Abstract
An effective technique for improving electrochemical efficiency is to rationally design hierarchical nanostructures that completely optimize the advantages of single components and establish an interfacial effect between structures. In this study, core–shell NiMoO4@Ni9S8/MoS2 hetero-structured nanorods are prepared via a facile hydrothermal process followed by a direct sulfurization. The resulting hierarchical architecture with outer Ni9S8/MoS2 nanoflakes shell on the inner NiMoO4 core offers plentiful active sites and ample charge transfer pathways in continuous heterointerfaces. Ascribing to the porous core–shell configuration and synergistic effect of bimetal sulfides, the obtained NiMoO4@Ni9S8/MoS2 as electrode material presents an unsurpassed specific capacity of 373.4 F g−1 at 10 A g−1 and remarkable cycling performance in the 6 M KOH electrolyte. This work delivers a rational method for designing highly efficient electrodes for supercapacitors, enlightening the road of exploring low-cost materials in the energy storage domain.
Graphical Abstract

Similar content being viewed by others
Introduction
Arising markets of high-energy density electronics and various electrical cars have prompted the production of efficient, cheap, and environmentally benign energy storage technologies and devices [1]. Among them, supercapacitors (SCs) have been attracted wide-ranging research interests because of their outstanding energy density, rating performance and superior cycling durability [2]. The SCs have filled the storage gap within traditional batteries devices and capacitors, so that they have been regarded as the promising and efficient storage devices. However, the energy density of supercapacitors still far below the Li-ion batteries which confines their large-print industrial employments [3]. Normally, supercapacitors are alienated to two types depending on different working principles: electrical double layer capacitors (EDLCs) and faradaic pseudocapacitors [4]. To boost the energy density and superior flexibility, asymmetric supercapacitors (ACS) consisted with the above two electrodes have aroused great interests, because they could be utilized the merits of both batteries and supercapacitors, and thus remarkably enhance their energy and power densities [5]. It’s known that carbon-based materials exhibit the excellent high conductivity and excellent density as battery-type electrodes. However, they still fail the expectation for long life durability. Compared to them, pseudo-capacitor materials possess rich Faradaic redox reactions active sites near the surface area of electrode, giving them excellent energy storage properties. Typical electrodes for pseudocapacitors are RuO2 and MnO2, but their usages are hindered because of their environmental harmfulness and low conductivity [6]. Recently, transition metal based materials, including oxides [6, 7], sulfides [8, 9] and hydroxides [10, 11] have become advanced pseudo-capacitor electrode materials. Because of their abundant oxidation states and multiple morphologies and structures, it’s favorable to facilitate the fast and reversible Faradaic redox reactions, thus resulting in high theoretical capacities. Particularly, NiMoO4 belonging to the binary oxide family is a good candidate because of its morphology diversity and outstanding capacitive efficiency [12]. Normally, NiMoO4 possesses two stable structures octahedral or tetrahedral configuration at low or high temperature, respectively [13]. Also, NiMoO4 is an unexpensive, plentiful, environmentally benign commodity and has significant nickel atomic capacity and high conductivity (10–6 S cm−1) from the molybdenum atom [14, 15]. For instance, Yun’s group published that gravel-like NiMoO4 grown on carbon cloth produced 970 F g−1 at 2.5 A g−1 [16]. Zhang, et al. synthesized hierarchical carbon sphere@NiMoO4 which displayed good specific capacity of 268.8 F g−1 at 1.0 A g−1 [17]. Interestingly, Wang’s group designed NiMoO4 with two morphology (nanospheres and nanorods), both of them showed the excellent specific capacitance, indicating NiMoO4 could be a promising candidate for supercapacitor [18]. Frustratingly, because of delayed kinetics reaction and intolerant morphology collapse during long charge/discharge procedure, NiMoO4 impedes low-rate capacity and poor cycling stability [19]. Even, it is still a big challenge to synthesize a NiMoO4 nanomaterials with large specific area and high electrical conductivity. So that not many reports are related to NiMoO4 electrode in application of supercapacitors. Consequently, structural modifications and constructions of NiMoO4 related to capacity and stability are of considerable importance to obtain advanced binary hybrids.
