). Os valores dos animais irradiados foram maiores em certos períodos da morte, na expressão do colágeno e no número de vasos em comparação com os grupos controles. Nenhuma diferença significativa foi encontrada na expressão do TGF-β entre os grupos nos períodos. Conclui-se que o tratamento com laser λ660 nm contribuiu para o aumento da angiogênese.
Introduction
Tissue repair is an interactive process, involving chemical mediators, cells, and the inflammatory response, characterized by the classic steps of repair: inflammation, granulation, and remodeling (11. Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med 1999;341:738-746.). The whole process is triggered by a tissue insult that is followed immediately by the migration of neutrophils into the injured site. Later, monocytes also migrate into the wound site in a similar manner to neutrophils. The proliferative phase occurs between the 5th and 14th days following injury; it is characterized by fibrogenesis. Fibroblasts, macrophages, and angiogenesis favor the formation of granulation tissue. Later, the wound enters a maturation period during which remodeling occurs, mainly by the deposition of a more mature and better-organized collagen fiber network (22. Enoch S, Grey JE, Harding KG. ABC of wound healing. Recent advances and emerging treatments. BMJ 2006;332:962-965.).
In systemic and local healing, neoangiogenesis and collagen matrix deposition are very important for the outcome of tissue repair. Angiogenesis restores the level of both oxygen and nutrients for the newly forming tissue, supplying the high metabolic demand, favoring cell proliferation and migration as well as protein synthesis (33. Schaffer CJ, Nanney LB. Cell biology of wound healing. Int Rev Cytol 1996;169:161-181.). Several growth factors show angiogenic potential. The transforming growth factor beta (TGF-β) is produced by most cells found in granulation tissue and increases the production of other cytokines, stimulates angiogenesis and the production of type 1 collagen, and inhibits the production of interstitial collagenase (11. Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med 1999;341:738-746.,22. Enoch S, Grey JE, Harding KG. ABC of wound healing. Recent advances and emerging treatments. BMJ 2006;332:962-965.).
It is known that low-level laser therapy (LLLT), using appropriate protocols, improves wound healing (44. Pinheiro AL, Meireles GC, de Barros Vieira AL, Almeida D, Carvalho CM, dos Santos JN. Phototherapy improves healing of cutaneous wounds in nourished and undernourished wistar rats. Braz Dent J 2004;15(Special Issue):SI21-SI28.). This study evaluated the effects of laser light (λ660 nm) on the maturation of granulation tissue, focusing on neoangiogenesis, TGF-β, and collagen matrix expression in cutaneous wounds in rodents.
Material and Methods
The Animal Experimentation Ethics Committee of the School of Dentistry of the Federal University of Bahia approved this work (Process #029/06).
Twenty-four young adult male Wistar rats, weighing 200-250 g, were obtained from the Central Animal House of the School of Veterinary Medicine of the Federal University of Bahia and kept in individual plastic cages, with wood-chip bedding, maintained at 22 °C on a day/night light cycle and fed with standard pellet laboratory diet (Labina, Agribrands-Purina Ltda., Paulínia, SP, Brazil) and had water available ad libitum at the Animal Experimentation Laboratory of the School of Dentistry of the Federal University of Bahia, Brazil.
After regular quarantining, the animals received intraperitoneal general anesthesia [60 mg/kg of ketamine chlorhydrate (Vetaset; Fort Dodge Animal Health, Campinas, SP, Brazil) and 10 mg/kg of xylazine (Coopazine; Intervet Schering-Plough, São Paulo, SP, Brazil)] and had their dorsum shaven and cleaned with chlorhexidine gluconate 10 mg/mL solution (Merthiolate; Hypermarcas S.A., Barueri, SP, Brazil). One excisional cutaneous wound (1 × 1 cm) was created with a scalpel on the dorsum of each animal and left without suturing or dressing. The animals were then randomly distributed into two groups with 12 animals in each (Control and Laser). Each group was subdivided into three subgroups according to the time of sacrifice (2, 4, or 6 days). Laser therapy was carried out using a Twin Flex Laser (MMOptics Ltda., São Carlos, SP, Brazil) and started immediately after surgery and was repeated at every other day during the experimental period. Laser light was applied at four points around the wound (4 × 2.5 J/cm2). The laser time was 62 s per point, with 248 s per session. The total dose time at 2, 4 and 6 days after surgery were respectively 248, 496 and 744 s (Table 1). The device automatically controlled the time of the application. The spatial average energy fluency (SAEF) per session used for all groups is summarized in Table 1. If the animal presented any evidence of pain, a non-steroid analgesic was ready for use, but this did not occur in any group.
