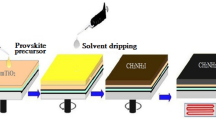
Abstract
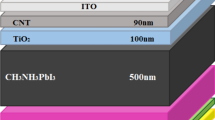
Three perovskite solar cells with a general architecture of FTO/GO-CuI/Perovskite/ZnO/Ag electrode were fabricated with different ratios of graphene oxide and copper iodide as hybrid hole transport layers in an open-air environment for the comparison purposes and to find better hole transport material. The obtained power conversion efficiencies were extrapolated to find the power conversion efficiencies of other compositions of these materials including graphene oxide and copper iodide only-based devices. Hence, enhanced information was obtained by just making three devices only, which allowed us to save a lot of time and resources as compared to making devices in the standard conditions. The power conversion efficiency was increased with the decrease in concentration of graphene oxide, hence copper iodide showed better hole transport characteristics. This simplistic approach to evaluate power conversion efficiencies for hybrid charge transport layers can be utilized for any combination of hole or electron hybrid transport layers in perovskite solar cells.
Graphical abstract












Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
L.M. Gonçalves et al., Dye-sensitized solar cells: a safe bet for the future. Energy Environ. Sci. 1(6), 655–667 (2008)
Z. Wu et al., Efficient planar heterojunction perovskite solar cells employing graphene oxide as hole conductor. Nanoscale 6(18), 10505–10510 (2014)
K. Ahmad, S.M. Mobin, Graphene oxide based planar heterojunction perovskite solar cell under ambient condition. New J. Chem. 41(23), 14253–14258 (2017)
J. Niu et al., Graphene-oxide doped PEDOT:PSS as a superior hole transport material for high-efficiency perovskite solar cell. Org. Electron. 48, 165–171 (2017)
Q.-D. Yang et al., Graphene oxide as an efficient hole-transporting material for high-performance perovskite solar cells with enhanced stability. Journal of Materials Chemistry A 5(20), 9852–9858 (2017)
A. Agresti et al., Graphene–perovskite solar cells exceed 18% efficiency: a stability study. Chemsuschem 9(18), 2609–2619 (2016)
M. Acik, S.B. Darling, Graphene in perovskite solar cells: device design, characterization and implementation. J. Mater. Chem. A 4(17), 6185–6235 (2016)
E. Kymakis, D. Konios, Graphene oxide-like materials in organic and perovskite solar cells, in The Future of Semiconductor Oxides in Next-Generation Solar Cells. (Elsevier, Amsterdam, 2018), pp. 357–394
M. Jawad et al., Effect of gold nanoparticles on transmittance and conductance of graphene oxide thin films and efficiency of perovskite solar cells. Appl. Nanosci. 10(2), 485–497 (2020)
M. Bidikoudi, E. Kymakis, Novel approaches and scalability prospects of copper based hole transporting materials for planar perovskite solar cells. J. Mater. Chem. C 7(44), 13680–13708 (2019)
A.M. Elseman et al., Recent progress concerning inorganic holetransport layers for efficient perovskite solar cells. Appl. Phys. A 125(7), 476 (2019)
R. Rajeswari et al., Emerging of inorganic hole transporting materials for perovskite solar cells. Chem. Rec. 17(7), 681–699 (2017)
S. Inudo, M. Miyake, T. Hirato, Electrical properties of Cu I films prepared by spin coating. Phys. Status Solidi A 210(11), 2395–2398 (2013)
Peng, Y., et al., Efficient organic solar cells using copper(I) iodide (CuI) hole transport layers. Applied Physics Letters, 2015. 106(24): p. 243302.