By contrast with metal oxides, transition metal sulfides (TMS) have higher conductivity, chemical durability or redox kinetics and are regarded as other potential pseudo-capacitance candidates [20]. Unlike oxygen, sulfur possesses comparatively low electronegativity, favoring for developing different nanostructures between metal ions and other species [5]. In particular, nickel sulfides have abundant redox active sites, forceful reducibility and inexpensive advantage exhibiting the excellent property for supercapacitor. For instance, Zhang’s group synthesized 3D flower-like nickel sulfide spheres with various morphologies exhibited initial discharge capacity of 550 mA h g−1 [21]. Osquian et al. reported three-dimensional starfish-like Ni3S4-NiS grown on graphene oxide with specific capacity of 1578 F g−1 at 0.5 A g−1 [22]. However, the cycle stability and rate performance of nickel sulfides still fail to reach the expectation. Wang’s group announced the provision of high capacitance three-dimensional Ni3S4 nanosheet electrodes but retained only around 60% capability after 2000 cycles [23]. However, another type of molybdenum disulfides (MoS2) displays superior cycling stability and pseudo-capacitance skills because of graphite-type layer structure and numerous valence states of Mo atoms, which can store charge between the different layers [24, 25]. In addition, atomic layers of MoS2 form a sandwich structure which molybdenum layer between two sulfur atomic layers bound together by Van der Waals forces favoring for ion intercalation and electron transfer [26]. Qi’s group synthesized hierarchical MoS2 nanospheres exhibited a specific capacitance of 142 F g−1at 0.59 A g−1 [27]. Kim et al. reported MoS2 spheres displayed capacitance retention of about 93.8% after 1000 cycles [28]. Unfortunately, chemically formulated MoS2 nanosheets are likely to aggregate, reducing surface area and leading to poor conductive ability. Hence, designing and developing of hetero-structured materials are necessary to enhance the conductivity, shortening the electron/ion transfer pathways and permit for high-rate capability.
One approach is to build hierarchical structures from multiple crystalline species that can effectively alternate electron configuration so that exhibit the superior electrochemical activity due to the synergistic effect of the heterostructure. A typical type of hierarchical structures is external structures which different porous structures such as nanowires, nanoparticles and nanosheets grown on the surface of the backbones such as nanorods or nanofibers owning higher specific surface current density and conductivity [29]. For example, hierarchical core–shell with strong interaction between the shell and core species greatly enhance the chemical and physical properties and offers the various interlayer [30]. Up to now, many contributions have been devoted in designing of the external hierarchical structures, consisting of metal sulfides with metal oxides. For instance, Jae Cheo’s group synthesized core–shell structural Co3O4@CdS showed 390 Fg−1 in symmetric supercapacitor device [31]. Tang et al. reported homogeneous core/shell NiMoO4-based nanowire arrayed on nickel foam showed amazing achievements with 47.2 Wh kg−1 at high energy density [32]. Hierarchical core–shell CoMoO4@NiMoO4 grown on nickel foam was synthesized by Zhang’s group, which showed a high capacity and excellent stability [33]. Qi et al. announced MnCo2S4@CoNi LDH core–shell heterostructures with high electrical conductivity and ample active sties offering better faradaic reactions as electrode for supercapacitor [34]. Therefore, construction of core–shell architecture is proved to be an effective strategy to utilize the merits of multiple components to the maximum to realize high-performance supercapacitors.
Herein, we develop a core–shell hierarchical architecture that core NiMoO4 nanorod is wrapped with Ni9S8/MoS2 nanoflakes, defined as NiMoO4@Ni9S8/MoS2, which is converted from the Mo/Ni precursor after sulfurization. This configuration offers mechanical protection and serves as the bridge connecting the outer metal sulfides and inner core. Due to the synergistic effect of its special multi-interfacial structure, fast ion/mass transportation in and ample active sites within 2D nanoflakes and 1D inner NiMoO4 nanorods, NiMoO4@Ni9S8/MoS2 nanorods demonstrate the excellent specific capacity of 373.4 F g−1 at 10 A g−1 and superior cycling property, implying that NiMoO4@Ni9S8/MoS2 can be a propitious electrode for high-efficiency energy storage system.
Results and discussion
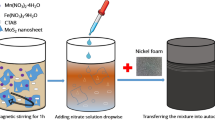
As illustrated in Fig. 1, the core–shell NiMoO4@Ni9S8/MoS2 heterostructure was converted from Mo/Ni precursor by a simple sulfurization method, as demonstrated in detail in the Materials and Method section. From the scanning electron microscope (SEM) image in Fig. 2a, it could be observed that Mo/Ni precursor possessed nanorod-like morphology with smooth exterior and a diameter of 130 nm. After the sulfurization method, the shell of Mo/Ni precursor converted to metal sulfides (Ni9S8/MoS2) with ultrathin nanoflakes shelling the inner core NiMoO4 to form a core–shell structure (Fig. 2b). By comparison, NiMoO4 nanorods without sulfurization remained the morphology with the smooth surface even some of the nanorods were broken, confirming that sulfurization could protect the nanorods from collapsing (Fig. S1). Figure 2c showed the clear structure and morphology of nanorods with a typical diameter of 180–200 nm with ultrathin nanoflakes and generated a hierarchical porous structure which further confirmed the formation of Ni9S8/MoS2. Such architecture was favorable for charge transfer and ion transportation to improve the electrochemical performance [35]. The detailed morphology of nanorods was discovered by transmission electron microscopy, which revealed that core–shell construction consisted of 2D Ni9S8/MoS2 nanoflakes as outer shell part and 1D nickel molybdate nanorods inner core (Fig. 2d). Coherent heterointerfaces could be observed in the entire nanorod between the outside metal sulfides and the inner metal oxide core, which could form the porous structure providing ample charge transfer channels.