Following macroscopic examination, each animal was sacrificed with an anesthetic overdose (300 mg/kg of ketamine hydrochloride and 50 mg/kg of xylazine hydrochloride) at 2, 4, or 6 days after surgery. Specimens were taken and kept in 10% phosphate-buffered formalin for 24 h and were then routinely cut and processed in wax. Five-micrometer-thick sections were stained with hematoxylin and eosin, and Sirius red for collagen identification. Antigen retrieval for the slides for anti-TGF-β staining was accomplished through incubation with pepsin 1%, pH 1.8, at approximately 37 °C for 30 min. For anti-von Willebrand factor, slides were steamed in Tris/EDTA solution, pH 9.0. They were then incubated overnight at 4 °C in a humidified chamber with polyclonal anti-von Willebrand factor antibody (A0082, DAKO-Denmark, 1:200) and a polyclonal anti-TGFβ antibody (1:4), with a background reducer (DAKO antibody diluent with background reducing components, S3022) using the EnVision system (DAKO, K4061), the DAB substrate chromogen system (DAKO, K3466), and Meyer's hematoxylin. Tissue sections were counterstained with hematoxylin, dehydrated, and mounted with Canada balsam. Granulation tissue acted as positive controls and negative control used PBS buffer instead of the primary antibody.
An experienced pathologist evaluated the immunostained sections histologically in a blind manner. A light microscope (Motic B5 Professional Series with a camera Moticam 2000 and the Motic Image Advance 3.0 software; Motic Instruments Inc., Richmond, BC, Canada) was used to capture images of three consecutive fields in each slide. The areas of collagen and TGF-β expression were measured using the Motic Image software. The number and area of vessels marked with anti-von Willebrand factor antibody were quantified. There was no restriction concerning the size of vessels; small endothelial cell islands were included, considering the bifurcations and longitudinal sections of microvessels. The mean area of each specimen was calculated using Excel for Mac. The mean area of each sub-group was calculated similarly. Data were analyzed using the Minitab software, version 15 (Globaltech, Belo Horizonte, MG, Brazil). For statistical analysis, ANOVA and Tukey's test were used to compare mean areas among groups with a significance level of 5%.
Results
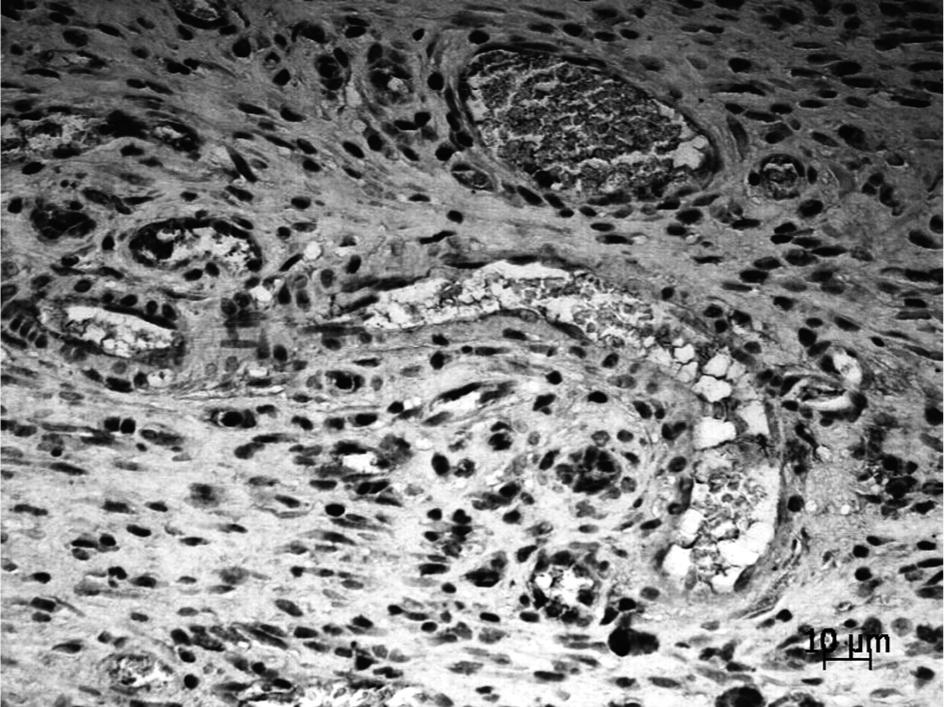
Immunohistochemical staining for von Willebrand Factor showed elevated numbers of vessels in surgical wounds subjected to laser therapy, compared with the controls, although the differences were only statistically significant on the second day (Table 2, Figs. 1 and 2). A statistically significant difference was found at days 2 and 4 between the control groups (p<0.001, ANOVA) and between the laser groups (p = 0.01, ANOVA). Table 2 shows the distributions of areas of vessels in the laser treatment versus control groups at days 2, 4, and 6 post-treatment. In animals treated with the laser, vasodilation was notable at every post-treatment time-point compared with control animals, although at days 2 and 4 the differences were statistically significant only between the laser groups. Table 2 shows the distribution of TGF-β area in laser-treated versus control groups at days 2, 4, and 6 