The introduction of lasers for the treatment of vascular atherosclerosis began in the 1980s, initially for the treatment of critical limb ischaemia,1 followed by trials that supported its use in coronary circulation.2–5 However, catheters and technique were rudimentary and associated with complications.6,7 Refinements in catheter technology8 and introduction of safe lasing techniques9,10 have led to improvements in clinical outcomes.11
The aim of this article is to describe the principles and practice of Excimer laser coronary atherectomy (ELCA), illustrating with case examples and relevant clinical data.
Excimer Laser Coronary Atherectomy
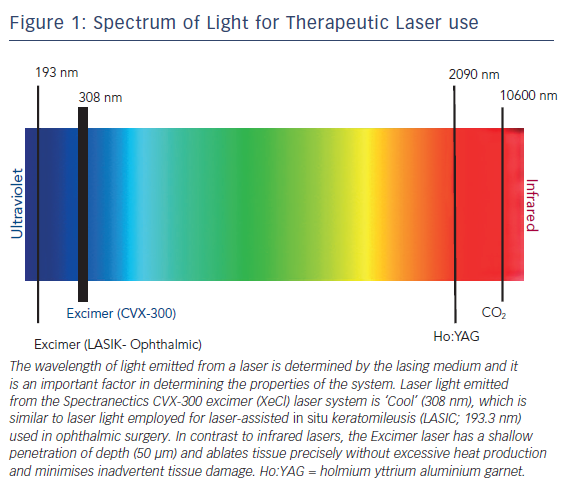
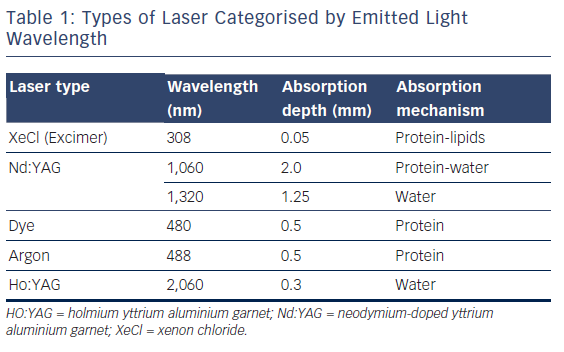
Excimer lasers are pulsed gas lasers that use a mixture of a rare gas and halogen as an active medium to generate pulses of short wavelength, high-energy ultraviolet (UV) light (see Figure 1). The depth of laser penetration is directly related to its wavelength, with UV laser (shorter wavelength) having less depth of penetration, less heat production and less unwanted tissue damage (see Table 1).
Excimer laser tissue ablation is mediated through three distinct mechanisms: photochemical, photo-thermal and photomechanical. UV laser light is absorbed by intra-vascular material and breaks carbon–carbon bonds (photochemical). It elevates the temperature of intra-cellular water, causing cellular rupture and generates a vapour bubble at the catheter tip (photo-thermal). Expansion and implosion of these bubbles disrupts the obstructive intra-vascular material (photomechanical). The fragments released are <10 μm in diameter, avoiding microvascular obstruction as they are absorbed by the reticulo-endothelial system.
The threshold energy required for the penetration of UV light into tissue and the creation of a steam bubble is called ‘fluence’ (range: 30–80 mJ/mm2). The number of pulses emitted during a 1–second period is the ‘pulse repetition rate’. The duration of each pulse is termed a ‘pulse width’, which is modified according to the nature of the treated lesion for example fibro-calcific lesions require higher fluence and repletion rate for effective ablation (see Figure 2).
Excimer Laser Equipment and General Technique
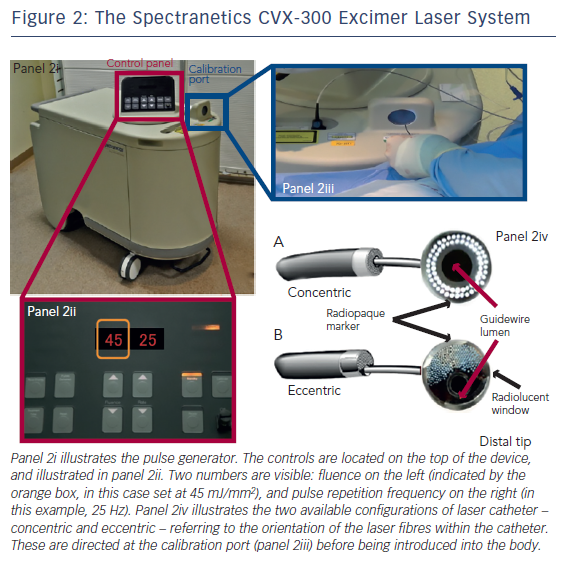
The CVX-300 cardiovascular laser Excimer system (Spectranetics; see Figure 2) uses Xenon chloride (XeCl) as the active medium. The light emitted has a wavelength of 308 nm (in the UVB spectrum) with a tissue penetration depth between 30–0 μm. It is the only coronary laser-emitting device currently approved by the US Food and Drug Administration.