The high-resolution TEM images of NiMoO4@Ni9S8/MoS2 in Fig. 3a-c which were selected from the upper (blue square) and lower part (orange square) from single nanorod. Figure 3b displays the crystalline spacings of 0.22 nm, 0.36 nm, and 0.27 nm, corresponding to (− 411) crystal lattice of NiMoO4, (022) crystal lattice of Ni9S8 and (101) lattice distance of MoS2, respectively, demonstrating ternary hybrids [36, 37] while Fig. 3c demonstrates the existence of abundant interfaces between these components [38]. The uniformed distribution of Mo, Ni, S and O is shown in Fig. 3d-e, confirming the binary compound of Ni9S8/MoS2. It's worth mentioning that most Mo and O elements mainly dispersed in the core center, indicating that the incorporation of NiMoO4 inside the composite. XRD was investigated to depict the crystalline shapes of NiMoO4@Ni9S8/MoS2, as presented in Fig. 3f. The characteristic peaks at 23.9°, 28.8°, 29.7°, 41.2° and 47.4° confirmed the formation of NiMoO4 (JCPDS No. 33-0948) [39]. The five sharp peaks located at 27.2°, 31.3°, 42.6°, 50.8° and 53.4° were attributed to (202), (222), (332), (153) and (261) planes of Ni9S8 (JCPDS No. 22-1193) [40]. The typical peaks placed at 33.5°, 35.8°, 44.1°, 49.7°, 55.9° and 58.3° corresponded to the (101), (102), (006), (105), (106) and (110) planes of MoS2 (JCPDS No. 37-1492), respectively, reinforcing formation of ternary composites [41]. However, in Fig. S2a, Mo/Ni without sulfurization formed the nickel molybdate displayed as XRD pattern of NiMoO4. Figure 3g represents N2 sorption isotherms of NiMoO4@Ni9S8/MoS2 with VI-type curve of a hysteresis loop. The NiMoO4@Ni9S8/MoS2 surface area was 27.9643 m2 g−1, above the NiMoO4 (20.5 m2 g−1). Pore distribution profile in Fig. 3g and Fig. S2b demonstrated main product possessed pores at 0.6 nm, 3 nm and 10 nm, confirming the existence of micropores from the Ni9S8/MoS2 nanoflakes and mesopores from inner core. This porous structure of the main product could result from the introduction of sulfur during the calcination process under the high temperature, further creating more active sites and fastening electron transportation during the electrochemical procedure. The above result confirmed that the formation of metal sulfides and nickel molybdate with abundant pores that such porous structure could create more active sites and offer more charge transfer channels, shortening the ion diffusion distance, favoring electrochemical process.
(a) TEM image of a single NiMoO4@Ni9S8/MoS2 nanorod; HRTEM images of (b) upper part (blue square) and (c) lower part (orange square) of NiMoO4@Ni9S8/MoS2 nanorod; (d) HAADF-STEM image of the selected part of NiMoO4@Ni9S8/MoS2 nanorod; and (e) the EDS element mapping spectra of element Mo, Ni, S and O; (f) XRD pattern and Rietveld refinement of NiMoO4@Ni9S8/MoS2; (g) N2 sorption isotherms of NiMoO4@Ni9S8/MoS2 (inset: pore size distribution profile).
The element state analysis of the electrode was conducted through X-ray spectroscopy. In Fig. S3, XPS survey spectra revealed co-occur of Ni, Mo, S, and O elements in the nanorods. The Mo 3d XPS spectrum at the core stage could be divided into six peaks. (Fig. 4a). The two signals at 235.5 and 232.2 eV were accredited to Mo 3d3/2 and 3d5/2 of nickel molybdate, and signals at 232.6 and 228.7 eV corresponded to Mo 3d3/2 and 3d5/2 of molybdenum disulfide, respectively [42, 43]. The other two signals at binding energy 226.5 and 225.6 eV also agreed to S 2 s orbital of metal sulfide bond (Mo-S and Ni-S), respectively [44]. Figure 4b shows the Ni 2p spectra of two spin–orbit doublets with its satellites. Distinct signals placed at binding energy 855.9 and 873.7 eV were credited to 2p3/2 and 2p1/2 of nickel ion (II), and the other two peaks at 856.4 and 875.3 eV were associated with nickel ion (III) (2p3/2 and 2p1/2), respectively [45]. As presented of S 2p spectrum in Fig. 3c, the binding energy signals at 163.6, 161.5 eV were allocated with the 2p1/2 and 2p3/2 of nickel-sulfur bonding, two signals at 163.5 and 161.6 eV were assigned to the molybdenum-sulfur bonding (2p1/2 and 2p3/2), respectively [42, 46]. Also, a typical peak of the sulfur-oxygen bond with oxidation state appeared at signal 168.4 eV [47]. O1s spectrum is displayed as Fig. 4d, the signals at 530.6 and 531.9 eV corresponded to metal–oxygen (M–O) bond in nickel molybdate and oxygen vacancy of composites, respectively [19].