post-treatment. At days 2 and 4, laser-treated wounds showed lower TGF-β levels, while at day 6, TGF-β synthesis was elevated in the laser treatment group compared with the control, although the differences were not significant (Figs. 3 and 4).Table 2 shows the distribution of collagen area in the laser and control groups at days 2, 4, and 6 post-treatment. Statistically significant differences were found at days 2 and 6 between the control (p=0.049) and the laser (p=0.049) groups. At the 6th day, laser-treated wounds showed elevated levels of collagen compared with the control group (Figs. 5 and 6, p = 0.012, ANOVA).
Expression pattern of endothelial von Willebrand positive cells in immunohistochemical staining. Control group showing a small number of vessels, day 2.
Von Willebrand positive cells in immunohistochemical staining. Laser group exhibiting elevated number of vessels and larger vessels lumen in comparison with the control group, day 2.
Discussion
This study assessed whether granulation tissue in cutaneous wounds would be influenced by the use of laser light because the formation of new blood vessels involves phenomena such as the migration of endothelial cells, proliferation, tube formation and survival (55. Melo VA, Anjos DC, Albuquerque Júnior R, Melo DB, Carvalho FU. Effect of low level laser on sutured wound healing in rats. Acta Cir Bras 2011;26:129-134.). The degradation involving mast cells represents an important step in the reduction of vessel formation in the first 24 h after laser application. Immunohistochemical staining of von Willebrand factor showed lower numbers of vessels until the first 24 h in surgical wounds submitted to laser therapy compared with the control group. In animals treated with laser, vasodilation was notable, especially at the first 12 h post-treatment, compared with control animals (66. Pereira MC, de Pinho CB, Medrado AR, Andrade Zde A, Reis SR. Influence of 670nm low-level laser theraphy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B 2010;98:188-192.).
In a study of the number of vessels formed with and without laser application (670 nm), there was an increase in vessels in the laser group compared to the control group on only the 3rd day (66. Pereira MC, de Pinho CB, Medrado AR, Andrade Zde A, Reis SR. Influence of 670nm low-level laser theraphy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B 2010;98:188-192.). In the present investigation, the number of vessels and relative area of the laser group was superior at all periods. The pro-angiogenic effect of the use of LLLT we report here was characterized by a higher number of newly formed blood vessels at the wound site, mainly on the 2nd day following injury. Our findings are consistent with the report of Corazza et al. (77. Corazza AV, Jorge J, Kurachi C, Bagnato VB. Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed Laser Surg 2007;25:102-106.), who used 5 J/cm2 and 20 J/cm2 (λ = 660 nm, 40 mW, 31 s and 126 s), also on cutaneous wounds. A recent study in Wistar rats found similar results (λ = 670 nm, 9 mW, 4 J/cm2 and 124 s) (66. Pereira MC, de Pinho CB, Medrado AR, Andrade Zde A, Reis SR. Influence of 670nm low-level laser theraphy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B 2010;98:188-192.).