It is essential that safety procedures should be observed when performing laser atherectomy. Prior to activation, all persons in the catheter lab, including the patient, must wear protective tinted spectacles to minimise the risk of retinal exposure to the UV light. All windows should be covered and doors locked. Following this safety checklist, the laser unit is warmed up and the selected catheter is connected and calibrated prior to being introduced into the body. Even when the catheter is in vivo, all staff in the vicinity should wear eye protection in case the catheter housing breaks, which could release UV light.
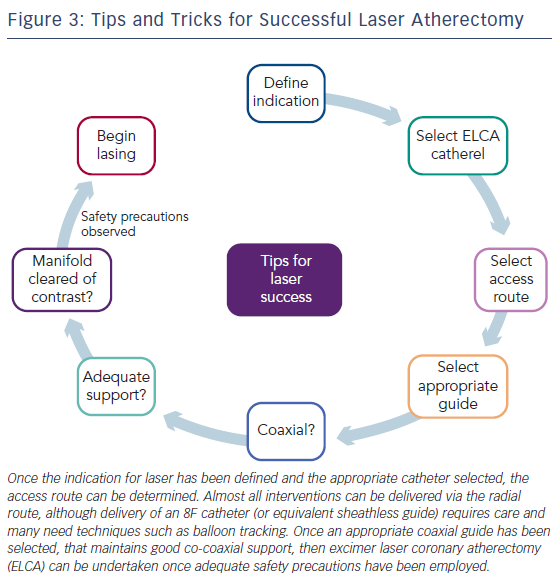
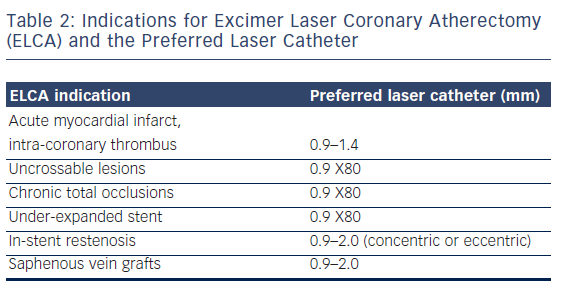
ELCA catheters are advanced on a short monorail segment (30 mm), compatible with any standard 0.014-inch Q2 guidewire. This is a major advantage over alternative coronary atherectomy techniques that require dedicated guidewires that are often more difficult to deliver distally. Coronary catheters are available in four diameters (0.9, 1.4, 1.7, 2.0 mm; see Table 2) and those most commonly used have a concentric array of laser fibres at the tip. The laser fibres of eccentric laser catheters are focused toward one hemisphere. These devices are primarily used for eccentric lesions or for extensive debulking of in-stent restenosis (ISR). The larger diameter laser catheters (1.7, 2.0 mm) are primarily used in straight sections of vessels with a diameter >3.0 mm and require 7F and 8F guide catheters, respectively. The 0.9- and 1.4-mm devices are used via a 6F guiding system. Care should be taken to select a guiding catheter that provides adequate support and that remains coaxial during lasing. Laser catheter size selection is primarily based on: (a) the severity of the lesion; (b) the reference vessel diameter and; (c) consistency of the target material12(see Table 2). The 0.9-mm X80 catheter is used in non-crossable, non-dilatable fibrocalcific lesions, due to its enhanced delivery and ability to emit laser energy at high power (80 mJ/mm2) at the highest repletion rate (80 Hz).
Saline Infusion Technique
Both blood and iodinated contrast media contain non-aqueous cellular macromolecules, such as proteins, and these absorb the majority of delivered Excimer laser energy creating cavitating microbubbles, which form at the site of energy delivery, increasing the likelihood of traumatic dissection.13 By contrast, saline permits passage of light from the catheter tip to the tissue without any interference so no microbubbles are formed in this milieu. Therefore, a saline flush/infusion technique is used to safely control energy delivery and minimise dissection risk.14,15 Application of laser in blood or contrast media is rarely performed in certain specific situations – such as treatment of an underexpanded stent – and should only be undertaken by experienced laser percutaneous coronary intervention (PCI) operators.16
To clear blood from the catheter–tissue interface, a 1-l bag of 0.9 % saline solution is connected to the manifold via a three-way tap, and a clean 20-ml Luer-Lok™ (Becton Dickinson) syringe replaces the contrast syringe. Once the system has been purged of contrast, confirmed by screening, 5 ml of saline solution should be infused followed by continued injection – at a rate of 1–2 ml/second – throughout laser activation. The guide catheter should be well intubated and coaxial within the artery, ensuring saline delivery to the catheter tip. For the standard coronary catheters activation will automatically cease after 5 seconds with a 10-second rest period. An audible alarm sounds at the end of the rest period to signal when to commence the next laser train. The 0.9-mm X80 catheter permits 10 seconds activation and 5 seconds rest, reflecting its use in more complex lesions.