The electrochemical outputs of all electrodes were delved by three-electrode configuration in alkaline solution. Figure 5a indicates CV curves of NiMoO4@Ni9S8/MoS2 from 5 to 200 mV s−1 in a voltage window of − 0.3 to 0.6 V (vs. SCE), respectively. Obviously, two redox summits were shown in a voltage window of 0.2–0.4 V and − 0.1 to 0 V, implying the typical valence state change of Ni2+/Ni3+ and faradaic redox between Mo4+ and Mo6+ during the electrochemical process [42]. Compared to the CV curves of NiMoO4 (Fig. S4a), the CV curves of the main product remained even at high 200 mV s−1, showing outstanding durability of NiMoO4@Ni9S8/MoS2. In addition, the sharp peaks slightly changed to a wide voltage window as the enlarging scan rate indicated fast faradaic reactions. It’s ascribed to the strong synergistic effect between Ni9S8/MoS2 and NiMoO4 due to the intimate hetero-interaction between them. It further verified the synthesis of metal sulfides hybrids and their typical pseudocapacitive behavior [24]. In Fig. 5b, CV plots of the main product and compared materials were illustrated to validate the merits of NiMoO4@Ni9S8/MoS2 nanorods for superior electrodes. Compared with NiMoO4 electrodes, the NiMoO4@Ni9S8/MoS2 nanorods had higher current densities and larger area, demonstrating a significant enhancement of capacitance due to multiple electron transport channels and synergistic effect within the metal sulfides and nickel molybdate [48]. Furthermore, the current density of the typical peak of NiMoO4@Ni9S8/MoS2 displayed a more linear and steep response than NiMoO4, suggesting larger surface area from the BET result, higher electrical conductivities, more active sites, and faster ion exchange due to the porous core–shell shape. Figure 5c displays the GCD plots of NiMoO4@Ni9S8/MoS2 nanorod with current densities ranging from 1 to 20 A g−1. Compared with NiMoO4 (Fig. S4b), NiMoO4@Ni9S8/MoS2 nanorod displayed a longer charge/discharge time of 446 s at 2 A g−1. The corresponding charge–discharge profiles of NiMoO4@Ni9S8/MoS2 and NiMoO4 were further provided in Fig. S4c and Fig. S4d. The obvious voltage plateau of NiMoO4@Ni9S8/MoS2 confirmed the “battery-type” pseudocapacitive behavior in comparison of NiMoO4 [49]. It’s reported that the bimetal precursor after sulfurization produced hierarchical Ni9S8/MoS2 nanoflakes with expanded interlayer spacing, which benefited to ion diffusion with enhanced capacitance [50]. The nonlinear shape of GCD plots further depicted the reversible pseudocapacitive properties which were mainly derived from the chemical conversion in NiMoO4, Ni9S8 and MoS2, which can be represented by the following equations [51, 52]:
Because of the sluggish potential reduction resulted from interior resistance and the faradaic chemical reaction, the discharge time decreased significantly as the current density increased [53]. More apparent results are presented in Fig. 5d. At 10 A g−1, the total charge/discharge duration of NiMoO4@Ni9S8/MoS2 composite was much superior to NiMoO4. Such excellent performance could be explained by more than one mechanism. First, the core–shell structure offered plentiful electron transportation pathways and functioned as a bridge connected the outer metal sulfides shell and inner NiMoO4 core, provided mechanical assistance to prevent the structure from collapsing and agglomerating during the faradic reaction, resulting in increased electrical conductivity. Second, the Ni9S8/MoS2 nanoflakes with abundant micropores and inner core NiMoO4 with mesopores possessed higher surface area and the heterogeneous increased electroactive sites for redox reaction enhanced the conductivity and prompted mass transport. Third, the synergistic effect between Ni9S8 and MoS2 contributed to high capacitance in the energy storage procedure.