Another finding in the irradiated animals was vasodilatation, which was observed at all time points in comparison with the control animals and was significantly higher in irradiated subjects on both days 2 and 4. Although the difference between the groups was not statistically significant, it is possible that the laser contributed to the increase in vessel area, possibly due to the effects of light on cellular receptors (88. Vasheghani MM, Bayat M, Rezaei F, Bayat A, Karimipour M. Effect of low-level laser therapy on mast cells in second-degree burns in rats. Photomed Laser Surg 2008;26:1-5.,99. Sawasaki I, Geraldo-Martins VR, Ribeiro MS, Marques MM. Effect of low-intensity laser therapy on mast cell degranulation in human oral mucosa. Lasers Med Sci 2009;24:113-116.). This phenomenon was also reported in subcutaneous tissue in which the inflammatory reaction was increased by laser irradiation (λ = 670 nm, 9 mW, 4 J/cm2, 124 s) (88. Vasheghani MM, Bayat M, Rezaei F, Bayat A, Karimipour M. Effect of low-level laser therapy on mast cells in second-degree burns in rats. Photomed Laser Surg 2008;26:1-5.).
Growth factors, proteins, components of the coagulation/fibrinolytic pathways, extracellular matrix proteins, and platelets interact with endothelial cells and pericytes adjacent to blood vessels, regulating the formation of new blood vessels (1010. Nienartowicz A, Sobaniec-Lotowska ME, Jarocka-Cyrta E, Lemancewicz D. Mast cells in neoangiogenesis. Med Sci Monit 2006;12:53-56.). Of the growth factors involved in angiogenesis, VEGF, PDGF, bFGF, TNFα, EGF, PDECGF and TGF-β were detected. VEGF is a determining factor for the differentiation of endothelial cells and for the development of vascularity in the wounded area. The role of the other growth factors, such as TGF-β, seems to be complementary (1010. Nienartowicz A, Sobaniec-Lotowska ME, Jarocka-Cyrta E, Lemancewicz D. Mast cells in neoangiogenesis. Med Sci Monit 2006;12:53-56.). It was found lower TGF-β expression in irradiated animals in comparison with the control groups during the early phases of the repair process. Increased expression of this factor was observed on only the sixth day. Despite the absence of a significant difference between the groups, TGF-β expression may be considered a signal of the inhibition of both immunological and inflammatory responses. A previous report of healing of gingival lesions in rodents showed that the use of LLLT (λ = 632.8 nm, 17 mW, 7.5 J/cm2, 300 s) increased PDGF and TGF-β gene expression. However, the expression of some cytokines, such as IL-1β and IFNγ, was reduced (1111. Safavi SM, Kazemi B, Esmaeili M, Fallah A, Modarresi A, Mir M. Effects of low-level He-Ne laser irradiation on the gene expression of IL-1β, TNF-α, IFN-γ, TGF-β. bFGF, and PDGF in rat's gingiva. Lasers Med Sci 2008;23:331-335.).
TGF-β acts as a regulator of growth and differentiation of cells, as well as playing an important role in the formation of the extracellular matrix (1212. Arany PR, Nayak RS, Hallikerimath S, Limaye AM, Kale AD, Kondaiah P. Activation of latent TGF-b1 by low-power laser in vitro correlates with increased TGF-b1 levels in laser-enhanced oral wound healing. Wound Rep Reg 2007;15:866-874.). In the present study, we found increased collagen deposition in irradiated subjects, which was significantly higher on the sixth day. This is consistent with previous reports in which LLLT accelerated both angiogenesis and wound healing (1313. Karu T. Photobiology of low-power laser effects. Health Phys 1989;56:691-704.). In vivo and in vitrostudies using different protocols have shown that LLLT modulates many of the cells involved in the healing process (66. Pereira MC, de Pinho CB, Medrado AR, Andrade Zde A, Reis SR. Influence of 670nm low-level laser theraphy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B 2010;98:188-192.,1414. Lopes-Martins RA, Albertini R, Martins PS, Bjordal JM, Faria Neto HC. Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by carrageenan. Photomed Laser Surg 2005;23:377-381.