The pulses of laser energy are delivered as the catheter is slowly (0.5 mm/second) advanced through the lesion, allowing adequate absorption and ablation. If the catheter is advanced too rapidly the tissue does not have time to absorb the light energy and ablation will be sub-optimal. On completion of several anterograde trains, retrograde lasing can be performed, particularly in severe lesions when there is anterograde resistance.
Contraindications and Avoiding Complications – Tips and Tricks (see Figure 3)
Other than lack of informed consent and unprotected left main disease (a relative contraindication) there are no absolute coronary contraindications for ELCA. ELCA complications are similar to those encountered during routine PCI. Specific issues may arise from interruption of the saline flush or contamination with contrast, which can generate excessive heat and increase the risk of vascular perforation. ELCA is not recommended when the operator is aware that there is a long length of sub-intimal guidewire positioning as may exist during hybrid PCI techniques for chronic total occlusions (CTOs).
Clinical Indications for Excimer Laser Coronary Atherectomy
As the application of ELCA has been refined, a number of indications have emerged for the technique:
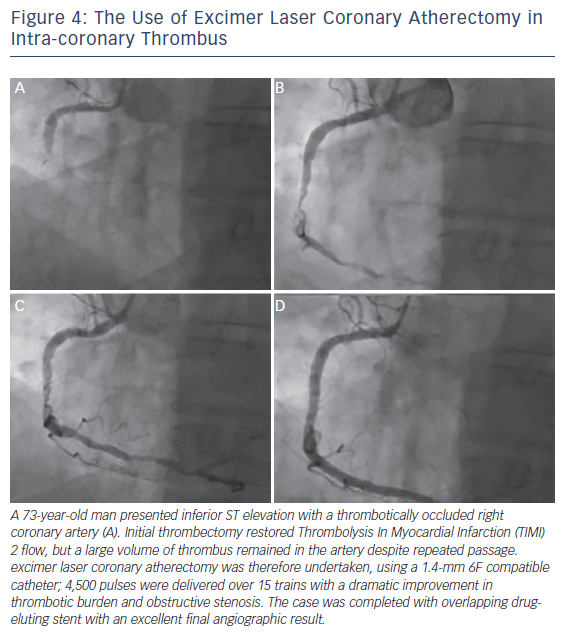
1. Acute Coronary Syndromes and Myocardial Infarction (see Figure 4)
The recommended treatment for acute myocardial infarction (AMI) associated with electrocardiogram (ECG) ST segment elevation is primary PCI.17,18 ELCA may be a beneficial given its potential for effective thrombus removal,19 promotion of fibrinolysis,20 plateletstunning effects21 and concomitant plaque debulking.22 We have published case reports of how effective ELCA can be at dealing with a large burden of intra-coronary thrombus providing excellent immediate and long-term results.23,24
However, clinical data supporting the use of ELCA in AMI remain limited. The largest study to date, the Cohort of Acute Revascularization of Myocardial infarction with Excimer Laser (CARMEL) multicentre registry, enrolled 151 AMI patients, 65 % of whom had large thrombus burden in the culprit artery.25 Following ELCA, Thrombolysis In Myocardial Infarction (TIMI) flow grade was significantly increased (1.2 to 2.8), with an associated reduction in angiographic stenosis (83 to 52 %).25 There was a low rate (8.6 %) of major adverse coronary events (MACE). The maximal effect was observed in arteries with a large angiographic thrombus burden.
A single randomised trial, the Laser AMI study, has been conducted. This study included 66 patients and sought to demonstrate safety and feasibility. They used optimal lasing technique (saline flushing with slow advancement [0.2–0.5 mm/second]) using the CVX-300 Excimer laser system and treated the majority of lesions with a laser-stent strategy (only two patients required balloon angioplasty prior to stenting). Primary angiographic endpoints were myocardial blush grade, TIMI flow and length-adjusted TIMI frame count. The TIMI score increased from 0.2±0.4 at baseline to 2.65±0.5 post-laser to 2.9±0.3 post-stent (both p<0.01 versus baseline). Similarly, myocardial blush grade increased from 0.12±0.4 to 2.5±0.6 post-laser, and to 2.8±0.4 post-stent. No reflow was observed in 11 % of cases after laser and a major dissection occurred in one case. There were no intraprocedural deaths and 95 % event-free survival at 6 months with LV remodelling occurring in 8 % patients.26 A second, larger study is ongoing and due to report in 2016.