Figure 6a exhibits the plot of log with peak current and log with scan speed at 5–200 mV·s−1 based on the CV plots of NiMoO4@Ni9S8/MoS2. Depending on the power-law [54]:
(a) Linear fitting plots of NiMoO4@Ni9S8/MoS2 for the log (peak current) and log (scan rate) for the cathodic and anodic peaks based on CV plots; (b) Specific capacitance of NiMoO4@Ni9S8/MoS2 and NiMoO4 at different current densities; (c) Cycling property and coulombic efficiency of NiMoO4@Ni9S8/MoS2 and NiMoO4 at a current density of 10 A g−1; (d) Comparison of Nyquist plots of NiMoO4@Ni9S8/MoS2 and NiMoO4.
where α was coefficient, i represented the maximum current, υ represented the scan rate. And β was the slope of the log (i) and log(v) plots. Accordingly, β = 0.5 indicated a diffusion-controlled process; β = 1 implied that the reaction was surface-controlled or had a capacitor-like feature [55]. Figure 6a shows that the value of β was close to 0.55, indicating that the redox process kinetics were determined by ion diffusion, further verifying the faradaic reaction in the NiMoO4@Ni9S8/MoS2 electrode. The special capacitances with different current densities depending on the GCD plot are presented in Fig. 6b. The specific capacitances of NiMoO4@Ni9S8/MoS2 electrode were 488.9, 467.6, 373.4, 240.1, 92.0, and 52.9 F g−1 at 1,2, 5, 10, 15, and 20 A g−1, respectively. By comparing with values of NiMoO4, it exhibited the superior rate performance. The cycling property of two electrodes is exhibited in Fig. 6c at 10 A g−1. After 10,000 cycles, the capacity retentiveness of the main product was 81.0% and coulombic efficiency remained almost at 100%, which is much better than NiMoO4 (40.1%) and other electrodes in Table S1, indicating its excellent cycling stability. The main reason of capacitance attenuation might be the difficulty of ions/electrons reaching to the thick area of material under 10 A g−1. However, the outstanding performance of NiMoO4@Ni9S8/MoS2 nanorods was ascribed to the core–shell structure which the outer metal sulfide shell protected the structure from collapsing and limiting fast electron transfer speed leading to the electrode decomposition, further confirming the merits of this architecture [13]. Figure 6d shows the typical EIS spectra of two electrodes before cycling. Obviously, the charge transfer resistance of the main product (0.7 Ω) was much smaller than the compared sample (2.41 Ω), which is correlated with electron transfer kinetics of redox reactions near the surface of electrolyte and electrode. According to the previous studies, such low charge transfer resistance was ascribed to plentiful electrochemical active sites and large cavities of micropores of the binary Ni9S8/MoS2 shell and interplayed with mass transport during the charge transport process [56]. It should also be mentioned that due to the lower electronegativity of sulfur than oxygen, bimetallic sulfides created a flexible space preventing the decomposition of structure caused by the elongation between atoms and made it easy for electrons to transport in the structure [57]. Consequently, after cycling, the resistance increasement of NiMoO4@Ni9S8/MoS2 from 0.7 Ω to 1.23 Ω (Fig. S4e). By comparison, NiMoO4 displayed the larger charge transfer resistance due to the collapsing structure in Fig. S4f. Depending on the above outcomes, the remarkable properties of NiMoO4@Ni9S8/MoS2 nanorods were credited to the subsequent explanations: (i) special core–shell architecture of nanorods protected the structure from collapsing and corrosion, serving as the bridge for outer metal sulfides and inner core leading to the high conductivity and supplied sufficient contact area between electrolyte and electrode; (ii) the ultrathin nanoflakes of Ni9S8/MoS2 provided more active sites and increased surface area and multiple micro/mesopores which provided the massive ion transport channels; (iii) the synergistic effect of typical heterostructure also favored the transport of ion and electrons.
Conclusion
In conclusion, special core–shell NiMoO4@Ni9S8/MoS2 heterostructured was converted from Mo/Ni precursor by a facile hydrothermal process and followed by sulfurization, which worked as an efficient electrode for supercapacitor. It unveiled unsurpassed specific capacity of 373.4 F g−1 at 10 A g−1. Furthermore, the hybrid electrode remained excellent durability at 81.0% after 10,000 charge/discharge cycles. Such excellent electrochemical property was ascribed to special porous core–shell structure, increased surface area and ample active sites. This work demonstrated a facile method to design the hierarchical structure nanomaterials for supercapacitors.
Materials and Methods
Chemicals
Sodium molybdate dihydrate (Na2MoO4·2H2O), Nickel (II) nitrate hexahydrate (Ni(NO3)2·6H2O), Sulfur (S), Potassium hydroxide (KOH) were purchased from Sigma-Aldrich.