15. Santos NR, dos Santos JN, dos Reis JA Jr, Oliveira PC, de Sousa AP, de Carvalho CM, et al.. Influence of the use of laser phototherapy (lambda660 or 790 nm) on the survival of cutaneous flaps on diabetic rats. Photomed Laser Surg 2009;15:763-769.
16. Pinheiro AL, Meireles GC, Carvalho CM, Ramalho LM, dos Santos JN. Biomodulative effects of visible and IR laser light on the healing of cutaneous wounds of nourished and undernourished Wistar rats. Photomed Laser Surg 2009;27:947-957.
17. Zhevago N, Samoilova KA. Pro and anti-inflammatory cytokine content in human peripheral blood after its transcutaneous (in vivo) and direct (in vitro) irradiation with polychromatic visible and infrared light. Photomed Laser Surg 2006;24:129-139.
18. Pires D, Xavier M, Araújo T, Silva JA Jr, Aimbire F, Albertini R. Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 2011;26:85-94.
19. Gonçalves RV, Novaes RD, Cupertino Mdo C, Moraes B, Leite JP, Peluzio Mdo C, et al.. Time-dependent effects of low-level laser therapy on the morphology and oxidative response in the skin wound healing in rats. Lasers Med Sci 2012;28:383-390.
20. Soleimani M, Abbasnia E, Fathi M, Sahraei H, Fathi Y, Kaka G. The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts–an in vitro study. Lasers Med Sci 2012;27:423-430.-2121. da Silva AP, Petri AD, Crippa GE, Stuani AS, Stuani AS, Rosa AL, et al.. Effect of low-level laser therapy after rapid maxillary expansion on proliferation and differentiation of osteoblastic cells. Lasers Med Sci 2012;27: 777-783.). A previous study using λ = 830 nm and/or λ = 685 nm laser light and doses of 20 J/cm2 and 50 J/cm2 showed increased collagen production in irradiated animals in comparison with the controls (λ = 830 nm, 50 J/cm2) (2222. Mendez TM, Pinheiro AL, Pacheco MT, Nascimento PM, Ramalho LM. Dose and wavelength of laser light have influence on the repair of cutaneous wounds. J Clin Laser Med Surg 2004;22:19-25.). The findings of a recent study evaluating the influence of LLLT on bone volume and bone implant contact interface around implants inserted in bovine or autologous bone grafts in the femurs of rabbits revealed that the use of LLLT stimulated new bone formation with consequent increase of bone-implant interface in both xenografts and autografts (2323. Soares LGP, Magalhaes Junior EB, Magalhaes CAB, Ferreira CF, Marques AMC, Pinheiro ALB. New Bone formation around implants inserted on autologous and xenografts irradiated or not with ir laser light: a histomorphometric study in rabbits. Braz Dent J 2013;24:218-223.).
The results of the present study indicate that LLLT positively influences angiogenesis, TGF-β expression and collagen deposition. However, the TGF-β area increased significantly only from the 6th day on, while the collagen area increased progressively throughout the study period. This finding suggests that other growth factors are involved in collagen synthesis.
References
-
1Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med 1999;341:738-746.
-
2Enoch S, Grey JE, Harding KG. ABC of wound healing. Recent advances and emerging treatments. BMJ 2006;332:962-965.
-
3Schaffer CJ, Nanney LB. Cell biology of wound healing. Int Rev Cytol 1996;169:161-181.
-
4Pinheiro AL, Meireles GC, de Barros Vieira AL, Almeida D, Carvalho CM, dos Santos JN. Phototherapy improves healing of cutaneous wounds in nourished and undernourished wistar rats. Braz Dent J 2004;15(Special Issue):SI21-SI28.
-
5Melo VA, Anjos DC, Albuquerque Júnior R, Melo DB, Carvalho FU. Effect of low level laser on sutured wound healing in rats. Acta Cir Bras 2011;26:129-134.
-
6Pereira MC, de Pinho CB, Medrado AR, Andrade Zde A, Reis SR. Influence of 670nm low-level laser theraphy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B 2010;98:188-192.