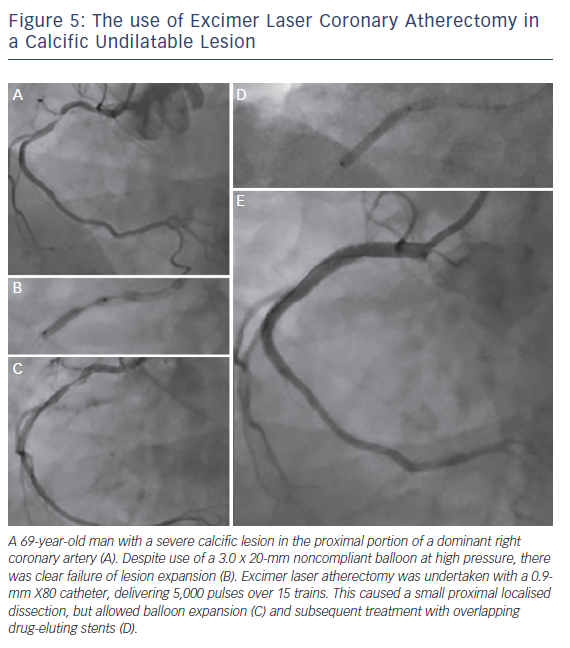
2. Excimer Laser Coronary Atherectomy for Non-crossable/Non-dilatable Lesions (Balloon Failure; see Figure 5)
Balloon failure occurs when a lesion cannot be crossed with a low-profile device, or when the balloon inadequately expands with dilatation. This is a situation where ELCA may be applied, with a high success rate in un-crossable or un-dilatable stenoses. However, in cases of significant calcification, the response is less favourable (calcified 79 % versus non-calcified 96 %; p<0.05).27,28 This is because the ablative effects of ELCA on calcium are minimal and success relies on the ablation of more pliable tissue within the calcific lesion, which will vary accordingly.
In heavily calcified coronary lesions the default technique for the majority of PCI operators remains rotational atherectomy (RA), even among proficient ELCA users. RA requires delivery of a dedicated 0.009-inch guidewire (Rotawire™) into the distal coronary vessel. This wire is less deliverable directly, and it may not be possible either independently or through a micro-catheter. When this situation arises, ELCA can be used to modify the lesion to create a channel through which a Rotawire™ can subsequently be delivered distally (usually via a microcatheter), to permit RA and case completion. We termed the combination of ELCA and RA the RASER technique and have developed this combination of atherectomy devices in a number of challenging cases.29–31 This combined use of atherectomy devices is particularly effective for non-crossable, non-dilatable calcified stenosis frequently encountered in daily PCI practice. The outcome is predictable and is associated with a low complication rate in experienced hands.29–31
The 0.9-mm X80 catheter is selected in the vast majority of balloon failure cases since this catheter provides the widest range of power and repetition rate to maximise the chances of procedural success. Given that this catheter only requires a 6F guiding system, the application of such techniques can easily be achieved through the transradial approach in keeping with contemporary PCI practice. Other devices that can be used to facilitate the PCI in these situations include the use of the GuideLiner® (Vascular Solutions) for the delivery of the ELCA, although care should be taken to retract the device prior to commencing lasing since the obstruction to blood flow may lead to ischaemia.32 Support strategies that require the use of additional wires (e.g., anchor wires, balloons, etc.) can also be used given that it is possible to safely laser with a second wire in place.
3. Excimer Laser Coronary Atherectomy for Chronic Total Occlusions
The role of ELCA in the treatment of CTOs is for resistant lesions: when equipment is unable to cross the lesion or proximal cap despite attaining distal wire position. It may also offer additional benefits as its ablative effect is transmitted through the lesion architecture, potentially weakening bonds between the constituent components of the CTO. In addition, the antithrombotic19,20 and platelet-suppressive21 effects of ELCA may reduce the risk of thrombotic complications during disobilteration. A success rate of 86–90 % for ELCA in CTO cases has been reported.16,29,33 From a technical perspective, saline is often not used at the laser–lesion interface for CTO cases as anterograde injections are usually avoided to prevent extending areas of dissection. In addition, it is unlikely that saline would reach the laser–tissue interface.
