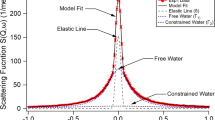
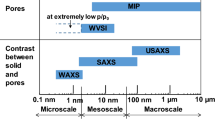
Abstract
The effects of different particle size distributions on the real-time hydration of tricalcium silicate cement paste were studied in situ by quasi-elastic neutron scattering. The changing state of water in the cement system was followed as a function both of cement hydration time and of temperature for different initial particle size distributions. It was found that the length of the initial, dormant, induction period, together with the kinetics of hydration product nucleation and growth, depends on the hydration temperature but not on the particle size distribution. However, initial particle size does affect the total amount of cement hydrated, with finer particle size producing more hydrated cement. Furthermore, the diffusion-limited rate of hydration at later hydration time is largely determined by the initial tricalcium silicate particle size distribution.
Similar content being viewed by others
References
H.L. Le Chatelier: In Experimental Researches on the Constitution of Hydraulic Mortars (McGraw Publishing Co., New York, 1905).
J. Skalny and I. Odler: Effect of heat treatment on the pore structure and drying shrinkage behavior of hydrated cement paste. J. Colloid Interface Sci. 40, 199 (1972).
I. Odler and H. Dorr: Tricalcium silicate formation by solid-state reactions. Am. Ceram. Soc. Bull. 56, 1086 (1977).
A. Bentur, R.L. Berger, J.H. Kung, N.B. Milestone, and J.F. Young: Structural-properties of calcium silicate pastes 2. Effect of curing temperature. J. Am. Ceram. Soc. 62, 362 (1979).
H.F.W. Taylor: In Cement Chemistry (Academic Press, London, U.K., 1990), pp. 153–156.
Cement chemistry notation: C = CaO, S = SiO2, H = H2O. http://www.wordiq.com/definition/cementchemistnotation.
R. Kondo and S. Ueda: Kinetics of Hydration of Cements, in Proc. 5th Int. Symposium on the Chemistry of Cement. Vol. II. (Cement Association of Japan Tokyo, Japan, 1969), p. 203.
J.H. Taplin: On the Hydration Kinetics of Hydraulic Cements, in Proc. 5th Int. Symposium on the Chemistry of Cement. Vol. II. (Cement Association of Japan Tokyo, Japan, 1969), p. 337.
J.M. Pommersheim, J.R. Clifton, and G.J. Frohnsdorff: Mathematical- modeling of tricalcium silicate hydration 2. Hydration sub-models and the effect of model parameters. Cem. Concr. Res. 12, 765 (1982).
J.M. Pommersheim: Effect of particle size distribution on hydration kinetics, in Microstructural Development During Hydration of Cement, edited by L.J. Struble and P.W. Brown (Mater. Res. Soc. Symp. Proc. 85, Pittsburgh, PA, 1987), p. 301.
T. Knudsen: The dispersion model for hydration of portlandcement 1 general concepts. Cem. Concr. Res., 14, 622 (1984).
T. Knudsen and M. Geiker: Obtaining hydration data by measurement of chemical shrinkage with an archimeter. Cem. Concr. Res. 15, 381 (1985).
P.W. Brown, J. Pommersheim, and G. Frohnsdorff: A kineticmodel for the hydration of tricalcium silicate. Cem. Concr. Res. 15, 35 (1985).
D.P. Bentz: Three-dimensional computer simulation of Portland cement hydration and microstructure development. J. Am. Ceram. Soc. 80, 3 (1997).
D.P. Bentz, E.J. Garboczi, C.J. Haecker, and O.M. Jensen: Effects of cement particle size distribution on performance properties of Portland cement-based materials. Cem. Concr. Res. 29, 1663 (1999).
A. Princigallo, P. Lura, K. van Breugel, and G. Levita: Early development of properties in a cement paste: A numerical and experimental study. Cem. Concr. Res. 33, 1013 (2003).
L.M. Parrott, M. Geiker, W.A. Gutteridge, and D. Killoh: Monitoring Portland-cement hydration - comparison of methods. Cem. Concr. Res. 20, 919 (1990).
G. Papavassiliou, M. Fardis, E. Laganas, A. Leventis, A. Hassanien, F. Milia, A. Papageorgiou, and E. Chaniotakis: Role of the surface morphology in cement gel growth dynamics: A combined nuclear magnetic resonance and atomic force microscopy study. J. Appl. Phys. 82, 449 (1997).
J. Greener, H. Peemoeller, C. Choi, R. Holly, E.J. Reardon, C.M. Hansson, and M.M. Pintar: Monitoring of hydration of white cement paste with proton NMR spin-spin relaxation. J. Am. Ceram. Soc. 83, 623 (2000).
A.J. Allen, R.C. Oberthur, D. Pearson, P. Schofield, and C.R. Wilding: Development of the fine porosity and gel structure of hydrating cement systems. Philos. Mag. B 56, 263 (1987).
J.J. Thomas, H.M. Jennings, and A.J. Allen: The surface area of cement paste as measured by neutron scattering: Evidence for two C-S-H morphologies. Cem. Concr. Res. 28, 897 (1998).
J.J. Thomas, H.M. Jennings, and A.J. Allen: The surface area of hardened cement paste as measured by various techniques. Concr. Sci. Eng. 1, 45 (1999).