W.-Y. Chen et al., Low-cost solution-processed copper iodide as an alternative to PEDOT:PSS hole transport layer for efficient and stable inverted planar heterojunction perovskite solar cells. J. Mater. Chem. A 3(38), 19353–19359 (2015)
W. Sun et al., Room-temperature and solution-processed copper iodide as the hole transport layer for inverted planar perovskite solar cells. Nanoscale 8(35), 15954–15960 (2016)
M. Huangfu et al., Copper iodide as inorganic hole conductor for perovskite solar cells with different thickness of mesoporous layer and hole transport layer. Appl. Surf. Sci. 357, 2234–2240 (2015)
J.A. Christians, R.C. Fung, P.V. Kamat, An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 136(2), 758–764 (2014)
J. Han et al., Enhancing the performance of perovskite solar cells by hybridizing SnS quantum dots with CH3NH3PbI3. Small 13(32), 1700953 (2017)
V. Srikant, D.R. Clarke, On the optical band gap of zinc oxide. J. Appl. Phys. 83(10), 5447–5451 (1998)
R. Azmi et al., High efficiency low-temperature processed perovskite solar cells integrated with alkali metal doped ZnO electron transport layers. ACS Energy Lett. 3(6), 1241–1246 (2018)
D. Bi et al., Efficient and stable CH3NH3PbI3-sensitized ZnO nanorod array solid-state solar cells. Nanoscale 5(23), 11686–11691 (2013)
D. Liu, T.L. Kelly, Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photon. 8(2), 133–138 (2014)
B. Gil et al., Recent progress in inorganic hole transport materials for efficient and stable perovskite solar cells. Electron. Mater. Lett. 15(5), 505–524 (2019)
J. Cao et al., Low-temperature solution-processed NiO x films for air-stable perovskite solar cells. J. Mater. Chem. A 5(22), 11071–11077 (2017)
D.-Y. Lee, S.-I. Na, S.-S. Kim, Graphene oxide/PEDOT: PSS composite hole transport layer for efficient and stable planar heterojunction perovskite solar cells. Nanoscale 8(3), 1513–1522 (2016)
Y. Wang et al., Ammonia-treated graphene oxide and PEDOT:PSS as hole transport layer for high-performance perovskite solar cells with enhanced stability. Org. Electron. 70, 63–70 (2019)
H. Luo et al., Efficient and air-stable planar perovskite solar cells formed on graphene-oxide-modified PEDOT:PSS hole transport layer. Nano-Micro Lett. 9(4), 39 (2017)
D. Li et al., Graphene oxide modified hole transport layer for CH3NH3PbI3 planar heterojunction solar cells. Sol. Energy 131, 176–182 (2016)
W.-D. Hu et al., Copper iodide-PEDOT:PSS double hole transport layers for improved efficiency and stability in perovskite solar cells. J. Photochem. Photobiol. A 357, 36–40 (2018)
X. Wang et al., Facile fabrication of reduced graphene oxide/CuI/PANI nanocomposites with enhanced visible-light photocatalytic activity. RSC Adv. 6(50), 44851–44858 (2016)
X. Ma et al., Enhanced bacterial disinfection by CuI–BiOI/rGO hydrogel under visible light irradiation. RSC Adv. 11(33), 20446–20456 (2021)
G. Niu, X. Guo, L. Wang, Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 3(17), 8970–8980 (2015)
Q. Wali et al., Advances in stability of perovskite solar cells. Org. Electron. 78, 105590 (2020)
N. Suresh Kumar, K. Chandra Babu Naidu, A review on perovskite solar cells (PSCs), materials and applications. J. Materiom. 7(5), 940–956 (2021)
Y. Cheng et al., Decomposition of organometal halide perovskite films on zinc oxide nanoparticles. ACS Appl. Mater. Interfaces 7(36), 19986–19993 (2015)
A. Kojima et al., Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131(17), 6050–6051 (2009)
Q. Chen et al., Planar heterojunction perovskite solar cells via vapor-assisted solution process. J. Am. Chem. Soc. 136(2), 622–625 (2014)
P. Luo et al., Uniform, stable, and efficient planar-heterojunction perovskite solar cells by facile low-pressure chemical vapor deposition under fully open-air conditions. ACS Appl. Mater. Interfaces 7(4), 2708–2714 (2015)
L.-L. Gao et al., Large-area high-efficiency perovskite solar cells based on perovskite films dried by the multi-flow air knife method in air. J. Mater. Chem. A 5(4), 1548–1557 (2017)
D.C. Marcano et al., Improved synthesis of graphene oxide. ACS Nano 4(8), 4806–4814 (2010)
A.K. Zak et al., Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 6, 1399 (2011)
S. Talam, S.R. Karumuri, N. Gunnam, Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. ISRN Nanotechnol. 2012, 372505 (2012)
L. Shahriary, A.A. Athawale, Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng 2(01), 58–63 (2014)
W. Muhammad et al., Optical, morphological and biological analysis of zinc oxide nanoparticles (ZnO NPs) using Papaver somniferum L. RSC Adv. 9(51), 29541–29548 (2019)
A. Alkhouzaam et al., Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram. Int. 46(15), 23997–24007 (2020)
S. Pan, I.A. Aksay, Factors controlling the size of graphene oxide sheets produced via the graphite oxide route. ACS Nano 5(5), 4073–4083 (2011)
R.M. Alwan et al., Synthesis of zinc oxide nanoparticles via sol–gel route and their characterization. Nanosci. Nanotechnol. 5(1), 1–6 (2015)