4. Excimer Laser Coronary Atherectomy in Underexpanded Stents (see Figure 6)
Stent under-expansion poses a significant risk for stent thrombosis. There are few PCI options available when this occurs. Maximal balloon dilatation (both diameter and pressure) has often already been undertaken, and RA risks metal fragment embolisation and burr stalling. ELCA remains the only technique that is able to modify the underlying resistant atheroma by delivering energy to the abluminal stent surface without disrupting the stent architecture.34,35 While having no impact on the calcification itself, ELCA modifies the plaque behind the stent, which weakens the overall resistance, thus enabling subsequent complete stent expansion.36–38
We have found that delivering high power laser energy (80 mJ/mm2/ 80 Hz), using the 0.9-mm X80 catheter in the absence of saline, or with contrast injection, amplifies the ablative effect. Within a stented environment ‘contrast-mileu’ lasing appears to be safe, facilitating high-pressure balloon stent expansion.36–39
This technique has been evaluated in the ELLEMENT registry of 28 patients.40 Procedural success was achieved in 96.4 % (27/28) of cases, using an increase of either 1 cm2 on intravenous ultrasound (IVUS) or 10 % using quantitative coronary angiography derived minimal stent diameter as a definition. This confirmed efficacy, with a low associated MACE rate.40
5. In-stent Restenosis
ISR remains a major limitation of PCI following stent implantation with rates of restenosis reported in 10–50 % of patients receiving a baremetal stent,41 although considerably less in the DES era.42 ELCA is a safe and effective technique in the treatment of ISR.43 Excimer laser did not alter stainless-steel stent endurance or liberate any significant material when five types of stainless-steel stents were subjected to 1,000 pulses of laser energy from a 2.0-mm eccentric Excimer laser catheter.35 In a clinical study, the examination of 107 re-stentoic lesions in 98 patients demonstrated that lesions treated with ELCA compared with balloon angioplasty alone, had a greater IVUS cross-sectional area and luminal gain, with more intimal hyperplasia ablation. There was a non-significant trend towards a less frequent need for target vessel revascularisation at 6 months (21 versus 38 %; p=0.083).43
We have observed, using optical coherence tomography (OCT) and optical frequency domain imaging, that during treatment of the restenotic segment, the laser therapy ablates both the luminal and abluminal atherosclerotic material.44 Therefore, if the mechanism of restenosis is in part stent under-expansion, ELCA increases the likelihood of achieving greater stent expansion and more durable longer-term outcomes.
6. Saphenous Vein Grafts
Occlusions in old saphenous vein grafts (SVGs) frequently consist of degenerative diffuse plaques often containing thrombus45,46 and prone to distal embolisation.47 Hence, distal protection devices (DPDs) are advocated when attempting SVG-PCI,46 but their bulky nature may prevent distal device delivery. ELCA is a safer alternative, allowing predictable debulking during SVG-PCI.48,49 The low rate of distal embolisation during ELCA of degenerative bypass grafts (1–5 %) may preclude the need for routine DPD in the majority of cases.48 However, OCT images post-SVG ELCA make it is clear that there remains friable fragments that could embolise and cause no-reflow.44 Therefore, when using ELCA for SVGPCI, it is advisable to stent on a DPD system to prevent no-reflow.44 Given advances in CTO success in recent years, SVG-PCI is likely to be less frequently undertaken as operators choose to treat the occluded native vessel. Nonetheless, if SVG-PCI is considered necessary, ELCA remains a useful adjunctive therapeutic intervention.
7. Bifurcations
Generally PCI for coronary bifurcation lesions is best treated with a main vessel (MV)-only stenting approach with preservation of side branch (SB), rather than an upstream two-stent strategy. However, in large vessels involving extensive SB disease it may be necessary to stent SB as well. ELCA could potentially be of value in these cases by debulking the SB lesion to permit more predictable success with the MV-only approach. However, in the few cases in our practice in which we have used this technique we have discovered SB dissection because of vessel angulation, which has necessitated SB stenting – thereby defeating the purpose of using ELCA. We have used ELCA more successfully in rare cases of SB restensosis (often due to stent under-expansion) guided by intra-coronary imaging with durable results.
Conclusion
The current indications for the use of Excimer laser atherectomy in modern interventional practice are described in this article. A detailed description of the ELCA technique and its potential pitfalls has been illustrated with complex interventional cases. This technology provides a solution to a variety of problems that may be encountered, including massive intra-coronary thrombus, un-crossable lesions and stent under-expansion. Careful case selection, proper use of equipment and safe, efficacious lasing technique all play crucial roles in successful ELCA interventions.