-
7Corazza AV, Jorge J, Kurachi C, Bagnato VB. Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed Laser Surg 2007;25:102-106.
-
8Vasheghani MM, Bayat M, Rezaei F, Bayat A, Karimipour M. Effect of low-level laser therapy on mast cells in second-degree burns in rats. Photomed Laser Surg 2008;26:1-5.
-
9Sawasaki I, Geraldo-Martins VR, Ribeiro MS, Marques MM. Effect of low-intensity laser therapy on mast cell degranulation in human oral mucosa. Lasers Med Sci 2009;24:113-116.
-
10Nienartowicz A, Sobaniec-Lotowska ME, Jarocka-Cyrta E, Lemancewicz D. Mast cells in neoangiogenesis. Med Sci Monit 2006;12:53-56.
-
11Safavi SM, Kazemi B, Esmaeili M, Fallah A, Modarresi A, Mir M. Effects of low-level He-Ne laser irradiation on the gene expression of IL-1β, TNF-α, IFN-γ, TGF-β. bFGF, and PDGF in rat's gingiva. Lasers Med Sci 2008;23:331-335.
-
12Arany PR, Nayak RS, Hallikerimath S, Limaye AM, Kale AD, Kondaiah P. Activation of latent TGF-b1 by low-power laser in vitro correlates with increased TGF-b1 levels in laser-enhanced oral wound healing. Wound Rep Reg 2007;15:866-874.
-
13Karu T. Photobiology of low-power laser effects. Health Phys 1989;56:691-704.
-
14Lopes-Martins RA, Albertini R, Martins PS, Bjordal JM, Faria Neto HC. Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by carrageenan. Photomed Laser Surg 2005;23:377-381.
-
15Santos NR, dos Santos JN, dos Reis JA Jr, Oliveira PC, de Sousa AP, de Carvalho CM, et al.. Influence of the use of laser phototherapy (lambda660 or 790 nm) on the survival of cutaneous flaps on diabetic rats. Photomed Laser Surg 2009;15:763-769.
-
16Pinheiro AL, Meireles GC, Carvalho CM, Ramalho LM, dos Santos JN. Biomodulative effects of visible and IR laser light on the healing of cutaneous wounds of nourished and undernourished Wistar rats. Photomed Laser Surg 2009;27:947-957.
-
17Zhevago N, Samoilova KA. Pro and anti-inflammatory cytokine content in human peripheral blood after its transcutaneous (in vivo) and direct (in vitro) irradiation with polychromatic visible and infrared light. Photomed Laser Surg 2006;24:129-139.
-
18Pires D, Xavier M, Araújo T, Silva JA Jr, Aimbire F, Albertini R. Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 2011;26:85-94.
-
19Gonçalves RV, Novaes RD, Cupertino Mdo C, Moraes B, Leite JP, Peluzio Mdo C, et al.. Time-dependent effects of low-level laser therapy on the morphology and oxidative response in the skin wound healing in rats. Lasers Med Sci 2012;28:383-390.
-
20Soleimani M, Abbasnia E, Fathi M, Sahraei H, Fathi Y, Kaka G. The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts–an in vitro study. Lasers Med Sci 2012;27:423-430.
-
21da Silva AP, Petri AD, Crippa GE, Stuani AS, Stuani AS, Rosa AL, et al.. Effect of low-level laser therapy after rapid maxillary expansion on proliferation and differentiation of osteoblastic cells. Lasers Med Sci 2012;27: 777-783.
-
22Mendez TM, Pinheiro AL, Pacheco MT, Nascimento PM, Ramalho LM. Dose and wavelength of laser light have influence on the repair of cutaneous wounds. J Clin Laser Med Surg 2004;22:19-25.
-
23Soares LGP, Magalhaes Junior EB, Magalhaes CAB, Ferreira CF, Marques AMC, Pinheiro ALB. New Bone formation around implants inserted on autologous and xenografts irradiated or not with ir laser light: a histomorphometric study in rabbits. Braz Dent J 2013;24:218-223.
Publication Dates
-
Publication in this collection
July-Aug 2013
History
-
Received
2 Sept 2012 -
Accepted
13 July 2013