Abstract
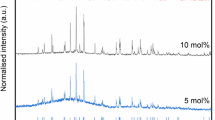
Due to their high actinide content MOX residues require immobilization within a robust host matrix. Although it is possible to immobilize actinides in vitreous wasteforms; ceramic phases, such as brannerite (UTi2O6), are attractive due to their high waste loading capacity and relative insolubility. Brannerites Gd0.1U0.9Ti2O6, Ce0.1U0.9Ti2O6 and Gd0.1U0.81Ce0.09Ti2O6 were prepared using an oxide route. Charge compensation of trivalent cations was expected to occur via the oxidation of U (IV) to higher valence states (U (V) or U (VI)). Gd was added to act as a neutron absorber in the final Pu bearing wasteform and Ce was used as a structural surrogate for Pu. X-ray absorption spectroscopy showed that Ce (IV) was reduced to Ce (III) in all cases. X-ray powder diffraction of synthesized specimens found that the final phase assemblage was strongly affected by processing atmosphere (air or argon). Prototypical brannerite was formed in all compositions, secondary phases observed were found to vary according to processing atmosphere and stoichiometry. Microstructural analysis (SEM) of the sintered samples confirmed the results of the X-ray powder diffraction.
Similar content being viewed by others
References
N. C. Hyatt, “Plutonium management policy in the United Kingdom: The need for a dual track strategy,” Energy Policy, 99, (In press, 2016).
P. D. Wilson, The Nuclear Fuel Cycle: From Ore to Waste, 1st ed (Oxford University Press, Oxford, 1996).
J. T. Szymanski and J. D. Scott, “A Crystal Structure Refinement of Synthetic Brannerite, UTi2O6 , and Its Bearing on Rate of Alkaline-Carbonate Leaching of Brannerite in Ore,” Can. Mineral., 20, 271–279 (1982).
F. J. Ryerson and B. Ebbinghaus, “Pyrochlore-Rich Titanate Ceramics for the Immobilization of Plutonium : Redox Effects on Phase Equilibria in Cerium- and Thorium- Substituted Analogs,” Lawrence Livermore National Laboratory Report, UCRL-ID-139092 (2000).
Y. Zhang, G. R. Lumpkin, H. Li, M. G. Blackford, M. Colella, M. L. Carter, and E. R. Vance, “Recrystallisation of amorphous natural brannerite through annealing : The effect of radiation damage on the chemical durability of brannerite,” J. Nucl. Mater., 350, 293–300 (2006).
F. A. Charalambous, R. Ram, M. I. Pownceby, J. Tardio, and S. K. Bhargava, “Chemical and microstructural characterisation studies on natural and heat treated brannerite samples,” Miner. Eng., 39, 276–288 (2012).
G. R. Lumpkin, “Alpha-decay damage and aqueous durability of actinide host phases in natural systems,” J. Nucl. Mater., 289 (1–2), 136–166 (2001).
M. C. Stennett, C. L. Freeman, A. S. Gandy, and N. C. Hyatt, “Crystal structure and nonstoichiometry of cerium brannerite: Ce0.975Ti 2O5.95,” J. Solid State Chem.,192, 172–178 (2012).
E. R. Vance, J. N. Watson, M. L. Carter, R. A. Day, and B. D. Begg, “Crystal Chemistry and Stabilization in Air of Brannerite, UTi2O6,” vol. 44, pp. 141–144, 2001.
M. James and J. N. Watson, “The Synthesis and Crystal Structure of Doped Uranium Brannerite Phases U1−xMxTi2O6 (M=Ca2+, La3+, and Gd3+),” J. Solid State Chem., 165, 261–265 (2002).
B. Ravel and M. Newville, “ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT,”Journal of Synchrotron Radiation, 12 (4), 537–541 (2005).
J. E. Patchettt and E. W. Nuffield, “Studies of Radioactive Compounds X- The Synthesis and Crystallography of Brannerite,” Can. Mineral., 6, 483–490 (1960).
D. J. Bailey, M. C. Stennett, and N. C. Hyatt, “Synthesis and Characterization of Brannerite Wasteforms for the Immobilization of Mixed Oxide Fuel Residues,” Procedia Chem., 21, 371–377 (2016).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bailey, D.J., Stennett, M.C. & Hyatt, N.C. Synthesis and Characterization of Brannerite Compositions for MOX Residue Disposal. MRS Advances 2, 557–562 (2017). https://doi.org/10.1557/adv.2016.631
Published:
Issue Date:
DOI: https://doi.org/10.1557/adv.2016.631