C. Xie et al., Novel surface modification of ZnO QDs for paclitaxel-targeted drug delivery for lung cancer treatment. Dose Response 18(2), 1559325820926739 (2020)
Z. Abbasi et al., Binding efficiency of functional groups towards noble metal surfaces using graphene oxide—metal nanoparticle hybrids. Colloids Surf. A 611, 125858 (2020)
W. Saeed et al., An insight into the binding behavior of graphene oxide and noble metal nanoparticles. J. Appl. Phys. 129(12), 125302 (2021)
A.J. Shaikh et al., Binding strength of porphyrin−gold nanoparticle hybrids based on number and type of linker moieties and a simple method to calculate inner filter effects of gold nanoparticles using fluorescence spectroscopy. J. Phys. Chem. A 119(7), 1108–1116 (2015)
A.J. Shaikh, Exploring the direction of charge transfer in Porphyrin—PbSe quantum dot hybrids. ChemistrySelect 1(8), 1678–1686 (2016)
M. Mathesh et al., Facile synthesis of graphene oxide hybrids bridged by copper ions for increased conductivity. J. Mater. Chem. C 1(18), 3084–3090 (2013)
V.A. Smirnov et al., Conductivity of graphene oxide films: Dependence from solvents and photoreduction. Chem. Phys. Lett. 583, 155–159 (2013)
C. Yang et al., Room-temperature synthesized copper iodide thin film as degenerate p-type transparent conductor with a boosted figure of merit. Proc. Natl. Acad. Sci. U.S.A. 113(46), 12929 (2016)
V.M. Le Corre et al., Charge transport layers limiting the efficiency of perovskite solar cells: how to optimize conductivity, doping, and thickness. ACS Appl. Energy Mater. 2(9), 6280–6287 (2019)
Y. Zhou, X. Li, H. Lin, To be higher and stronger—metal oxide electron transport materials for perovskite solar cells. Small 16(15), 1902579 (2020)
M.S. Khan et al., Graphene quantum dot and iron co-doped TiO2 photocatalysts: Synthesis, performance evaluation and phytotoxicity studies. Ecotoxicol. Environ. Saf. 226, 112855 (2021)
Q. Yuan et al., Cu2O/BiVO4 heterostructures: synthesis and application in simultaneous photocatalytic oxidation of organic dyes and reduction of Cr(VI) under visible light. Chem. Eng. J. 255, 394–402 (2014)
J. Peng et al., Developing an efficient NiCo2S4 cocatalyst for improving the visible light H2 evolution performance of CdS nanoparticles. Phys. Chem. Chem. Phys. 19(38), 25919–25926 (2017)
Y. Xu, M.A.A. Schoonen, The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 85(3–4), 543–556 (2000)
S. Li et al., A brief review of hole transporting materials commonly used in perovskite solar cells. Rare Met. 40(10), 2712–2729 (2021)
D. Saranin et al., Copper iodide interlayer for improved charge extraction and stability of inverted perovskite solar cells. Materials 12(9), 1406 (2019)
S. Uthayaraj et al., Powder pressed cuprous iodide (CuI) as a hole transporting material for perovskite solar cells. Materials 12(13), 2037 (2019)
G. Venugopal et al., An investigation of the electrical transport properties of graphene-oxide thin films. Mater. Chem. Phys. 132(1), 29–33 (2012)
Q. Shao, H. Lin, M. Shao, Determining locations of conduction bands and valence bands of semiconductor nanoparticles based on their band gaps. ACS Omega 5(18), 10297–10300 (2020)
P.-H. Lee et al., High-efficiency perovskite solar cell using cobalt doped nickel oxide hole transport layer fabricated by NIR process. Sol. Energy Mater. Solar Cells. 208, 110352 (2020)
W.S. Hummers Jr., R.E. Offeman, Preparation of graphitic oxide. J. Am. Chem. Soc. 80(6), 1339–1339 (1958)
W.J. Beek et al., Hybrid zinc oxide conjugated polymer bulk heterojunction solar cells. J. Phys. Chem. B 109(19), 9505–9516 (2005)
J.B. Coulter, D.P. Birnie, Assessing Tauc Plot slope quantification: ZnO thin films as a model system. Physica Status Solidi b 255(3), 1700393 (2018)
Acknowledgments
The research described in this paper was partially financially supported by the Higher Education Commission of Pakistan under National Research Program for Universities with Reference No. 20-3369/R&D/HEC/14/978, which was awarded to Dr. A. J. Shaikh. Francis Agada is thankful to Queen Elizabeth Commonwealth Scholarship for his graduate studies, with reference no. FE-2019-100.
Author information
Authors and Affiliations
Contributions
ZA and FA performed bench work, ZA wrote the initial draft; AHK provided all help related to fabrication of perovskite solar cells, AMK provided help with the UV–Vis experiments; MB and MA helped with the SEM images and XRD experiments, AJS conceived the idea and was the major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abbas, Z., Agada, F., Kamboh, A.H. et al. A simplistic approach to evaluate the power conversion efficiencies for hybrid charge transport layers in open-air fabricated perovskite solar cells. Journal of Materials Research 37, 1323–1340 (2022). https://doi.org/10.1557/s43578-022-00537-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00537-x