Synthesis of Mo/Ni precursor
The conventional hydrothermal approach was used to prepare Mo/Ni precursors, followed by sulfurization. Sodium molybdate dihydrate (0.2 g) and nickel nitrate hexahydrate (0.2 g) were dissolved in deionized water (70 mL). Then the green transparent solution was moved to a 100 mL Telon autoclave and held at 180 °C for 12 h. The green final material was cleaned with deionized water and kept at 50 ℃ overnight, giving rise to Mo/Ni precursor.
Synthesis of nanowire NiMoO4@Ni9S8/MoS2
Mo/Ni precursor and sulfur (mass ratio = 1:3) were put in one porcelain boat with the downstream and upstream side and calcinated at 350℃ for 2 h under flowing Ar. The obtained material was NiMoO4@Ni9S8/MoS2. For comparison, NiMoO4 was also synthesized by calcining Mo/Ni precursor without sulfur in the same calcination process.
Characterization
FEI Quanta 450 Environmental Scanning Electron Microscope was utilized to examine the anatomy of materials. Transmission electron microscopy (TEM, Thermo Scientific Talos F200X G2 S/TEM) was tested to examine the crystal details of the materials. X-ray diffraction (XRD) measurements were used by a monochromatic Bruker D8 advance diffractometer. The composition and valence states were performed on a Thermo Scientific X-ray photoelectron spectroscopy. N2 sorption measurements were utilized using Micromeritics 3Flex Surface Characterization Analyzer with N2 as the adsorbent.
Electrochemical Measurements
All electrocatalytic tests were conducted by Biologic VMP300 electrochemical workstation in 6.0 M KOH with three-electrode equipment including the glassy carbon (GC) electrode (area: 0.196 cm2) as the working electrode, platinum wire as the counter electrode and the saturated calomel electrode (SCE) as the reference electrode. Working electrode was prepared by adding the active nanomaterial and super P in 600 µL of 10% polyvinylidene difluoride (PVDF) dissolved in N-methyl pyrrolidinone (NMP). Then slurry (120 µL) was spread on a clean nickel foam (1 \(\times\) 1 cm2) by drop casting and dried overnight. The dried electrode was then pressed using a hydraulic press at a pressure of 10 MPa. The mass loading of active material in single nickel foam was about 2 mg. The CV and GCD were determined within a possible window of − 0.3 to 0.6 V, and the material's specific capacity was estimated using GCD curves. The specific capacitance, Csc (F g−1), were measured by the equation:
where I (A) was the discharge current, Δt (s) represented the discharge time, ΔV (V) was the potential window, and m (g) was the mass of the active materials.
References
X. Liu, Z. Ye, Adv. Energy Mater. 11, 2003281 (2020)
K. Jayaramulu, D.P. Dubal, B. Nagar, V. Ranc, O. Tomanec, M. Petr, K.K.R. Datta, R. Zboril, P. Gomez-Romero, R.A. Fischer, Adv. Mater. 30, e1705789 (2018)
N. Wang, Q. Pan, X. Yang, H. Zhu, G. Ding, Z. Jia, Y. Wu, L. Zhao, ACS Appl. Nano Mater. 2, 4910–4920 (2019)
R. Yang, X. Guo, K. Song, X. Bai, L. Jia, X. Chen, X. Wang, J. Wang, Ceram. Int. 47, 11349–11357 (2021)
S. Guan, X. Fu, Z. Lao, C. Jin, Z. Peng, ACS Sustain. Chem. Eng. 7, 11672–11681 (2019)
K. Allado, M. Liu, A. Jayapalan, D. Arvapalli, K. Nowlin, J. Wei, Energy Fuels 35, 8396–8405 (2021)
S. Xiong, S. Jiang, J. Wang, H. Lin, M. Lin, S. Weng, S. Liu, Y. Jiao, Y. Xu, J. Chen, Electrochim. Acta 340, 135956 (2020)
