Abstract
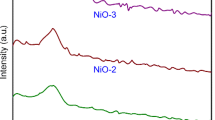
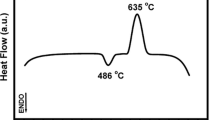
A borate glass, phosphate glass, and silicate glass were converted to hydroxyapatite (HA) by soaking the substrates in a solution of K2HPO4 with a pH value of 9.0 at 37 °C. The weight loss of the substrates was studied as a function of time. Unlike the silicate glasses, the reaction processes of the borate glasses and phosphate glasses were bulk dissolution. X-ray diffraction and scanning electron microscopy revealed an initially amorphous product that subsequently crystallized to HA. The data suggest good bioactive characteristics for the borate and phosphate glass and the potential use of them as a favorable template for bone-tissue formation.
Similar content being viewed by others
References
K. Tadashi, K. Hyun-Min and K. Masakazu: Novel bioactive materials with different mechanical properties. Biomater. 24, 2161 (2003).
E.N. Özgür and T.A. Cüneyt: Preparation of porous Ca10(PO4)6(OH)2 and β–Ca3(PO4)2. J. Am. Ceram. Soc. 83, 1581 (2000).
H.K.X. Hockin, B.Q. Janet, T. Shozo and C.C. Laurence: Synergistic reinforcement of in situ hardening calcium phosphate composite scaffold for bone tissue engineering. Biomaterials 25, 1029 (2004).
S. Lossdörfer, Z. Schwartz, C.H. Lohmann, D.C. Greenspan, D.M. Ranly and B.D. Boyan: Osteoblast response to bioactive glasses in vitro correlates with inorganic phosphate content. Biomaterials 25, 2547 (2004).
H. Ylanen and K.H. Karlsson: Effect of immersion in SBF on porous bioactive bodies made by sintering bioactive glass microspheres. J. Non-Cryst. Solids 275, 107 (2000).
N. Olmo, A.I. Martín, A.J. Salinas, J. Turnay, M. Vallet-Regí and M.A. Lizarbe: Bioactive sol-gel glasses with and without a hydroxycarbonate apatite layer as substrates for osteoblast cell adhesion and proliferation. Biomaterials 24, 3383 (2003).
H. Oonishi, L.L. Hench, J. Wilson, F. Sugihara, E. Tsuji and M. Matsuura: Quantitative comparison of bone growth behavior in granules of Bioglass, A-W glass-ceramic, and hydroxyapatite. J. Biomed. Mater. Res. 51, 37 (2000).
M. Brink, T. Turunen, R.P. Happonen and Y.U. Antti: Compositional dependence of bioactivity of glasses in the system Na2O–K2O–MgO–CaO–B2O3–P2O5–SiO2. J. Biomed. Mater. Res. 37, 114 (1997).
F. Shunsuke and N. Masashi: A comparative study between in vivo bone ingrowth and in vitro apatite formation on Na2O–CaO–SiO2 glasses. Biomaterials 24, 1349 (2003).
J.E. Gougha, J.R. Jones and L.L. Hench: Nodule formation and mineralisation of human primary osteoblasts cultured on a porous bioactive glass scaffold. Biomaterials 25, 2039 (2004).
J.R. Jones and L.L. Hench: Effect of surfactant concentration and composition on the structure and properties of sol-gel-derived bioactive glass foam scaffolds for tissue engineering. J. Mater. Sci. 38, 3783 (2003).
P. Li, C. Ohtsuki and T. Kokubo: Apatite formation induced by silica gel in a simulated body fluid. J. Am. Ceram. Soc. 75, 2094 (1992).
K.H. Karlsson, K. Froberg and T. Ringbom: A structure approach to bone adhering of bioactive glasses. J. Non-Cryst. Solids 112, 69 (1989).
L.A. Mortin and R.M. Shelton: Primary bone-derived cell colonization of unconditioned and pre-conditioned bioglass 45S5 surface in vitro. J. Mater. Sci.-Mater. Med. 14, 297 (2003).
J.A. Juhasz, S.M. Best, W. Bonfield, M. Kawashita, N. Miyata, T. Kokubo and T. Nakamura: Apatite-forming ability of glass-ceramic apatite-wollastonite-polythylene composites: Effect of filler content. J. Mater. Sci.-Mater. Med. 14, 489 (2003).
D.C. Clupper, J.E. Gough, P.M. Embanga, I. Notingher, L.L. Hench and M.M. Hall: Bioactive evaluation of 45S5 bioactive glass fibres and preliminary study of human osteoblast attachment. J. Mater. Sci.-Mater. Med. 15, 803 (2004).
I. Ahmed, M. Lewis, I. Olsen and J.C. Knowles: Phosphate glasses for tissue engineering: Part 2. Processing and characterisation of a ternary-based P2O5–CaO–Na2O glass fiber system. Biomaterials 25, 501 (2004).
D.E. Day, J.E. White, R.F. Brown and K.D. McMenamin: Transformation of borate glasses into biologically useful materials. Glass Technol. 44, 75 (2003).
X. Han Reaction of sodium calcium borate glass to form hydroxyapatite and preliminary evolution of hydroxyapatite microspheres used to absorb and separate proteins. Master’s Thesis, University of Missouri-Rolla, Rolla, MO (2002).
M.N. Richard Reaction of a borate glass with K2HPO4 solutions. Master’s Thesis, University of Missouri-Rolla, MO (2000).
F. Delahaye, L. Montagne and G. Palavit: Acid dissolution of sodium calcium metaphosphate glasses. J. Non-Cryst. Solids 242, 25 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Address all correspondence to this author.
Rights and permissions
About this article
Cite this article
Liang, W., Christian, R., Day, D.E. et al. Bioactive comparison of a borate, phosphate and silicate glass. Journal of Materials Research 21, 125–131 (2006). https://doi.org/10.1557/jmr.2006.0025
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2006.0025