D.R. Vollet and A.F. Craievich: Effects of temperature and of the addition of accelerating and retarding agents on the kinetics of hydration of tricalcium silicate. J. Phys. Chem. B 104, 12143 (2000).
D.H.C. Harris, C.G. Windsor, and C.D. Lawrence: Free and bound water in cement pastes. Mag. Concr. Res. 26, 65 (1974).
S.A. FitzGerald, D.A. Neumann, J.J. Rush, D.P. Bentz, and R.A. Livingston: In situ quasi-elastic neutron scattering study of the hydration of tricalcium silicate. Chem. Mater. 10, 397 (1998).
R. Berliner, M. Popvici, K.W. Herwig, M. Berliner, H.M. Jennings, and J.J. Thomas: Quasielastic neutron scattering study of the effect of water-to-cement ratio on the hydration kinetics of tricalcium silicate. Cem. Concr. Res. 28, 231 (1998).
E. Fratini, S-H. Chen, P. Baglioni, and M-C. Bellissent-Funel: Dynamic scaling of quasielastic neutron scattering spectra from interfacial water. Phys. Rev. E 64, 020201 (2001).
E. Fratini, A. Faraone, P. Baglioni, M-C. Bellissent-Funel, and S-H. Chen: Dynamic scaling of QENS spectra of glassy water in aging cement paste. Physica A 304, 1 (2002).
E. Fratini, S-H. Chen, P. Baglioni, and M-C. Bellissent-Funel: Quasi-elastic neutron scattering study of translational dynamics of hydration water in tricalcium silicate. J. Phys. Chem. B 106, 158 (2002).
S.A. FitzGerald, D.A. Neumann, J.J. Rush, R.J. Kirkpatrick, X. Cong, and R.A. Livingston: Inelastic neutron scattering study of the hydration of tricalcium silicate. J. Mater. Res. 14, 1160 (1999).
J. J. Thomas, S.A. FitzGerald, D.A. Neumann, and R.A. Livingston: State of water in hydrating tricalcium silicate and portland cement pastes as measured by quasi-elastic neutron scattering. J. Am. Ceram. Soc. 84, 1811 (2001).
S.A. FitzGerald, J.J. Thomas, D.A. Neumann, and R.A. Livingston: A neutron scattering study of the role of diffusion in the hydration of tricalcium silicate. Cem. Concr. Res. 32, 409 (2002).
R. Berliner, M. Popovici, K. Herwig, H.M. Jennings, and J. Thomas: High-resolution neutron scattering with commercial thin silicon wafers as focusing monochromators. Physica B 241, 1237 (1997).
R.A. Livingston: Fractal nucleation and growth model for the hydration of tricalcium silicate. Cem. Concr. Res. 30, 1853 (2000).
J.J. Thomas and H.M. Jennings: Effects of D2O and mixing on the early hydration kinetics of tricalcium silicate. Chem. Mater. 11, 1907 (1999).
A.J. Allen, I.G. Richardson, and G.N. Kearley: (Institute Laue-Langevin: Grenoble, France, 1988) Unpublished results.
J.J. Thomas, J. Chen, H.M. Jennings, and A.J. Allen: Effects of decalcification on the microstructure and surface area of cement and tricalcium silicate pastes. Cem. Concr. Res. (2004, in press).
A.J. Allen, J.J. Thomas, and H.M. Jennings: Composition and density of amorphous calcium–silicate–hydrate gel in cement from combined neutron and x-ray small-angle scattering. J. Am. Ceram. Soc., (2004) (in press).
L.F. Brown: LANL Report LA-UR-01-1005 (Los Alamos, NM, 2001).
A. Jillavenkatesa, S.J. Dapkunas, and L-S.H. Lum: In Particle Size Characterization, NIST Special Publication 960-1 (National Institute of Standards and Technology: Gaithersburg, MD, 2001), p. 93.
W.K. Brown and K.H. Wohletz: Derivation of the Weibull distribution based on physical principles and its connection to the Rosin-Rammler and lognormal distributions. J. Appl. Phys. 78, 2758 (1995).
H.C. Van de Hulst: In Light Scattering by Small Particles (John Wiley and Sons, New York, 1962).
J.R.D. Copley and T.J. Udovic: Neutron time-of-flight spectroscopy. J. Res. Natl. Inst. Stand. Technol. 98, 71 (1993).
J.S. Langer and A.J. Schwartz: Kinetics of nucleation in nearcritical fluids. Phys. Rev. A 21, 948 (1980).
A.J. Allen, D. Gavillet, and J.R. Weertman: SANS and TEM studies of isothermal M2C carbide precipitation in ultrahigh strength AF1410 steels. Acta Metall. Mater. 41, 1869 (1993).
A.J. Allen and R.A. Livingston: The relationship between differences in silica fume additives and the fine scale microstructural evolution in cement-based materials, Adv. Cement-Based Mater. 8, 118 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allen, A.J., McLaughlin, J.C., Neumann, D.A. et al. In situ quasi-elastic scattering characterization of particle size effects on the hydration of tricalcium silicate. Journal of Materials Research 19, 3242–3254 (2004). https://doi.org/10.1557/JMR.2004.0415
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2004.0415