L. Yue, X. Wang, T. Sun, H. Liu, Q. Li, N. Wu, H. Guo, W. Yang, Chem. Eng. J. 375, 121959 (2019)
Z. Dai, X. Zang, J. Yang, C. Sun, W. Si, W. Huang, X. Dong, ACS Appl. Mater. Interfaces 7, 25396–25401 (2015)
D. Zhang, X. Guo, X. Tong, Y. Chen, M. Duan, J. Shi, C. Jiang, L. Hu, Q. Kong, J. Zhang, J. Alloys Compd. 837, 155529 (2020)
A. Elsonbaty, A.M. Elshaer, M. Harb, M. Soliman, S. Ebrahim, A. Eltahan, Electrochim. Acta 368, 137577 (2021)
N.S. Neeraj, B. Mordina, A.K. Srivastava, K. Mukhopadhyay, N.E. Prasad, Appl. Surf. Sci. 473, 807–819 (2019)
K. Seevakan, A. Manikandan, P. Devendran, A. Shameem, T. Alagesan, Ceram. Int. 44, 13879–13887 (2018)
D. Cai, D. Wang, B. Liu, L. Wang, Y. Liu, H. Li, Y. Wang, Q. Li, T. Wang, ACS Appl. Mater. Interfaces 6, 5050–5055 (2014)
R. Xu, L. Du, D. Adekoya, G. Zhang, S. Zhang, S. Sun, Y. Lei, Adv. Energy Mater. 11, 2001537 (2020)
S. Hussain, M.S. Javed, S. Asim, A. Shaheen, A.J. Khan, Y. Abbas, N. Ullah, A. Iqbal, M. Wang, G. Qiao, S. Yun, Ceram. Int. 46, 6406–6412 (2020)
C. Wei, Y. Huang, J. Yan, X. Chen, X. Zhang, Ceram. Int. 42, 15694–15700 (2016)
D. Cai, D. Wang, B. Liu, Y. Wang, Y. Liu, L. Wang, H. Li, H. Huang, Q. Li, T. Wang, ACS Appl. Mater. Interfaces 5, 12905–12910 (2013)
D. Chen, M. Lu, L. Li, D. Cai, J. Li, J. Cao, W. Han, J. Mater. Chem. A 7, 21759–21765 (2019)
B. Jiang, X. Ban, Q. Wang, K. Cheng, K. Zhu, K. Ye, G. Wang, D. Cao, J. Yan, J. Mater. Chem. A 7, 24374–24388 (2019)
L. Mi, Q. Ding, W. Chen, L. Zhao, H. Hou, C. Liu, C. Shen, Z. Zheng, Dalton Trans. 42, 5724–5730 (2013)
S. Azizi Darsara, M. Seifi, M.B. Askari, M. Osquian, Ceram. Int. 47, 20992–20998 (2021)
L. Wang, J. Liu, L.L. Zhang, B. Dai, M. Xu, M. Ji, X.S. Zhao, C. Cao, J. Zhang, H. Zhu, RSC Adv. 5, 8422–8426 (2015)
J. Yan, S. Wang, Y. Chen, M. Yuan, Y. Huang, J. Lian, J. Qiu, J. Bao, M. Xie, H. Xu, H. Li, Y. Zhao, J. Alloys Compd. 811, 151915 (2019)
W. Wu, L. Wang, Y. Li, F. Zhang, L. Lin, S. Niu, D. Chenet, X. Zhang, Y. Hao, T.F. Heinz, J. Hone, Z.L. Wang, Nature 514, 470–474 (2014)
L. Huang, H. Hou, B. Liu, K. Zeinu, X. Zhu, X. Yuan, X. He, L. Wu, J. Hu, J. Yang, Appl. Surf. Sci. 425, 879–888 (2017)
L. Wang, Y. Ma, M. Yang, Y. Qi, Electrochim. Acta 186, 391–396 (2015)
K. Krishnamoorthy, G.K. Veerasubramani, S. Radhakrishnan, S.J. Kim, Mater. Res. Bull. 50, 499–502 (2014)
Q. Liu, X. Hong, X. You, X. Zhang, X. Zhao, X. Chen, M. Ye, X. Liu, Energy Storage Mater. 24, 541–549 (2020)
X. Zhang, H. Liang, H. Li, Y. Xia, X. Zhu, L. Peng, W. Zhang, L. Liu, T. Zhao, C. Wang, Z. Zhao, C.T. Hung, M.M. Zagho, A.A. Elzatahry, W. Li, D. Zhao, Angew. Chem. Int. Ed. Engl. 59, 3287–3293 (2020)
D.S. Patil, S.A. Pawar, J.C. Shin, Chem. Eng. J. 335, 693–702 (2018)
J.Y. Dong, J.C. Xu, K.N. Hui, Y. Yang, S.C. Su, L. Li, X.T. Zhang, K.W. Ng, S.P. Wang, Z.K. Tang, Nanomaterials 9, 1033 (2019)
Z. Zhang, H. Zhang, X. Zhang, D. Yu, Y. Ji, Q. Sun, Y. Wang, X. Liu, Facile synthesis of hierarchical CoMoO4@NiMoO4 core-shell nanosheet arrays on nickel foam as an advanced electrode for asymmetric supercapacitors. J. Mater. Chem. A 4, 18578–18584 (2016)
H. Liang, T. Lin, S. Wang, H. Jia, C. Li, J. Cao, J. Feng, W. Fei, J. Qi, Dalton Trans. 49, 196–202 (2020)
S. Li, T. Chen, J. Wen, P. Gui, G. Fang, Nanotechnology 28, 445407 (2017)
X. Luo, Q. Zhou, S. Du, J. Li, J. Zhong, X. Deng, Y. Liu, ACS Appl. Mater. Interfaces 10, 22291–22302 (2018)
H. Li, X. Qian, C. Xu, S. Huang, C. Zhu, X. Jiang, L. Shao, L. Hou, ACS Appl. Mater. Interfaces 9, 28394–28405 (2017)
S. Chen, M. Zhang, G. Jiang, Z. Zhang, X. Zhou, J. Alloys Compd. 814, 152253 (2020)
J. Hong, Y.W. Lee, B. Hou, W. Ko, J. Lee, S. Pak, J. Hong, S.M. Morris, S. Cha, J.I. Sohn, J.M. Kim, ACS Appl. Mater. Interfaces 8, 35227–35234 (2016)
A. Khalil, Q. Liu, Z. Muhammad, M. Habib, R. Khan, Q. He, Q. Fang, H.T. Masood, Z.U. Rehman, T. Xiang, C.Q. Wu, L. Song, Langmuir 33, 5148–5153 (2017)
Q. Qin, L. Chen, T. Wei, X. Liu, Small 15, e1803639 (2019)
X. Yang, J. Mao, H. Niu, Q. Wang, K. Zhu, K. Ye, G. Wang, D. Cao, J. Yan, Chem. Eng. J. 406, 126713 (2021)
B. Yu, G. Jiang, W. Xu, C. Cao, Y. Liu, N. Lei, U. Evariste, P. Ma, J. Alloys Compd. 799, 415–424 (2019)
S. Guan, X. Fu, Z. Lao, C. Jin, Z. Peng, Sustain. Energy Fuels 3, 2056–2066 (2019)
X. Yang, L. Zhao, J. Lian, J. Power Sources 343, 373–382 (2017)
Z. Ma, H. Meng, M. Wang, B. Tang, J. Li, X. Wang, ChemElectroChem 5, 335–342 (2018)
S. Song, Y. Wang, W. Li, P. Tian, S. Zhou, H. Gao, X. Tian, J. Zang, Electrochim. Acta 332, 135454 (2020)
Y. Li, L. Cao, L. Qiao, M. Zhou, Y. Yang, P. Xiao, Y. Zhang, J. Mater. Chem. A 2, 6540–6548 (2014)
L. Yu, G.Z. Chen, Electron. Energy Rev. 3, 271–285 (2020)
D. Sarkar, D. Das, S. Das, A. Kumar, S. Patil, K.K. Nanda, D.D. Sarma, A. Shukla, ACS Energy Lett. 4, 1602–1609 (2019)
Y. Ruan, J. Jiang, H. Wan, X. Ji, L. Miao, L. Peng, B. Zhang, L. Lv, J. Liu, J. Power Sources 301, 122–130 (2016)
V.S. Kumbhar, V.Q. Nguyen, Y.R. Lee, C.D. Lokhande, D.H. Kim, J.J. Shim, New J. Chem. 42, 14805–14816 (2018)
H. Pang, C. Wei, X. Li, G. Li, Y. Ma, S. Li, J. Chen, J. Zhang, Sci. Rep. 4, 3577 (2014)
S.S. Karade, S. Lalwani, J.H. Eum, H. Kim, J. Electroanal. Chem. 864, 114080 (2020)
N.R. Chodankar, I.V. Bagal, S.W. Ryu, D.H. Kim, Chem. Eng. J. 362, 609–618 (2019)
R. Seeber, C. Zanardi, G. Inzelt, ChemTexts (2016). https://doi.org/10.1007/s40828-016-0027-3
P.E. Lokhande, U.S. Chavan, A. Pandey, Electron. Energy Rev. 3, 155–186 (2019)
Acknowledgments
This work was financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), through the Discovery Grant Program (RGPIN-2018-06725) and the Discovery Accelerator Supplement Grant program (RGPAS-2018-522651), and by the New Frontiers in Research Fund-Exploration program (NFRFE-2019-00488). Lu Chen, Wenjing Deng, Prof. Zhi Chen and Prof. Xiaolei Wang also acknowledge support from Concordia University, the University of Alberta, and the Future Energy Systems (FES).
Funding
Natural Sciences and Engineering Research Council of Canada (NSERC); Discovery Grant Program (RGPIN-2018–06725) (Recipient: Xiaolei Wang); Discovery Accelerator Supplement Grant program (RGPAS-2018–522651) (Recipient: Xiaolei Wang); New Frontiers in Research Fund (NFRF); Exploration program (NFRFE-2019–00488) (Recipient: Xiaolei Wang).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Availability of Data and Material
Data will be made available upon reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, L., Deng, W., Chen, Z. et al. Hetero-architectured core–shell NiMoO4@Ni9S8/MoS2 nanorods enabling high-performance supercapacitors. Journal of Materials Research 37, 284–293 (2022). https://doi.org/10.1557/s43578-021-00318-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-021-00318-y